The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials
Abstract
1. Introduction
- The freezing of the product, most often under atmospheric pressure.
- Primary drying—proper freeze-drying—ice sublimation, most often at reduced pressure.
- Secondary drying—desorption drying—drying the product to the required final humidity.
2. The Characteristics of the Freeze-Drying Process
- The phase transition of the water contained in the product into ice.
- The ice to vapor phase transition.
- The desorption of water molecules from material structures.
- The obtainment of a sufficiently low pressure.
- The re-sublimation of water vapor removed from the material on the surface of the condenser.
- The removal of a layer of ice from the surface of the capacitor.
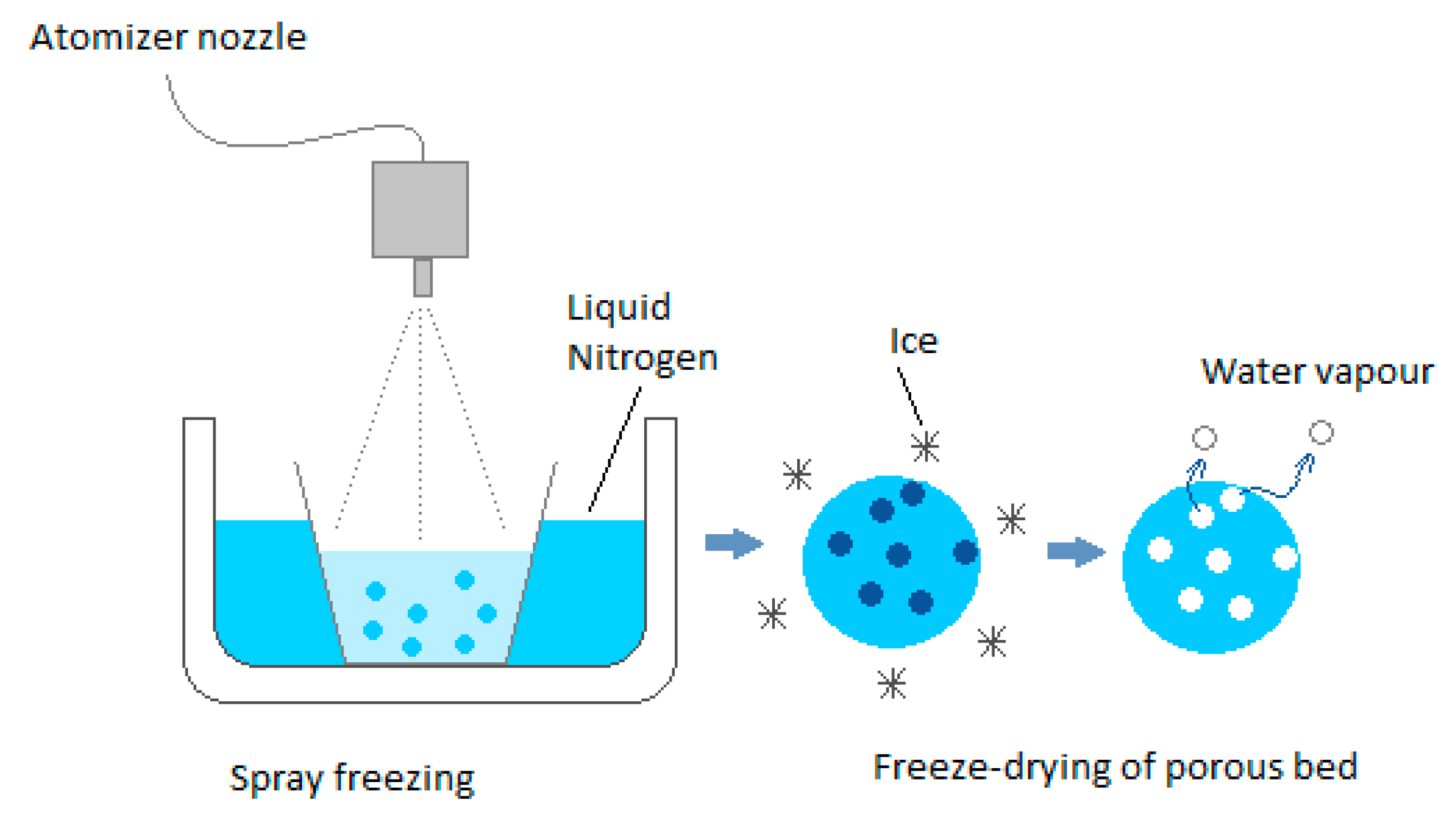
2.1. The First Stage of the Freeze-Drying Process—Freezing the Raw Material
- Immobilizing the ingredients in solution and preventing foaming occurring during pressure reduction in the freeze-dryer chamber.
- Limiting the chemical, biochemical, and microbiological changes taking place in the material.
- Creating a specific structure of ice crystals in the frozen product, which, in the next step, facilitates or limits the migration of water vapor from the dried material; the structure of ice formed during freezing determines the intensity of mass movement and, as a result, shapes the final morphology of the dried material [23].
- Stiffening of the structure, counteracting contraction of the cells of plant or animal tissue caused by the removal of water from them, which is possible due to the plasticization of the material by liquid water.
