A Nanoengineered Stainless Steel Surface to Combat Bacterial Attachment and Biofilm Formation
Abstract
1. Introduction
2. Materials and Methods
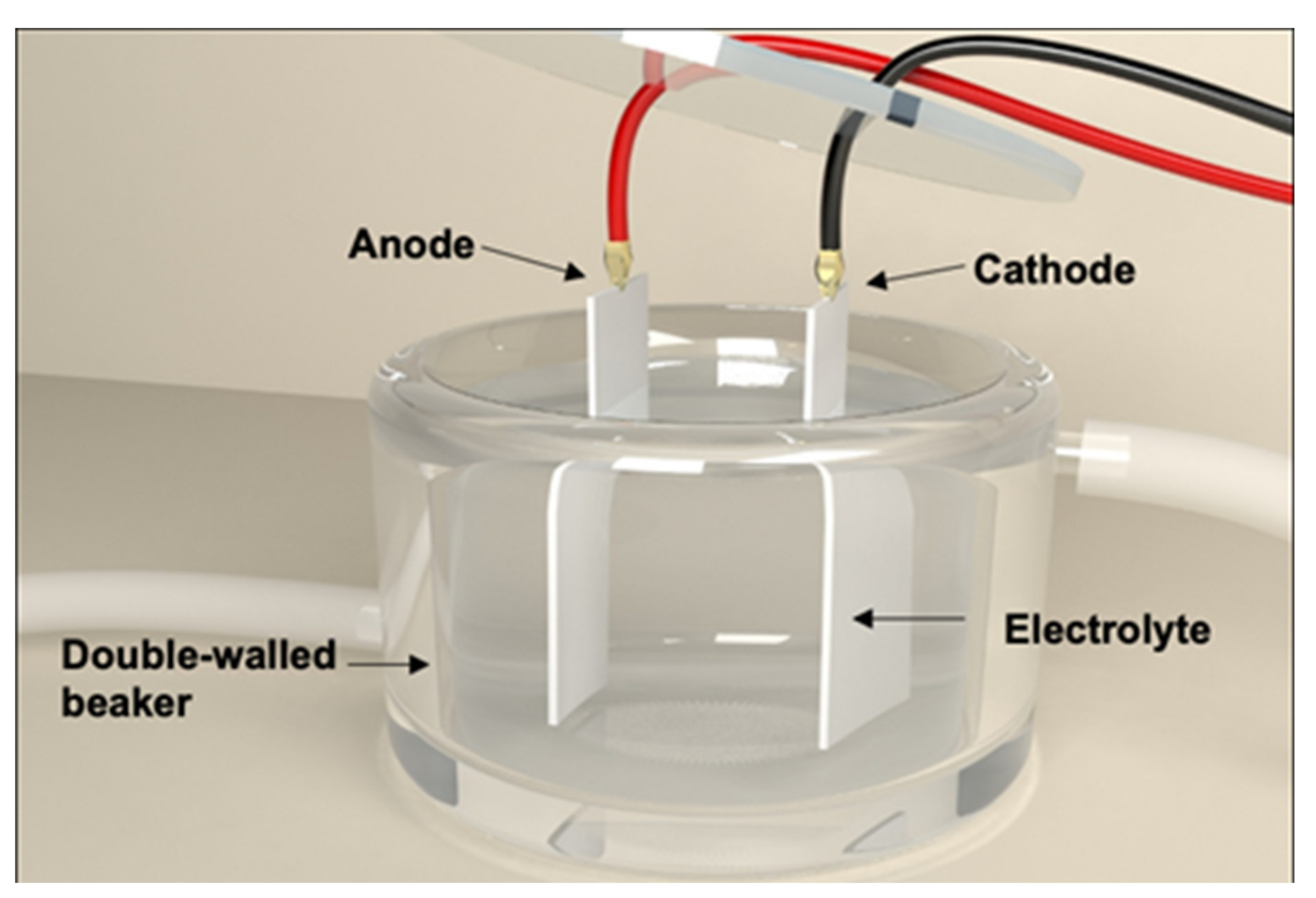
2.1. Fabrication of Nanoengineered Stainless Steel Surface Using Electrochemical Etching
2.2. Creation of Superhydrophobic Surface on Stainless Steel
2.3. Bacterial Strains and Culture Preparation
2.4. Bacterial Attachment and Biofilm Forming Ability Test
2.5. Bacterial Enumeration
2.6. Field Emission Scanning Electron Microscopy (FE-SEM)
2.7. Statistical Analysis
3. Results and Discussion
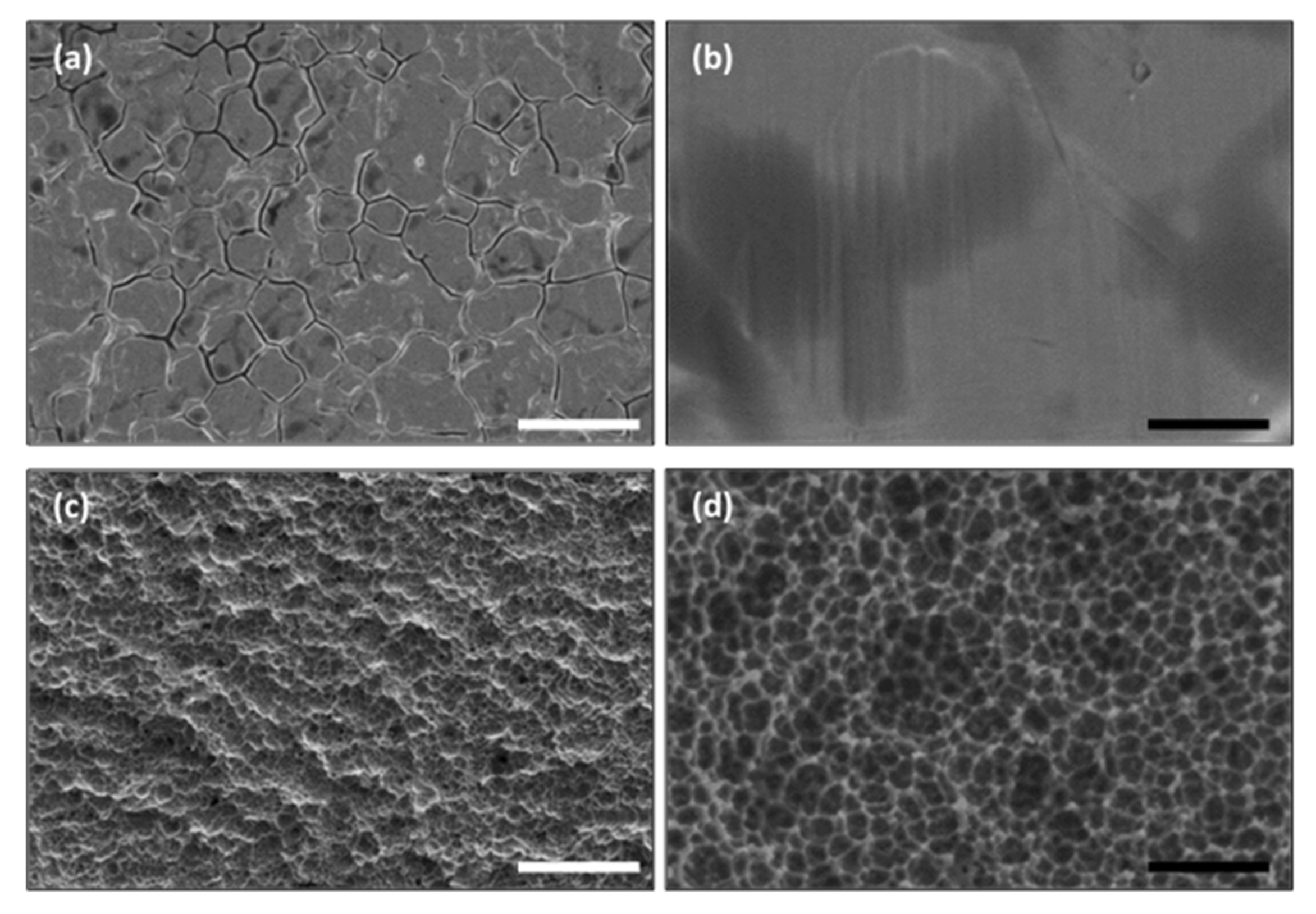
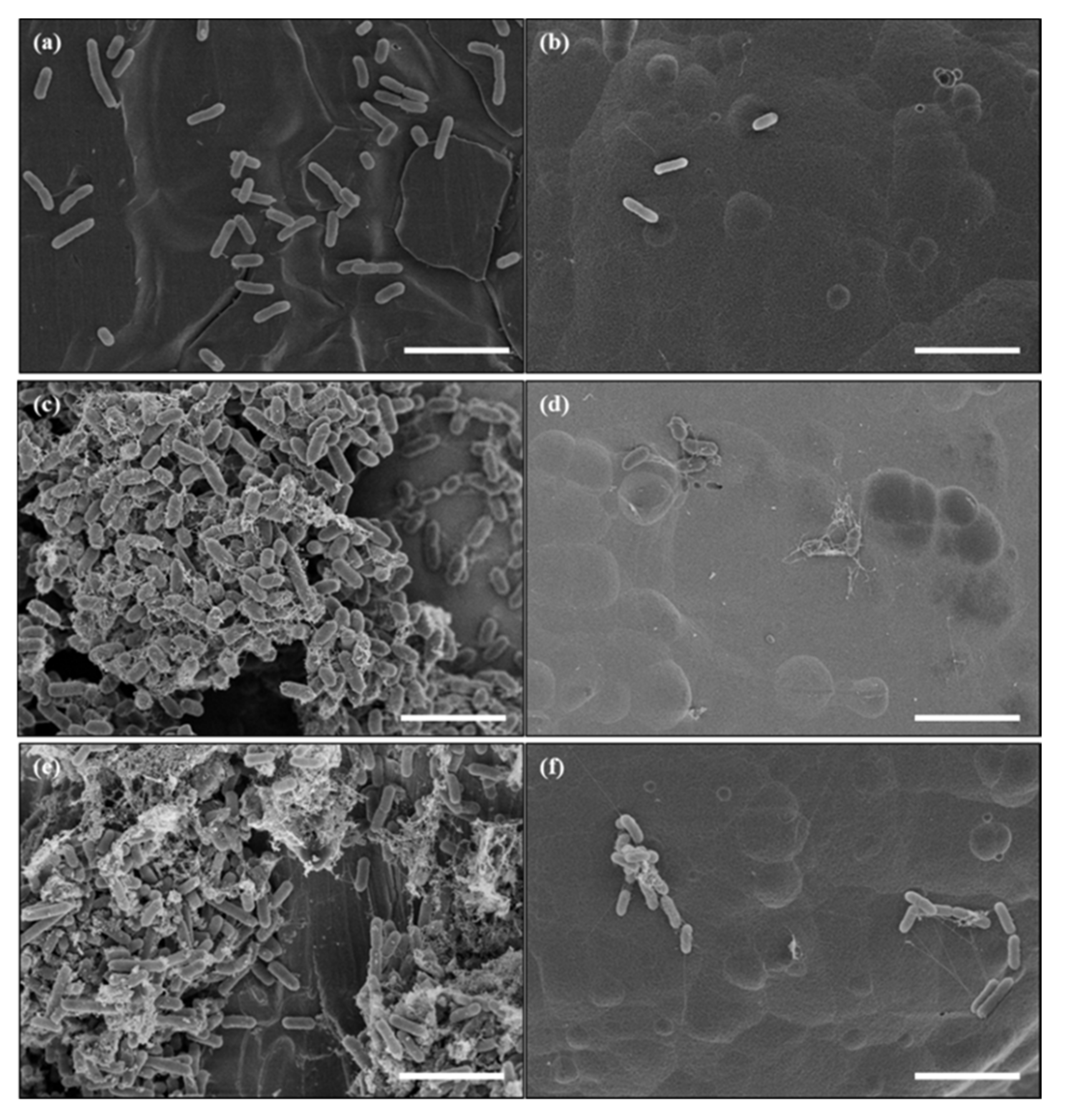
3.1. Surface Morphology of Nanoengineered Stainless Steel Surface
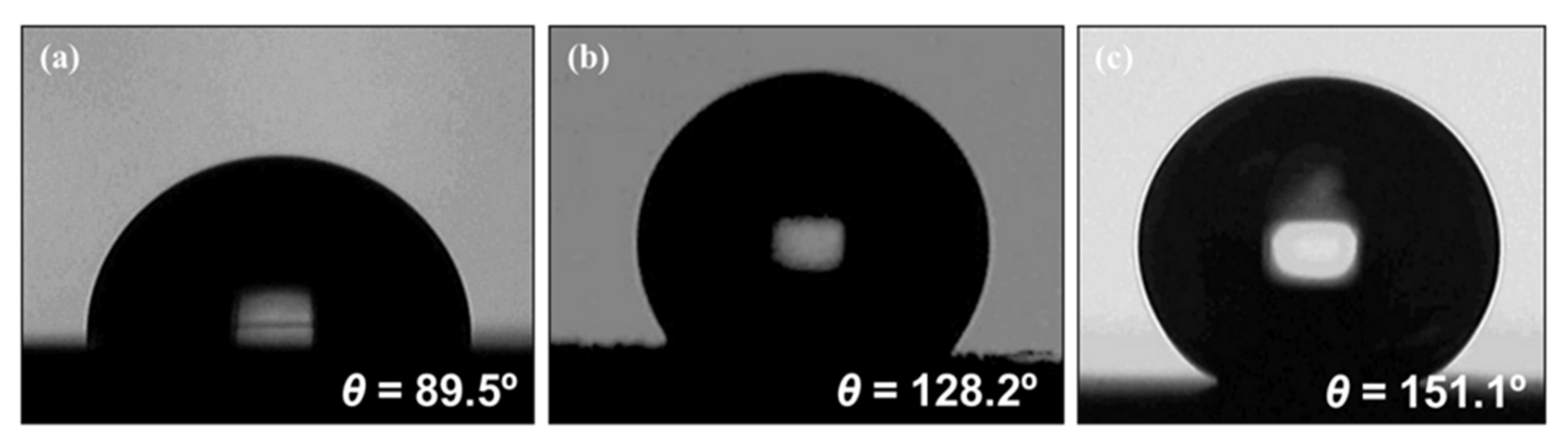
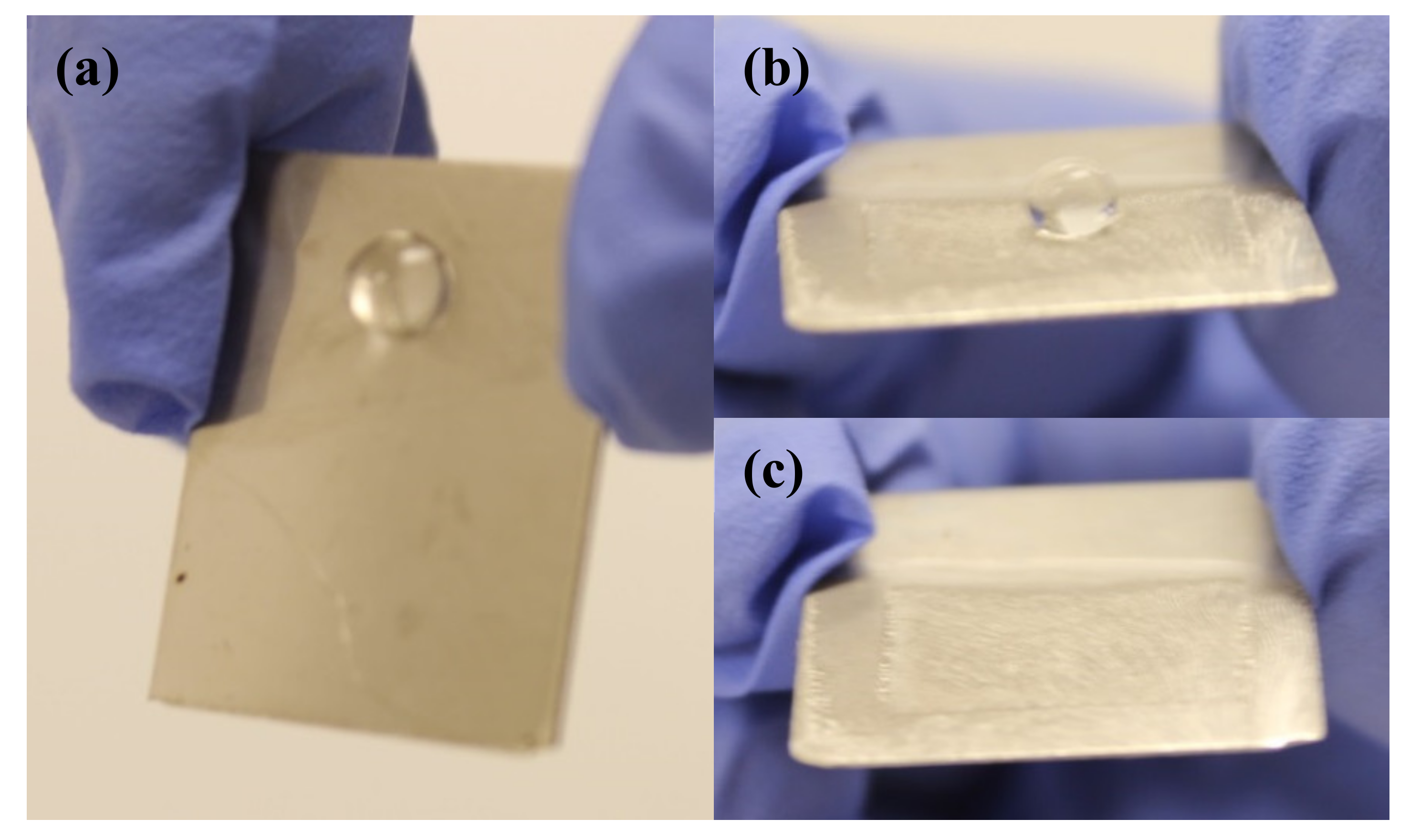
3.2. Wetting Properties of Nanoengineered Stainless Steel Surface
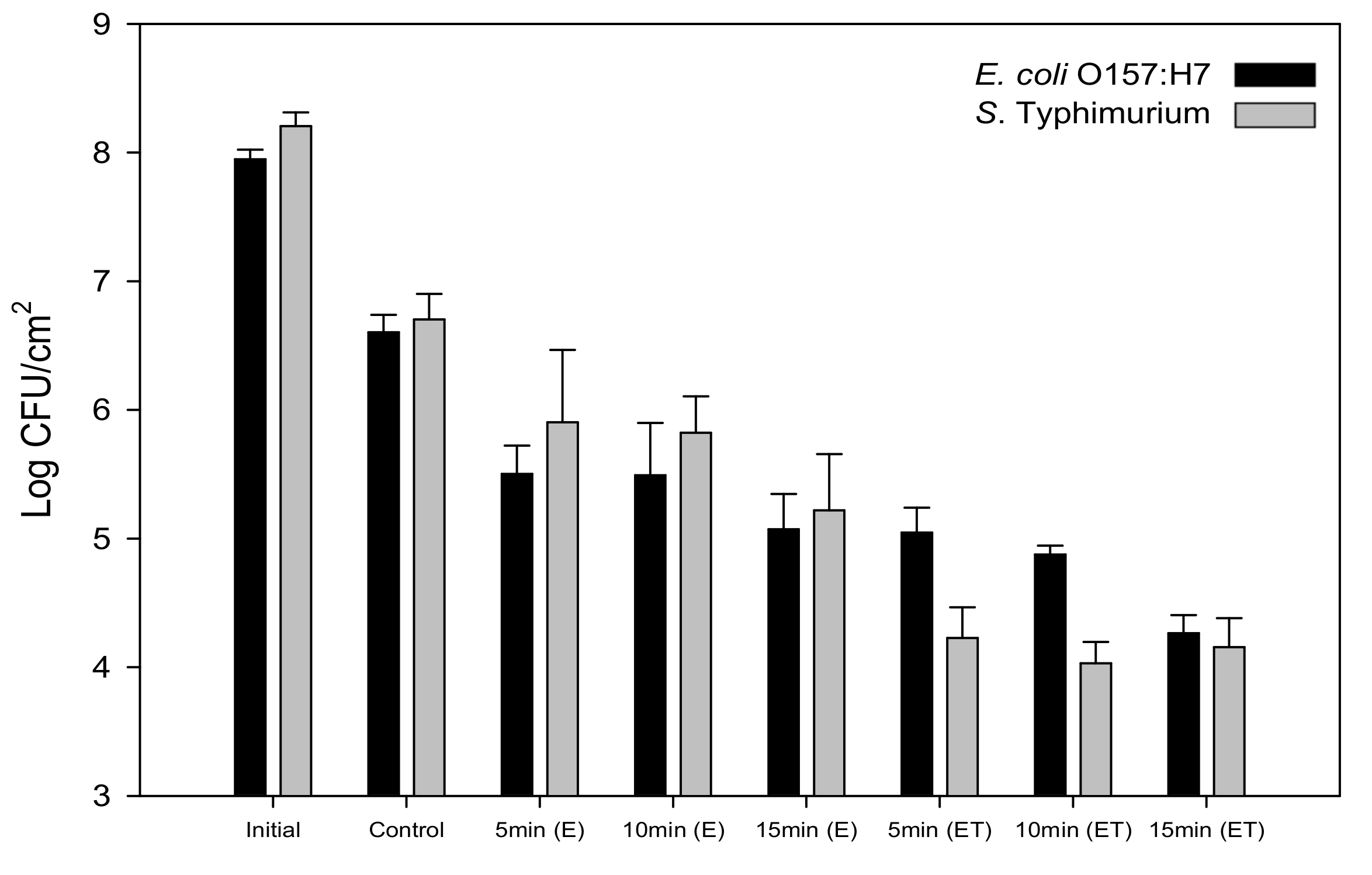
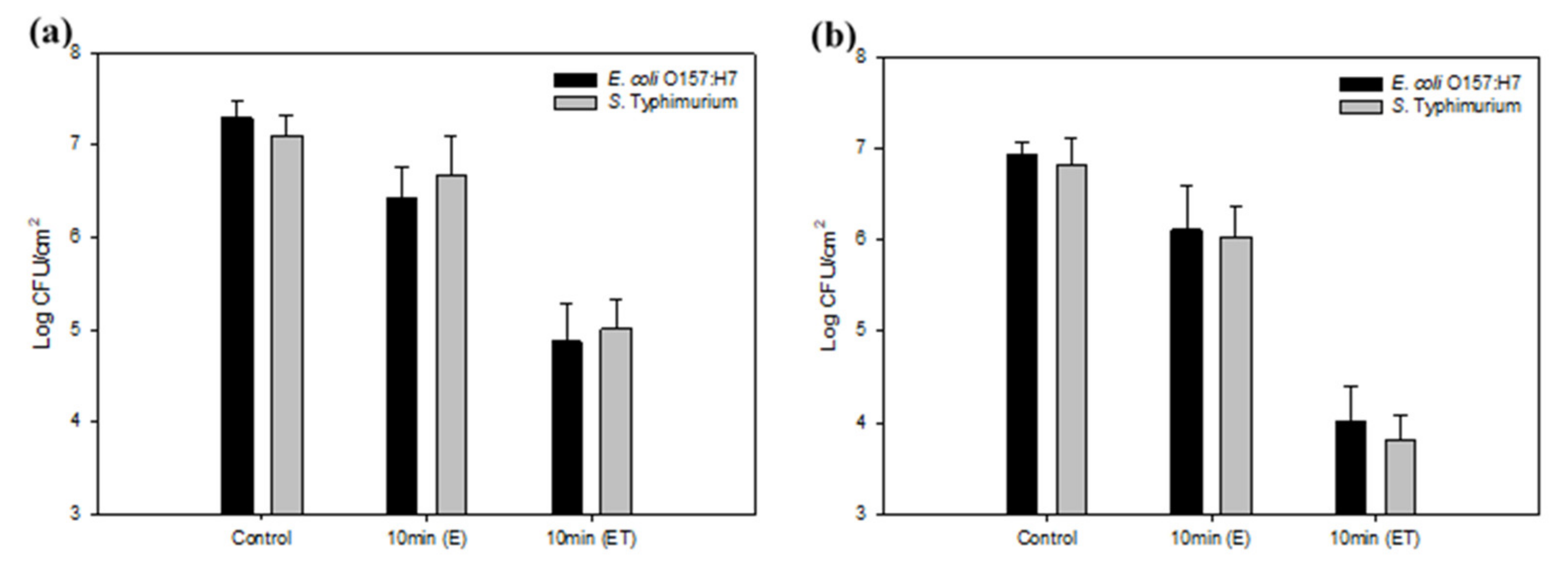
3.3. Reduced Bacterial Adhesion and Biofilm Formation on a Nanoengineered Stainless Steel Surface
Author Contributions
Funding
Conflicts of Interest
References
- Davies, D. Understanding Biofilm Resistance to Antibacterial Agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Genet. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.K.; Zottola, E.A. Adherence to Stainless Steel by Foodborne Microorganisms During Growth in Model Food Systems. Int. J. Food Microbiol. 1997, 37, 145–153. [Google Scholar] [CrossRef]
- Sinde, E.; Carballo, J. Attachment of Salmonella spp. and Listeria Monocytogenes to Stainless Steel, Rubber and Polytetrafluorethylene: The Influence of Free Energy and the Effect of Commercial Sanitizers. Food Microbiol. 2000, 17, 439–447. [Google Scholar] [CrossRef]
- Stepanovic, S.; Cirkovic, I.; Ranin, L.; Svabić-Vlahović, M. Biofilm Formation by Salmonella spp. and Listeria monocytogenes on Plastic Surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Hyde, F.W.; Alberg, M.; Smith, K. Comparison of Fluorinated Polymers Against Stainless Steel, Glass and Polypropylene in Microbial Biofilm Adherence and Removal. J. Ind. Microbiol. Biotechnol. 1997, 19, 142–149. [Google Scholar] [CrossRef]
- Vogeleer, P.; Tremblay, Y.D.; Mafu, A.A.; Jacques, M.; Harel, J. Life on the Outside: Role of Biofilms in Environmental Persistence of Shiga-Toxin Producing Escherichia coli. Front. Microbiol. 2014, 5, 317. [Google Scholar] [CrossRef]
- Steenackers, H.; Hermans, K.; Vanderleyden, J.; De Keersmaecker, S.C.J. Salmonella Biofilms: An Overview on Occurrence, Structure, Regulation and Eradication. Food Res. Int. 2012, 45, 502–531. [Google Scholar] [CrossRef]