2.2. Primary Drying—Sublimation
2.3. Second Drying—Desorption
2.4. Methods of Determining the End of Primary and Secondary Drying
3. Effect of Freeze-Drying Conditions of the Selected Physical Properties of Materials
3.1. The Shelf Temperature
3.2. The Pressure Chamber
3.3. The Freezing Rate
4. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haseley, P.; Oetjen, G.W. Freeze-Drying; Wiley-VCH: Veinheim, Germany, 2018; p. 421. [Google Scholar]
- Mellor, J.D. Fundamentals of Freeze-Drying; Academic Press Inc.: London, UK, 1978; p. 386. [Google Scholar]
- Adams, G.D.J.; Cook, I.; Ward, K.R. The Principles of Freeze-Drying. In Cryopreservation and Freeze-Drying Protocols, 3rd ed.; Wolkers, W.F., Oldenhof, H., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2015; Volume 1257, pp. 121–143. [Google Scholar]
- Franks, F.; Auffret, T. Freeze-Drying of Pharmaceuticals and Biopharmaceuticals; RSC Publishing: Cambridge, UK, 2008; p. 218. [Google Scholar]
- Liu, Y.Z.; Zhao, Y.F.; Feng, X. Exergy analysis for a freeze-drying process. Appl. Therm. Eng. 2008, 28, 675–690. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Assegehegn, G.; Brito-de la Fuente, E.; Franco, J.M.; Gallegos, C. The importance of understanding the freezing step and its impact on freeze-drying process performance. J. Pharm. Sci. 2019, 108, 1378–1395. [Google Scholar] [CrossRef] [PubMed]
- Meda, L.; Ratti, C. Rehydration of freeze-dried strawberries at varying temperatures. J. Food Process Eng. 2005, 28, 233–246. [Google Scholar] [CrossRef]
- Jia, Y.; Khalifa, I.; Hu, L.; Zhu, W.; Li, J.; Li, K.; Li, C. Influence of three different drying techniques on persimmon chips’ characteristics: A comparison study among hot-air, combined hot-air-microwave, and vacuum-freeze drying techniques. Food Bioprod. Process. 2019, 118, 67–76. [Google Scholar] [CrossRef]
- Oikonomopoulou, V.P.; Krokida, M.K.; Karathanos, V.T. The influence of freeze drying conditions on microstructural changes of food products. Procedia Food Sci. 2011, 1, 647–654. [Google Scholar] [CrossRef]
- Krzykowski, A.; Dziki, D.; Rudy, S.; Gawlik-Dziki, U.; Polak, R.; Biernacka, B. Effect of pre-treatment conditions and freeze-drying temperature on the process kinetics and physicochemical properties of pepper. LWT 2018, 98, 25–30. [Google Scholar] [CrossRef]
- Martinez-Navarrete, N.; Salvador, A.; Oliva, C.; Camacho, M.M. Influence of biopolymers and freeze-drying shelf temperature on the quality of a mandarin snack. LWT 2019, 99, 57–61. [Google Scholar] [CrossRef]
- Wu, X.-f.; Zhang, M.; Bhandari, B. A novel infrared freeze drying (IRFD) technology to lower the energy consumption and keep the quality of Cordyceps militaris. Innov. Food Sci. Emerg. 2019, 54, 34–42. [Google Scholar] [CrossRef]
- Silva-Espinoza, M.A.; Ayed, C.; Foster, T.; Camacho, M.D.M.; Martinez-Navarrete, N. The impact of freeze-drying conditions on the physico-chemical properties and bioactive compounds of a freeze-dried orange puree. Foods 2019, 9, 32. [Google Scholar] [CrossRef]
- Khalloufi, S.; Ratti, C. Quality deterioration of freeze-dried foods as explained by their glass transition temperature and internal structure. J. Food Sci. 2003, 68, 892–903. [Google Scholar] [CrossRef]
- Egas-Astudillo, L.A.; Martínez-Navarrete, N.; Camacho, M.M. Impact of biopolymers added to a grapefruit puree and freeze-drying shelf temperature on process time reduction and product quality. Food Bioprod. Process. 2020, 120, 143–150. [Google Scholar] [CrossRef]
- Flink, J.M. Energy analysis in dehydration processes. Food Technol. 1977, 31, 77–84. [Google Scholar]
- Flink, J.M. Simplified cost comparison of a freeze-dried food with its canned and frozen counterparts. Food Technol. 1977, 31, 50–56. [Google Scholar]
- Nowak, D. The Innovative Measurement System of the Kinetic of Freeze-Drying and Sorption Properties of Dried Products as a Tool for Controlling and Assessing the Course of Freeze-drying; Warsaw University of Life Sciences Press: Warsaw, Poland, 2017; p. 217. [Google Scholar]
- Patel, S.M.; Doen, T.; Pikal, M.J. Determination of end point of primary drying in freeze-drying process control. AAPS PharmSciTech 2010, 11, 73–84. [Google Scholar] [CrossRef]
- Liu, J.S.; Viverette, T.; Virgin, M.; Anderson, M.; Dalal, P. A study of the impact of freezing on the lyophilization of a concentrated formulation with a high fill depth. Pharm. Dev. Technol. 2005, 10, 261–272. [Google Scholar] [CrossRef]
- Petzold, G.; Aguilera, J.M. Ice morphology: Fundamentals and technological applications in foods. Food Biophys. 2009, 4, 378–396. [Google Scholar] [CrossRef]
- Geidobler, R.; Winter, G. Controlled ice nucleation in the field of freeze-drying: Fundamentals and technology review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef]
- Kasper, J.C.; Friess, W. The freezing step in lyophilization: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 78, 248–263. [Google Scholar] [CrossRef]
- Hottot, A.; Vessot, S.; Andrieu, J. Freeze drying of pharmaceuticals in vials: Influence of freezing protocol and sample configuration on ice morphology and freeze-dried cake texture. Chem. Eng. Process. 2007, 46, 666–674. [Google Scholar] [CrossRef]
- Ceballos, A.M.; Giraldo, G.I.; Orrego, C.E. Effect of freezing rate on quality parameters of freeze dried soursop fruit pulp. J. Food Eng. 2012, 111, 360–365. [Google Scholar] [CrossRef]
- Nowak, D.; Piechucka, P.; Witrowa-Rajchert, D.; Wiktor, A. Impact of material structure on the course of freezing and freeze-drying and on the properties of dried substance, as exemplified by celery. J. Food Eng. 2016, 180, 22–28. [Google Scholar] [CrossRef]