- Doyle, M.P. Escherichia coli O157: H7 and Its Significance in Foods. Int. J. Food Microbiol. 1991, 12, 289–301. [Google Scholar] [CrossRef]
- Baird-Parker, A. Foodborne Salmonellosis. Lancet 1990, 336, 1231–1235. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G. Prevention and Control of Biofilm-Based Medical-Device-Related Infections. FEMS Immunol. Med. Microbiol. 2010, 59, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Michiels, C.W. Biofilm Formation and the Food Industry: A Focus on the Bacterial Outer Surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef]
- Lellouch, J.; Friedman, A.; Lahmi, R.; Gedanken, A.; Banin, E. Antibiofilm Surface Functionalization of Catheters by Magnesium Fluoride Nanoparticles. Int. J. Nanomed. 2012, 7, 1175–1188. [Google Scholar] [CrossRef][Green Version]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-Biofilm Properties of Chitosan-Coated Surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2010, 23, 690–718. [Google Scholar] [CrossRef]
- Yoon, S.H.; Rungraeng, N.; Song, W.; Jun, S. Superhydrophobic and Superhydrophilic Nanocomposite Coatings for Preventing Escherichia coli K-12 Adhesion on Food Contact Surface. J. Food Eng. 2014, 131, 135–141. [Google Scholar] [CrossRef]