- Wanning, S.; Süverkrüp, R.; Lamprecht, A. Jet-vortex spray freeze drying for the production of inhalable lyophilisate powders. Eur. J. Pharm. Sci. 2017, 96, 1–7. [Google Scholar] [CrossRef]
- Hu, J.H.; Johnston, K.P.; Williams, R.O. Spray freezing into liquid (SFL) particle engineering technology to enhance dissolution of poorly water soluble drugs: Organic solvent versus organic/aqueous co-solvent systems. Eur. J. Pharm. Sci. 2003, 20, 295–303. [Google Scholar] [CrossRef]
- Rogers, T.L.; Hu, J.H.; Yu, Z.S.; Johnston, K.P.; Williams, R.O. A novel particle engineering technology: Spray-freezing into liquid. Int. J. Pharm. 2002, 242, 93–100. [Google Scholar] [CrossRef]
- Adali, M.B.; Barresi, A.A.; Boccardo, G.; Pisano, R. Spray freeze-drying as a solution to continuous manufacturing of pharmaceutical products in bulk. Processes 2020, 8, 709. [Google Scholar] [CrossRef]
- Rogers, T.L.; Nelsen, A.C.; Sarkari, M.; Young, T.J.; Johnston, K.P.; Williams, R.O. Enhanced aqueous dissolution of a poorly water soluble drug by novel particle engineering technology: Spray-freezing into liquid with atmospheric freeze-drying. Pharm. Res. 2003, 20, 485–493. [Google Scholar] [CrossRef]
- Barron, M.K.; Young, T.J.; Johnston, K.P.; Williams, R.O. Investigation of processing parameters of spray freezing into liquid to prepare polyethylene glycol polymeric particles for drug delivery. AAPS PharmSciTech 2003, 4, 1–13. [Google Scholar] [CrossRef]
- Liang, W.; Chan, A.Y.L.; Chow, M.Y.T.; Lo, F.F.K.; Qiu, Y.; Kwok, P.C.L.; Lam, J.K.W. Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery. Asian J. Pharm. 2018, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, L.; Van Bockstal, P.J.; Corver, J.; Vervaet, C.; Remon, J.P.; De Beer, T. Evaluation of spin freezing versus conventional freezing as part of a continuous pharmaceutical freeze-drying concept for unit doses. Int. J. Pharm. 2015, 496, 75–85. [Google Scholar] [CrossRef]
- Searles, J.A.; Carpenter, J.F.; Randolph, T.W. The ice nucleation temperature determines the primary drying rate of lyophilization for samples frozen on a temperature-controlled shelf. J. Pharm. Sci. 2001, 90, 860–871. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.J.; Tessier, S.N.; Banik, P.D.; Nagpal, S.; Cronin, S.E.J.; Ozer, S.; Hafiz, E.O.A.; van Gulik, T.M.; Yarmush, M.L.; Markmann, J.F.; et al. Supercooling extends preservation time of human livers. Nat. Biotechnol. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Jha, P.K.; Xanthakis, E.; Jury, V.; Havet, M.; Le-Bail, A. Advances of electro-freezing in food processing. Curr. Opin. Food Sci. 2018, 23, 85–89. [Google Scholar] [CrossRef]
- Orlowska, M.; Havet, M.; Le-Bail, A. Controlled ice nucleation under high voltage DC electrostatic field conditions. Food Res. Int. 2009, 42, 879–884. [Google Scholar] [CrossRef]
- Fernandez, P.P.; Otero, L.; Guignon, B.; Sanz, P.D. High-pressure shift freezing versus high-pressure assisted freezing: Effects on the microstructure of a food model. Food Hydrocoll. 2006, 20, 510–522. [Google Scholar] [CrossRef]
- Elia, A.M.; Barresi, A.A. Intensification of transfer fluxes and control of product properties in freeze-drying. Chem. Eng. Processin. Process Intensif. 1998, 37, 347–358. [Google Scholar] [CrossRef]
- Silva, A.C.C.; Schmidt, F.C. vacuum freezing of coffee extract under different process conditions. Food Bioprocess Technol. 2019, 12, 1683–1695. [Google Scholar] [CrossRef]
- Lammerskitten, M.; Mykhailyk, V.; Wiktor, A.; Toepfl, S.; Nowacka, M.; Bialik, M.; Czyzewski, J.; Witrowa-Rajchert, D.; Parniakov, O. Impact of pulsed electric fields on physical properties of freeze-dried apple tissue. Innov. Food Sci. Emerg. 2019, 57, 7. [Google Scholar] [CrossRef]
- Parniakov, O.; Bals, O.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted vacuum freeze-drying of apple tissue. Innov. Food Sci. Emerg. 2016, 35, 52–57. [Google Scholar] [CrossRef]
- Nowak, D.; Lewicki, P.P. Quality of infrared dried apple slices. Dry. Technol. 2005, 23, 831–846. [Google Scholar] [CrossRef]
- Assegehegn, G.; Brito-de la Fuente, E.; Franco, J.M.; Gallegos, C. Freeze-drying: A relevant unit operation in the manufacture of foods, nutritional products, and pharmaceuticals. Adv. Food Nutr. Res. 2020, 93, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Ciurzyńska, A.; Lenart, A. Freeze-drying-application in food processing and biotechnology—A Review. Polish J. Food Nutr. Sci. 2011, 61, 165–171. [Google Scholar] [CrossRef]
- Li, R.J.; Huang, L.L.; Zhang, M.; Mujumdar, A.S.; Wang, Y.C. Freeze drying of apple slices with and without application of microwaves. Dry. Technol. 2014, 32, 1769–1776. [Google Scholar] [CrossRef]
- Pei, F.; Shi, Y.; Gao, X.Y.; Wu, F.N.; Mariga, A.M.; Yang, W.J.; Zhao, L.Y.; An, X.X.; Xin, Z.H.; Yang, F.M.; et al. Changes in non-volatile taste components of button mushroom (Agaricus bisporus) during different stages of freeze drying and freeze drying combined with microwave vacuum drying. Food Chem. 2014, 165, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Bera, M.; Mukhopadhyay, P.; Bhattacharya, P. Prediction of optimal conditions of infrared assisted freeze-drying of aloe vera (Aloe barbadensis) using response surface methodology. Sep. Purif. Technol. 2011, 80, 375–384. [Google Scholar] [CrossRef]
- Lin, Y.P.; Tsen, J.H.; King, V.A.E. Effects of far-infrared radiation on the freeze-drying of sweet potato. J. Food Eng. 2005, 68, 249–255. [Google Scholar] [CrossRef]