- Epstein, A.K.; Hochbaum, A.I.; Kim, P.; Aizenberg, J. Control of Bacterial Biofilm Growth on Surfaces by Nanostructural Mechanics and Geometry. Nanotechnology 2011, 22, 494007. [Google Scholar] [CrossRef]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.S.; Israelachvili, J.N.; Full, R.J. Evidence for Van Der Waals Adhesion in Gecko Setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef]
- Hazan, Z.; Zumeris, J.; Jacob, H.; Raskin, H.; Kratysh, G.; Vishnia, M.; Dror, N.; Barliya, T.; Mandel, M.; Lavie, G. Effective Prevention of Microbial Biofilm Formation on Medical Devices by Low-Energy Surface Acoustic Waves. Antimicrob. Agents Chemother. 2006, 50, 4144–4152. [Google Scholar] [CrossRef]
- Harris, J.M. Poly (Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Epstein, A.K.; Wong, T.-S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-Infused Structured Surfaces with Exceptional Anti-Biofouling Performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, D.; Lu, Z. Slippery Liquid-Infused Porous Surface Bio-Inspired by Pitcher Plant for Marine Anti-Biofouling Application. Colloids Surf. B Biointerfaces 2015, 136, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Indirect Food Additives: Paper and Paperboard Components. Fed. Regist 2016, 81, 5–8. [Google Scholar]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Marmur, A. The Lotus Effect: Superhydrophobicity and Metastability. Langmuir 2004, 20, 3517–3519. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Zhang, W.; Wang, Y.; Zhang, B.; Wang, H.; Lin, C.; Zhang, L. Effect of Superhydrophobic Surface of Titanium on Staphylococcus aureus Adhesion. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Hizal, F.; Rungraeng, N.; Lee, J.; Jun, S.; Busscher, H.J.; Van Der Mei, H.C.; Choi, C.-H. Nanoengineered Superhydrophobic Surfaces of Aluminum with Extremely Low Bacterial Adhesivity. ACS Appl. Mater. Interfaces 2017, 9, 12118–12129. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Y. Preparation and Physical Properties of Superhydrophobic Papers. J. Colloid Interface Sci. 2008, 325, 588–593. [Google Scholar] [CrossRef]
- Hong, I.; Koo, C. Antibacterial Properties, Corrosion Resistance and Mechanical Properties of CU-Modified SUS 304 Stainless Steel. Mater. Sci. Eng. A 2005, 393, 213–222. [Google Scholar] [CrossRef]
- Anselme, K.; Davidson, P.; Popa, A.; Giazzon, M.; Liley, M.; Ploux, L. The Interaction of Cells and Bacteria with Surfaces Structured at the Nanometre Scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef]
- Lee, C.; Kim, A.; Kim, J. Electrochemically Etched Porous Stainless Steel for Enhanced Oil Retention. Surf. Coat. Technol. 2015, 264, 127–131. [Google Scholar] [CrossRef]