- Wang, H.C.; Zhang, M.; Adhikari, B. Drying of shiitake mushroom by combining freeze-drying and mid-infrared radiation. Food Bioprod. Process. 2015, 94, 507–517. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Mujumdar, A.S.; Wang, S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Duan, X.; Liu, W.C.; Ren, G.Y.; Liu, L.L.; Liu, Y.H. Browning behavior of button mushrooms during microwave freeze-drying. Dry. Technol. 2016, 34, 1373–1379. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Mujumdar, A.S. Studies on the microwave freeze drying technique and sterilization characteristics of cabbage. Dry. Technol. 2007, 25, 1725–1731. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Li, X.L.; Mujumdar, A.S. Microwave freeze drying of sea cucumber coated with nanoscale silver. Dry. Technol. 2008, 26, 413–419. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Mujumdar, A.S.; Wang, R. Trends in microwave-assisted freeze drying of foods. Dry. Technol. 2010, 28, 444–453. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, M.; Mujumdar, A.S. Effects of vacuum and microwave freeze drying on microstructure and quality of potato slices. J. Food Eng. 2010, 101, 131–139. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Mujumdar, A.S. Microwave freeze-drying characteristics of banana crisps. Dry. Technol. 2010, 28, 1377–1384. [Google Scholar] [CrossRef]
- Cao, X.H.; Zhang, M.; Mujumdar, A.S.; Zhong, Q.F.; Wang, Z.S. Effect of microwave freeze drying on quality and energy supply in drying of barley grass. J. Sci. Food Agric. 2018, 98, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Duan, X.; Ren, G.Y.; Liu, L.L.; Liu, Y.H. Optimization of microwave freeze drying strategy of mushrooms (Agaricus bisporus) based on porosity change behavior. Dry. Technol. 2017, 35, 1327–1336. [Google Scholar] [CrossRef]
- Hua, T.C.; Liu, B.L.; Zhang, H. Freeze-Drying of Pharmaceutical and Food Products. In Freeze-Drying of Pharmaceutical and Food Products; Elsevier Science Bv: Amsterdam, The Netherlands, 2010; Volume 198, pp. 1–257. [Google Scholar]
- Goldblith, S.A.; Rey, L.R.; Rothmayr, W. Freeze Drying and Advanced Food Technology; Academic Press: London, UK, 1975; p. 730. [Google Scholar]
- Genin, N.; Rene, F. Influence of freezing rate and the ripeness state of fresh courgette on the quality of freeze-dried products and freeze-drying time. J. Food Eng. 1996, 29, 201–209. [Google Scholar] [CrossRef]
- Bruttini, R.; Rovero, G.; Baldi, G. Experimentation and modelling of pharmaceutic lyophilization using a pilot plant. Chem. Eng. J. 1991, 45, 175–177. [Google Scholar] [CrossRef]
- Pikal, M.J.; Roy, M.L.; Shah, S. mass and heat-transfer in vial freeze-drying of pharmaceuticals—Role of the vial. J. Pharm. Sci. 1984, 73, 1224–1237. [Google Scholar] [CrossRef]
- Tang, X.L.; Pikal, M.J. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.; McMinn, W.; Magee, T. Moisture sorption isotherm characteristics of food products: A review. Food Bioprod. Process. 2002, 80, 118–128. [Google Scholar] [CrossRef]
- Aviara, N.A. Moisture Sorption Isotherms and Isotherm Model Performance Evaluation for Food and Agricultural Products. In Sorption in 2020s; Kyzas, G., Lazaridis, N., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Trelea, I.C.; Fonseca, F.; Passot, S. Dynamic modeling of the secondary drying stage of freeze drying reveals distinct desorption kinetics for bound water. Dry. Technol. 2016, 34, 335–345. [Google Scholar] [CrossRef]
- Abdul-Fattah, A.M.; Lechuga-Ballesteros, D.; Kalonia, D.S.; Pikal, M.J. The impact of drying method and formulation on the physical properties and stability of methionyl human growth hormone in the amorphous solid state. J. Pharm. Sci. 2008, 97, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Colandene, J.D.; Maldonado, L.M.; Creagh, A.T.; Vrettos, J.S.; Goad, K.G.; Spitznagel, T.M. Lyophilization cycle development for a high-concentration monoclonal antibody formulation lacking a crystalline bulking agent. J. Pharm. Sci. 2007, 96, 1598–1608. [Google Scholar] [CrossRef]
- Pikal, M.J.; Shah, S.; Roy, M.L.; Putman, R. The secondary drying stage of freeze-drying—Drying kinetics as a function of temperature and chamber pressure. Int. J. Pharm. 1990, 60, 203–217. [Google Scholar] [CrossRef]
- Liapis, A.I.; Bruttini, R. A Theory for the primary and secondary drying stages of the freeze-drying of pharmaceutical crystalline and amorphous solutes—Comparison between experimental-data and theory. Sep. Technol. 1994, 4, 144–155. [Google Scholar] [CrossRef]
- Millman, M.J.; Liapis, A.I.; Marchello, J.M. An analysis of the lyophilization process using a sorption-sublimation model and various operational policies. Aiche J. 1985, 31, 1594–1604. [Google Scholar] [CrossRef]
- Sadikoglu, H.; Liapis, A.I. Mathematical modelling of the primary and secondary drying stages of bulk solution freeze-drying in trays: Parameter estimation and model discrimination by comparison of theoretical results with experimental data. Dry. Technol. 1997, 15, 791–810. [Google Scholar] [CrossRef]
- Sadikoglu, H.; Liapis, A.I.; Crosser, O.K. Optimal control of the primary and secondary drying stages of bulk solution freeze drying in trays. Dry. Technol. 1998, 16, 399–431. [Google Scholar] [CrossRef]
- Sadikoglu, H. Optimal control of the secondary drying stage of freeze drying of solutions in vials using variational calculus. Dry. Technol. 2005, 23, 33–57. [Google Scholar] [CrossRef]
- Gan, K.H.; Bruttini, R.; Crosser, O.K.; Liapis, A.I. Heating policies during the primary and secondary drying stages of the lyophilization process in vials: Effects of the arrangement of vials in clusters of square and hexagonal arrays on trays. Dry. Technol. 2004, 22, 1539–1575. [Google Scholar] [CrossRef]
- Fissore, D.; Pisano, R.; Barresi, A.A. Monitoring of the secondary drying in freeze-drying of pharmaceuticals. J. Pharm. Sci. 2011, 100, 732–742. [Google Scholar] [CrossRef]
- Pisano, R.; Fissore, D.; Barresi, A.A. Quality by design in the secondary drying step of a freeze-drying process. Dry. Technol. 2012, 30, 1307–1316. [Google Scholar] [CrossRef]
- Sheehan, P.; Liapis, A.I. Modeling of the primary and secondary drying stages of the freeze drying of pharmaceutical products in vials: Numerical results obtained from the solution of a dynamic and spatially multi-dimensional lyophilization model for different operational policies. Biotechnol. Bioeng. 1998, 60, 712–728. [Google Scholar] [CrossRef]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 22. [Google Scholar] [CrossRef]
- Andrieu, J.; Vessot, S. A review on experimental determination and optimization of physical quality factors during pharmaceuticals freeze-drying cycles. Dry. Technol. 2018, 36, 129–145. [Google Scholar] [CrossRef]
- Hammami, C.; Rene, F.; Marin, M. Process-quality optimization of the vacuum freeze-drying of apple slices by the response surface method. Int. J. Food Sci. Technol. 1999, 34, 145–160. [Google Scholar] [CrossRef]
- Alves, O.; Roos, Y.H. Advances in multi-purpose drying operations with phase and state transitions. Dry. Technol. 2006, 24, 383–396. [Google Scholar] [CrossRef]
- Antelo, L.T.; Passot, S.; Fonseca, F.; Trelea, I.C.; Alonso, A.A. Toward optimal operation conditions of freeze-drying processes via a multilevel approach. Dry. Technol. 2012, 30, 1432–1448. [Google Scholar] [CrossRef]
- Malik, N.; Muttakin, S.; Lopez-Quiroga, E.; Watson, N.J.; Fryer, P.J.; Bakalis, S.; Gouseti, O. Microstructure and reconstitution of freeze-dried gum Arabic at a range of concentrations and primary drying temperatures. Food Hydrocoll. 2020, 104, 11. [Google Scholar] [CrossRef]
- Barresi, A.A.; Pisano, R.; Fissore, D.; Rasetto, V.; Velardi, S.A.; Vallan, A.; Parvis, M.; Galan, M. Monitoring of the primary drying of a lyophilization process in vials. Chem. Eng. Process. 2009, 48, 408–423. [Google Scholar] [CrossRef]
- Liapis, A.I.; Bruttini, R. Freeze Drying. In Handbook of Industrial Drying, 4th ed.; Mujumdar, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 259–282. [Google Scholar]
- Nireesha, G.; Divya, L.; Sowmya, C.; Venkateshan, N.; Babu, M.; Lavakumar, V. Lyophilization/freeze drying—An review. IJNTPS 2013, 3, 87–98. [Google Scholar]
- Sadikoglu, H.; Ozdemir, M.; Seker, M. Freeze-drying of pharmaceutical products: Research and development needs. Dry. Technol. 2006, 24, 849–861. [Google Scholar] [CrossRef]
- Sablani, S.S.; Rahman, M.S. Pore formation in selected foods as a function of shelf temperature during freeze drying. Dry. Technol. 2002, 20, 1379–1391. [Google Scholar] [CrossRef]
- Sablani, S.S.; Rahman, M.S.; Al-Kuseibi, M.K.; Al-Habsi, N.A.; Al-Belushi, R.H.; Al-Marhubi, I.; Al-Amri, I.S. Influence of shelf temperature on pore formation in garlic during freeze-drying. J. Food Eng. 2007, 80, 68–79. [Google Scholar] [CrossRef]
- Krokida, M.K.; Karathanos, V.T.; Maroulis, Z.B. Effect of freeze-drying conditions on shrinkage and porosity of dehydrated agricultural products. J. Food Eng. 1998, 35, 369–380. [Google Scholar] [CrossRef]
- Rahman, M.S. Toward prediction of porosity in foods during drying: A brief review. Dry. Technol. 2001, 19, 1–13. [Google Scholar] [CrossRef]
- Hussain, M.A.; Rahman, M.S.; Ng, C.W. Prediction of pores formation (porosity) in foods during drying: Generic models by the use of hybrid neural network. J. Food Eng. 2002, 51, 239–248. [Google Scholar] [CrossRef]
- Khalloufi, S.; Kharaghani, A.; Almeida-Rivera, C.; Nijsse, J.; van Dalen, G.; Tsotsas, E. Monitoring of initial porosity and new pores formation during drying: A scientific debate and a technical challenge. Trends Food Sci. Technol. 2015, 45, 179–186. [Google Scholar] [CrossRef]
- Shishehgarha, F.; Makhlouf, J.; Ratti, C. Freeze-drying characteristics of strawberries. Dry. Technol. 2002, 20, 131–145. [Google Scholar] [CrossRef]
- Rudy, S.; Dziki, D.; Krzykowski, A.; Gawlik-Dziki, U.; Polak, R.; Różyło, R.; Kulig, R. Influence of pre-treatments and freeze-drying temperature on the process kinetics and selected physico-chemical properties of cranberries (Vaccinium macrocarpon Ait.). LWT 2015, 63, 497–503. [Google Scholar] [CrossRef]
- Hammami, C.; Rene, F. Determination of freeze-drying process variables for strawberries. J. Food Eng. 1997, 32, 133–154. [Google Scholar] [CrossRef]
- Zghal, M.C.; Scanlon, M.G.; Sapirstein, H.D. Cellular structure of bread crumb and its influence on mechanical properties. J. Cereal Sci. 2002, 36, 167–176. [Google Scholar] [CrossRef]
- Agbisit, R.; Alavi, S.; Cheng, E.Z.; Herald, T.; Trater, A. Relationships between microstructure and mechanical properties of cellular cornstarch extrudates. J. Texture Stud. 2007, 38, 199–219. [Google Scholar] [CrossRef]
- Le-Bail, A.; Boumali, K.; Jury, V.; Ben-Aissa, F.; Zuniga, R. Impact of the baking kinetics on staling rate and mechanical properties of bread crumb and degassed bread crumb. J. Cereal Sci. 2009, 50, 235–240. [Google Scholar] [CrossRef]
- Gulati, T.; Datta, A.K. Mechanistic understanding of case-hardening and texture development during drying of food materials. J. Food Eng. 2015, 166, 119–138. [Google Scholar] [CrossRef]
- Kanit, T.; N’Guyen, F.; Forest, S.; Jeulin, D.; Reed, M.; Singleton, S. Apparent and effective physical properties of heterogeneous materials: Representativity of samples of two materials from food industry. Comput. Meth. Appl. Mech. Eng. 2006, 195, 3960–3982. [Google Scholar] [CrossRef]
- Dintwa, E.; Jancsok, P.; Mebatsion, H.K.; Verlinden, B.; Verboven, P.; Wang, C.X.; Thomas, C.R.; Tijskens, E.; Ramon, H.; Nicolai, B. A finite element model for mechanical deformation of single tomato suspension cells. J. Food Eng. 2011, 103, 265–272. [Google Scholar] [CrossRef]
- Guessasma, S.; Chaunier, L.; Della Valle, G.; Lourdin, D. Mechanical modelling of cereal solid foods. Trends Food Sci. Technol. 2011, 22, 142–153. [Google Scholar] [CrossRef]