- Kim, H.; Ryu, J.-H.; Beuchat, L.R. Attachment of and Biofilm Formation by Enterobacter sakazakii on Stainless Steel and Enteral Feeding Tubes. Appl. Environ. Microbiol. 2006, 72, 5846–5856. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pitts, B.; Stewart, P.S.; Camper, A.; Yoon, J. Comparison of the Antimicrobial Effects of Chlorine, Silver Ion, and Tobramycin on Biofilm. Antimicrob. Agents Chemother. 2008, 52, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.D.; Verran, J.; Hall, K.; Underhill, C.; Hibbert, S.; West, R. The Cleanability of Stainless Steel as Determined by X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. 2001, 172, 135–143. [Google Scholar] [CrossRef]
- Sulka, G.D.; Parkoła, K. Temperature Influence on Well-Ordered Nanopore Structures Grown by Anodization of Aluminium in Sulphuric Acid. Electrochim. Acta 2007, 52, 1880–1888. [Google Scholar] [CrossRef]
- Song, H.-J.; Kim, M.-K.; Jung, G.-C.; Vang, M.-S.; Park, Y.-J. The Effects of Spark Anodizing Treatment of Pure Titanium Metals and Titanium Alloys on Corrosion Characteristics. Surf. Coat. Technol. 2007, 201, 8738–8745. [Google Scholar] [CrossRef]
- Wang, Y.; Subbiahdoss, G.; Swartjes, J.; Van Der Mei, H.C.; Busscher, H.; Libera, M. Length-Scale Mediated Differential Adhesion of Mammalian Cells and Microbes. Adv. Funct. Mater. 2011, 21, 3916–3923. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, A.F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Quéré, D.; Reyssat, M. Non-Adhesive Lotus and Other Hydrophobic Materials. Philos. Trans. R. Soc. A 2008, 366, 1539–1556. [Google Scholar] [CrossRef]
- Martin, S.; Brown, P.S.; Bhushan, B. Fabrication Techniques for Bioinspired, Mechanically-Durable, Superliquiphobic Surfaces for Water, Oil, and Surfactant Repellency. Adv. Colloid Interface Sci. 2017, 241, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef] [PubMed]
- Ban, G.-H.; Lee, J.; Choi, C.-H.; Li, Y.; Jun, S. Nano-Patterned Aluminum Surface with Oil-Impregnation for Improved Antibacterial Performance. LWT 2017, 84, 359–363. [Google Scholar] [CrossRef]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef]
- Wang, R.; Kalchayanand, N.; Schmidt, J.W.; Harhay, D.M. Mixed Biofilm Formation by Shiga Toxin–Producing Escherichia coli and Salmonella enterica serovar Typhimurium Enhanced Bacterial Resistance to Sanitization due to Extracellular Polymeric Substances. J. Food Prot. 2013, 76, 1513–1522. [Google Scholar] [CrossRef][Green Version]
- Purevdorj, B.; Costerton, J.W.; Stoodley, P. Influence of Hydrodynamics and Cell Signaling on the Structure and Behavior of Pseudomonas aeruginosa Biofilms. Appl. Environ. Microbiol. 2002, 68, 4457–4464. [Google Scholar] [CrossRef]