- Rahman, M.S. A theoretical model to predict the formation of pores in foods during drying. Int. J. Food Prop. 2003, 6, 61–72. [Google Scholar] [CrossRef]
- Krokida, M.K.; Maroulis, Z.B.; Marinos-Kouris, D. Viscoelastic behavior of dehydrated carrot and potato. Dry. Technol. 1998, 16, 687–703. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Lukaszuk, A. Changes of rheological properties of apple tissue undergoing convective drying. Dry. Technol. 2000, 18, 707–722. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Jakubczyk, E. Effect of hot air temperature on mechanical properties of dried apples. J. Food Eng. 2004, 64, 307–314. [Google Scholar] [CrossRef]
- Gabas, A.L.; Menegalli, F.C.; Ferrari, F.; Telis-Romero, J. Influence of drying conditions on the rheological properties of prunes. Dry. Technol. 2002, 20, 1485–1502. [Google Scholar] [CrossRef]
- Iwaniw, D.C.; Mittal, G.S. Process optimization of freeze-dried strawberries. Can. Agric. Eng. 1990, 32, 323–328. [Google Scholar]
- Mawilai, P.; Chaloeichitratham, N.; Pongsuttiyakorn, T.; Pornchaloempong, P. Effect of Final Drying Condition on Qualities of Freeze Dry Dragon Fruit (Hylocercus undatus). In Proceedings of the 18th TSAE National Conference and 10th TSAE International Conference, Bangkok, Thailand, 7–9 September 2017; pp. 66–70. [Google Scholar]
- Djekic, I.; Tomic, N.; Bourdoux, S.; Spilimbergo, S.; Smigic, N.; Udovicki, B.; Hofland, G.; Devlieghere, F.; Rajkovic, A. Comparison of three types of drying (supercritical CO2, air and freeze) on the quality of dried apple—Quality index approach. LWT 2018, 94, 64–72. [Google Scholar] [CrossRef]
- Reyes-Alvarez, C.A.; Lanari, M.C. Storage stability of freeze-dried araza (Eugenia stipitata Mc Vaugh) powders. Implications of carrier type and glass transition. LWT 2020, 118, 9. [Google Scholar] [CrossRef]
- Bui, L.T.T.; Coad, R.A.; Stanley, R.A. Properties of rehydrated freeze dried rice as a function of processing treatments. LWT 2018, 91, 143–150. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Lenart, A. Effect of the aerated structure on selected properties of freeze-dried hydrocolloid gels. Int. Agrophys. 2016, 30, 9–17. [Google Scholar] [CrossRef]
- Lombrana, J.I.; Villaran, M.C. The influence of pressure and temperature on freeze-drying in an adsorbent medium and establishment of drying strategies. Food Res. Int. 1997, 30, 213–222. [Google Scholar] [CrossRef]
- Domin, M.; Dziki, D.; Kłapsia, S.; Blicharz-Kania, A.; Biernacka, B.; Krzykowski, A. Influence of the freeze-drying conditions on the physicochemical properties and grinding characteristics of kiwi. Int. J. Food Eng. 2020, 16. [Google Scholar] [CrossRef]
- Udomkun, P.; Argyropoulos, D.; Nagle, M.; Mahayothee, B.; Oladeji, A.E.; Müller, J. Changes in microstructure and functional properties of papaya as affected by osmotic pre-treatment combined with freeze-drying. J. Food Meas. Charact. 2018, 12, 1028–1037. [Google Scholar] [CrossRef]
- Harnkarnsujarit, N.; Charoenrein, S.; Roos, Y.H. Microstructure formation of maltodextrin and sugar matrices in freeze-dried systems. Carbohydr. Polym. 2012, 88, 734–742. [Google Scholar] [CrossRef]
- Regier, M.; Hardy, E.H.; Knoerzer, K.; Leeb, C.V.; Schuchmann, H.P. Determination of structural and transport properties of cereal products by optical scanning, magnetic resonance imaging and Monte Carlo simulations. J. Food Eng. 2007, 81, 485–491. [Google Scholar] [CrossRef]
- Reyes, A.; Vega, R.; Bustos, R.; Araneda, C. Effect of processing conditions on drying kinetics and particle microstructure of carrot. Dry. Technol. 2008, 26, 1272–1285. [Google Scholar] [CrossRef]
- Antal, T.; Sikolya, L.; Kerekes, B. Assessment of freezing pre-treatments for the freeze dried of apple slices. Acta Univ. Cibiniensis Ser. E Food Technol. 2013, 17, 3–14. [Google Scholar] [CrossRef][Green Version]
- Kang, H.W.; Tabata, Y.; Ikada, Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials 1999, 20, 1339–1344. [Google Scholar] [CrossRef]
- Ma, P.X.; Zhang, R.Y. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 1999, 46, 60–72. [Google Scholar] [CrossRef]
- Shapiro, L.; Cohen, S. Novel alginate sponges for cell culture and transplantation. Biomaterials 1997, 18, 583–590. [Google Scholar] [CrossRef]
- Jakubczyk, E.; Ostrowska-Ligeza, E.; Gondek, E. Moisture sorption characteristics and glass transition temperature of apple puree powder. Int. J. Food Sci. Technol. 2010, 45, 2515–2523. [Google Scholar] [CrossRef]
- Malik, N.; Gouseti, O.; Bakalis, S. Effect of freezing on microstructure and reconstitution of freeze-dried high solid hydrocolloid-based systems. Food Hydrocoll. 2018, 83, 473–484. [Google Scholar] [CrossRef]
- Harguindeguy, M.; Bobba, S.; Colucci, D.; Fissore, D. Effect of vacuum freeze-drying on the antioxidant properties of eggplants (Solanum melongena L.). Dry. Technol. 2019, 1–16. [Google Scholar] [CrossRef]
- Acevedo, B.A.; Chaves, M.G.; Avanza, M.V.; Dellacassa, E.S. Freeze-drying concentration of Rangpur lime juice. Int. J. Food Sci. Technol. 2014, 49, 423–428. [Google Scholar] [CrossRef]
- Reyes, A.; Pérez, N.; Mahn, A. Theoretical and experimental study of freeze-drying of “loco” (Concholepas concholepas). Dry. Technol. 2011, 29, 1386–1395. [Google Scholar] [CrossRef]

| Material | Initial Moisture Content | Water Content After Drying | Water Activity of Freeze-Dried Product | Ref. |
|---|---|---|---|---|
| Grapefruit puree | 83.0–86.7% | 0.013–0.030 g water/g sample | n/a | [16] |
| Orange puree | n/a | <4% | n/a | [14] |
| Strawberry | 90.8% | 2% | n/a | [15] |
| Apple | 86.3% | <0.5% | n/a | [15] |
| n/a | n/a | 0.14 | [116] | |
| Garlic | 73.2% | 0.061–0.095 g water/g sample | n/a | [94] |
| Arazá (Eugenia stipitata McVaugh) paste | 96.0% | 0.02 kg/kg d.m. | 0.08 | [117] |
| Pear | 84.3% | <0.5% | n/a | [15] |
| Dragon fruit | 86.5–87.5% | 8.53–9.87% | 0.08–0.16 | [115] |
| Cooked rice | 60.0% | 1.69–2.09% | n/a | [118] |
| Hydrocolloid gels | 83.6–87.5% | 1.4–4.0% | 0.14–0.330 | [119] |
| Dried Material | Material Size and Form | Freezing Parameters | Shelf Temperature | Pressure of the Chamber | Drying Time | Properties of Material | Ref. |
|---|---|---|---|---|---|---|---|
| Abalone | Cylindrical disks (2.5 cm diameter and 0.7 cm height) | −40 °C | −45, −30, −20, −10, −5, 0, 10, and 15 °C | 100 Pa | 72 h | The increase of a shelf temperature in the range of −45–15 °C caused an increase of the apparent density of dried abalone from 372.9 to 472.1 kg/m3 and a decrease of apparent porosity from 0.733 to 0.664. | [93] |
| Apple | Cylindrical disks (2.5 cm diameter and 0.7 height) | −40 °C | −45, −30, −20, −10, −5, 0, 10, and 15 °C | 100 Pa | 72 h | The increase of a shelf temperature in the range of −45–15 °C caused an increase of apparent porosity from 0.876 to 0.910. | [93] |
| Semi-circular slices (55 mm in length, 2.2–2.5 mm thick) | −25 °C | −25 °C at 1st step drying, 40 °C at 2nd drying | 20 Pa at 1st step drying, 5 Pa at 2nd drying | 24 h | The moisture content was negatively correlated with hardness of freeze-dried apples. The application of freeze-drying resulted in a lower springiness compared to that of the air-drying method. Additionally, the color difference ΔE of freeze-dried apples (11.37) was lower than that obtained for air-dried apples (21.11). | [116] | |
| Puree—layer with thickness of 4 mm | −40 °C | 20 °C | 63 Pa | 26 h | The apple puree freeze-dried at 20 °C absorbed less water than air-dried samples. The application of freeze-drying method enabled the obtainment of powder with a slightly lower hygroscopicity than after microwave-drying. | [130] | |
| Banana | Cylinders with diameter of 20 mm and 8 mm height | −35 °C (48 h), tempered for 1 h in liquid N2 | Product temperature from −50 to −8 °C | 3–300 Pa | 24 h | The values of the bulk density of the banana decreased after freeze-drying from 1900 kg·m−3 to values lower than 400 kg·m−3. The values of bulk density increased (about ~30%) as the temperature of process was increased from −50 to −8 °C. The porosity of freeze-dried banana was the highest at the low temperature of −50 °C (~0.9). | [95] |
| Carrot | Cylinders with diameter of 20 mm and 8 mm height | −35 °C (48 h), tempered for 1 h in liquid N2 | Product temperature from −50 to −5 °C | 3–300 Pa | 24 h | The values of bulk density of carrot tissue decreased after freeze-drying from 1750 kg·m−3 to values lower than 250 kg·m−3. The bulk density values increased (about ~40%) as the temperature of process was increased from −50 to −8 °C. The porosity of freeze-dried carrot was reduced by about 10% after drying at higher temperatures. | [95] |
| Coffee solutions | Layer with thickness of 20 mm | 1 set: −40 °C at 1 °C/min 2 set: fluctuation of temperature between −40 and −20 °C | −40 °C at the primary drying, 20 °C at the secondary drying | 10 Pa | 18 h | Samples freeze-dried with temperature oscillations (−20 and −40 °C) had larger pores than material frozen at −40 °C. Temperature fluctuations during freezing promoted large crystal formation and resulted in a higher total porosity by, on average, 18%. The application of freezing cycles led to faster reconstruction rates. | [131]: |
| Dragon fruit | Pieces with thickness of 1 cm | −40 °C fast freezing (an air blast freezer and a contact plate freezer) | −5 °C at the primary drying, 30, 40, and 50 °C at the secondary drying | 40 Pa | 50 h at 30 °C, 55 h at 40 °C, 60 h at 50 °C | The apparent densities of freeze-dried dragon fruits were 0.16, 0.19, and 0.08 g × cm−3 at the drying temperatures 30, 40, and 50 °C, respectively. The hardness of dried fruit decreased from 9.26 to 4.33 N and crispness increased from 6.83 to 10.56 with the increase of the heating temperature. | [115]: |
| Eggplant | Cubes of 9 mm side | −40 °C | 1 set: −30, −15, and 0 °C at 1st step drying, 20 °C at 2nd step drying 2 set: −30 and 0 °C at 1st drying, 20 °C at 2nd step drying | 1 set: 10 Pa 2 set: 10, 20, and 40 Pa | 1 set: 7–15.3 h 2 set: 14–20.9 h | The loss of antioxidant capacity was 49.9 and 68.6% for freeze-dried samples dried at −30 and 0 °C, respectively. The increase of drying temperature from −30 to 0 °C caused the loss of ascorbic acid from 37.9 to 12.2%. Total polyphenol content—TPC—in dried product was retained at higher pressures. The loss of TPC was 32.5% at 40 Pa and 47.7% at 10 Pa. | [132]: |
| Garlic | Brick shaped samples (20 × 10 × 10 mm) | −40 °C | −5, −15, and −25 °C | 108 Pa | 72 h | The decrease of shelf temperature from −5 to −25 °C during the freeze-drying of garlic resulted in a decrease of the apparent density from 469 to 431 kg/m3 and an increase of the shrinkage expansion between 0.44 and 0.52, as well as the true density decreased in the range of 1534–1504 kg/m3. | [94]: |
| Grapefruit puree | 1-cm layer | −45 °C | room temperature, 40 °C | 9 Pa | 1.5−21 h | The increase of temperature promoted an increase in the porosity of freeze-dried puree (from 0.78 to 0.83) and a decrease in the number of pores formed from 415to 312. | [16]: |
| Gum Arabic solutions | Layer with a height of 0.5 cm | −40 °C (at 1 °C/min) | −20, −30, and −40 °C at primary during, 20 °C at secondary drying | 10 Pa | 18 h | The degree of puffing was stronger for samples dried at higher (−20 and −30 °C) compared to lower (−40 °C) temperatures of the shelf. The primary drying temperature did not affect the size of pores and pore distribution for solutions with concentrations of 20, 30, 40, and 50%. The mean pore diameter of 60% freeze-dried gum hydrocolloid system increased from 745 to 973 µm with the increase of shelf temperature from −40 to −20 °C. | [88]: |