- Nejadnik, M.R.; Van Der Mei, H.C.; Busscher, H.J.; Norde, W. Determination of the Shear Force at the Balance between Bacterial Attachment and Detachment in Weak-Adherence Systems, Using a Flow Displacement Chamber. Appl. Environ. Microbiol. 2007, 74, 916–919. [Google Scholar] [CrossRef]
- Yin, J.; Mei, M.L.; Li, Q.; Xia, R.; Zhang, Z.; Chu, C.H. Self-Cleaning and Antibiofouling Enamel Surface by Slippery Liquid-Infused Technique. Sci. Rep. 2016, 6, 25924. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, G.-H.; Li, Y.; Wall, M.M.; Jun, S. A Nanoengineered Stainless Steel Surface to Combat Bacterial Attachment and Biofilm Formation. Foods 2020, 9, 1518. https://doi.org/10.3390/foods9111518
Ban G-H, Li Y, Wall MM, Jun S. A Nanoengineered Stainless Steel Surface to Combat Bacterial Attachment and Biofilm Formation. Foods. 2020; 9(11):1518. https://doi.org/10.3390/foods9111518
Chicago/Turabian StyleBan, Ga-Hee, Yong Li, Marisa M. Wall, and Soojin Jun. 2020. "A Nanoengineered Stainless Steel Surface to Combat Bacterial Attachment and Biofilm Formation" Foods 9, no. 11: 1518. https://doi.org/10.3390/foods9111518
APA StyleBan, G.-H., Li, Y., Wall, M. M., & Jun, S. (2020). A Nanoengineered Stainless Steel Surface to Combat Bacterial Attachment and Biofilm Formation. Foods, 9(11), 1518. https://doi.org/10.3390/foods9111518