| Kiwi | Whole fruit (without peel) | −40 °C | n/a | 12, 20, 42, 85, and 103 Pa | n/a | The increase of pressure in the range of 12–100 Pa resulted in a decrease of L* from 65.3 to 58.3, as well as a* values from −2.7 to −6.8, and the increase of b* from 22.3 to 28.3. The higher pressure affected the increase of penetration force for freeze-dried kiwi fruit from 4.3 to 16.2 N. | [121]: |
| Lime juice | Sample juice layer with a thickness from 0.3 to 1.1. cm | −30 °C | −61 °C | 3 Pa | 1–10 h | The freeze-drying of lime juice did not affect acidity (4.10–4.15 g citric ac./100 mL), antioxidant activity (17.5–18.3 mg ascorbic ac./100 mL), and carotenoids content (0.61–0.64 mg ∙100 mL-). Fresh juice and reconstituted freeze-dried juice did not significantly differ in relation to sensory attributes. | [133]: |
| Loco (Concholepas concholepas) (boiled) | Samples 1 × 1 × 0.5 cm Cubes 0.5 side cm | −25 °C | n/a | 6,7 Pa and 9.6·10−4 Pa (AFD- atmospheric freeze-drying) | 6.7−12 h | The pore surface of freeze-dried loco obtained at a low pressure was 0.32 m2 pores/m2, while after AFD, this value was half (0.16 pores/m2 material surface). The water absorption capacity of the freeze-dried sample was higher than 1.0 at a low pressure, while at AFD conditions, it was lower than 1.0. | [134]: |
| Maltodextrin sugar–agar solutions | Cube (10 × 10 × 10 mm) samples | −20, −40, and −80 °C, tempered at −80 °C before drying | Room temperature | 10 Pa | 48 h | The pore size and thickness of pore membranes of the freeze-dried system were reduced with a decrease of the freezing temperature. The system frozen at −80 °C was more resistant to compression than samples frozen at −40 and −20 °C. | [123]: |
| Orange puree | Puree, layer with a thickness of 0.5 mm | −45 °C—slow rate: a conventional freezer −38 °C—fast rate—a blast freezer | 30, 40, and 50 °C | 5 and 100 Pa | 25 h at 30 °C, 7 h at 40 °C, 6 h at 50 °C | The color attributes L, C*, and h* of freeze-dried orange puree were affected by working pressure. The lower values of L* and higher C* were characteristic for samples dried at the high pressure of 100 Pa. The lower range of h* values between 80.3 and 82.6 was registered for the samples dried at higher pressure (100 Pa) and the temperature of the shelf below 50 °C. The lower pressure of 5 Pa and a higher temperature of 50 °C created more resistant to fracture a freeze-dried sample. The lower degradation of vitamin C was observed for samples dried at 40 and 50 °C than at 30 °C. | [14]: |
| Pepper | Samples and puree with layer of 5 mm | −25 °C | 20, 40, and 60 °C | 63 Pa | 290 min (60 °C) 900 min (20 °C) | The red pepper freeze-dried at higher temperature 60 °C was characterized by lower values of L* (lightness =35.5), a* (redness =27.6), and b* (yellowness =23.8) than the sample dried at 20 °C (L* = 39.2, a* = 34.8, and b* = 27.0). Additionally, the increase in drying temperature caused a decrease of the total phenolic content (from 12.6 to 11.8 mg GAE/g d.m.) and antioxidant activity (EC50- concentration required to obtain a 50% antioxidant effect) from 21.7 to 26.1 mg d.m./mL). | [11]: |
| Potato | Cylindrical disks (2.5 cm diameter and 0.7 height) | −40 °C | −45, −30, −20, −10, −5, 0, 10, and 15 °C | 100 Pa | 72 h | The increase of a shelf temperature in the range from −45–15 °C caused an increase of apparent density of dried potato from 204.2 to 452.2 kg × m−3 and a decrease of apparent porosity from 0.863 to 0.698. | [93] |
| Rice (cooked) | The layer of 1.8 mm | −18 °C | 90 °C | 80 Pa (initial) and 20 Pa (final) | 12 h | The freeze-dried rice had a better rehydration capacity than the freshly cooked sample. Freeze-drying caused the extensive breakage of the grains. The extent of breakage was dependent on the cooking method and was lower in freeze-dried parboiled rice (3.6–36.9%) than non-parboiled grain (50%). | [118]: |
| n/a | −30 °C for 72 h, tempered for 1 h in liquid N2 | n/a | 4, 13, and 125 Pa | 24 h | The bulk density of freeze-dried rice decreased from ~0.9 to ~0.8 for kernels boiled for 4 min and from ~0.6 to ~0.5 for kernels boiled for longer than 20 min with the decrease of applied pressure from 125 to 4 Pa. The porosity of dried kernels was the highest at low pressures. | [10]: | |
| Strawberry | Whole fruits and slices (5 and 10 mm thick) | −40 °C | 30, 40, 50, 60, and 70 °C | n/a | 12–48 h | The color of strawberries and the volume reduction of fruits did not change in case of different drying temperatures. The percentage of collapsed samples exceeded 20% at drying temperatures higher than 50 °C. | [99]: |
| Yellow dates | Halves | −40 °C | −45, −30, −20, −10, −5, 0, 10, and 15 °C | 100 Pa | 72 h | The increase of a shelf temperature in the range from −45–15 °C caused a decrease of the apparent density of dried dates from 485.1.2 to 205.5 kg·× m−3, as well as an increase of apparent porosity from 0.709 to 0.864. | [93]: |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. https://doi.org/10.3390/foods9101488
Nowak D, Jakubczyk E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods. 2020; 9(10):1488. https://doi.org/10.3390/foods9101488
Chicago/Turabian StyleNowak, Dorota, and Ewa Jakubczyk. 2020. "The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials" Foods 9, no. 10: 1488. https://doi.org/10.3390/foods9101488
APA StyleNowak, D., & Jakubczyk, E. (2020). The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods, 9(10), 1488. https://doi.org/10.3390/foods9101488