Hydroxycinnamic Acids and Their Derivatives in Broa, a Traditional Ethnic Maize Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maize Flour and Broas Preparation
2.2. Reagents
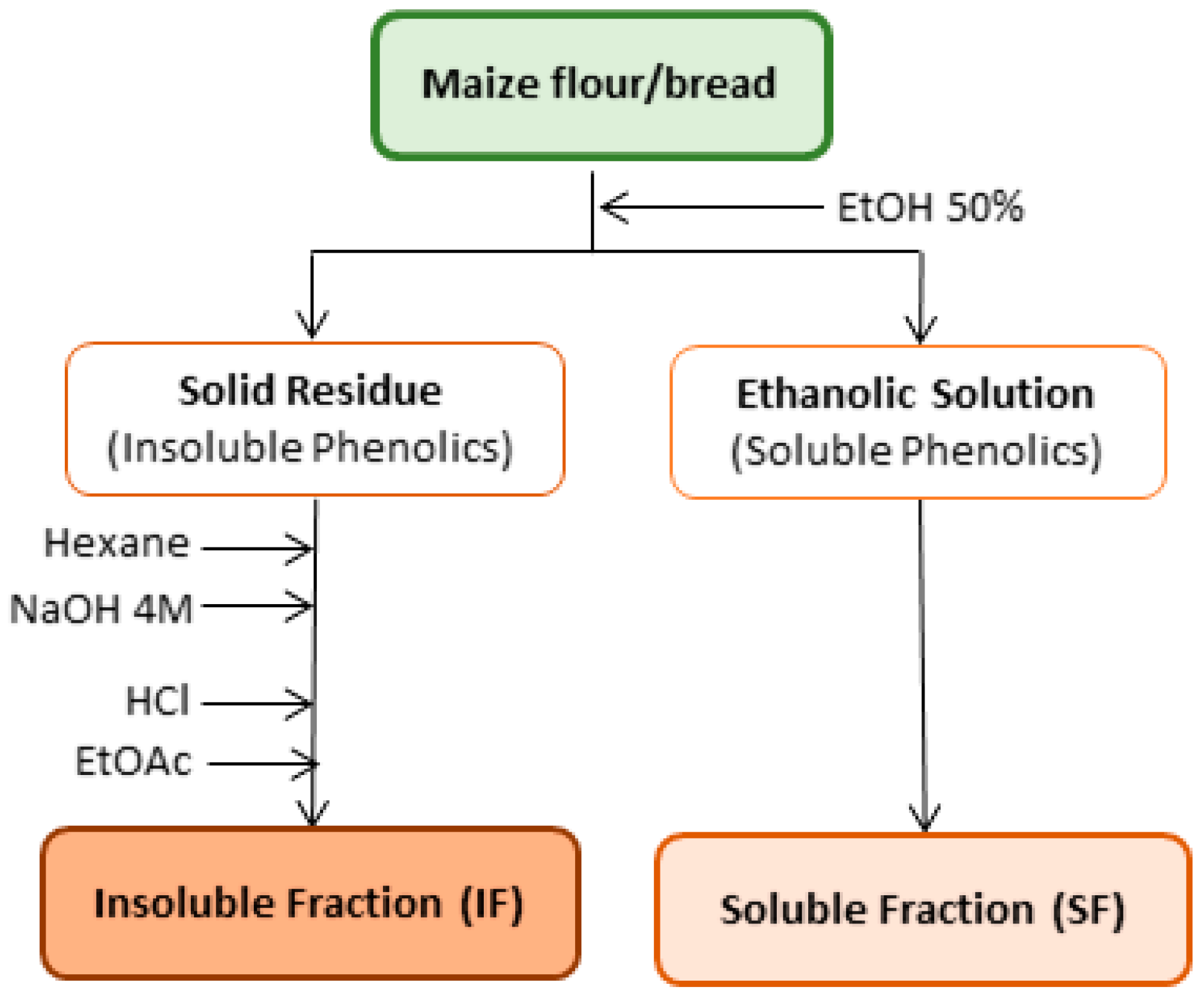
2.3. Extraction of Phenolic Compounds
2.4. Analysis of Phenolic Compounds by Liquid Chromatography
2.5. Data Analysis
3. Results and Discussion
3.1. Small Phenolic Compounds
3.2. Ferulic Acid Dehydrodimers
3.3. Ferulic Acid Dehydrotrimers and Tetramers
3.4. Soluble Hydroxycinnamic Acid Amides
3.5. Insoluble Hydroxycinnamic Acid Amides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix B
References
- Singh, N.; Singh, S.; Shevkani, K. Maize: Composition, bioactive constituents, and unleavened bread. In Flour and Breads and Their Fortification in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–121. [Google Scholar]
- Vaz Patto, M.; Alves, M.; Almeida, N.; Santos, C.; Mendes Moreira, P.; Satovic, Z.; Brites, C. Is the bread making technological ability of Portuguese traditional maize landraces associated with their genetic diversity. Maydica 2009, 54, 297–311. [Google Scholar]
- Smith, J.R. 50 of the World’s Best Breads. Available online: https://edition.cnn.com/travel/article/world-50-best-breads/index.html (accessed on 30 April 2020).
- Bento-Silva, A.; Patto, M.C.V.; do Rosário Bronze, M. Relevance, structure and analysis of ferulic acid in maize cell walls. Food Chem. 2018, 246, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Moreno, Y.; García-Salinas, C.; Ramírez-Díaz, J.L.; Alemán-de la Torre, I. Phenolic Compounds in Maize Grains and Its Nixtamalized Products. In Phenolic Compounds-Natural Sources, Importance and Applications; InTech: London, UK, 2017. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Dietary Phenolic Compounds, Health Benefits and Bioaccessibility; Archivos Latinoamericanos de Nutrición: Caracas, Venezuela, 2016; Volume 66. [Google Scholar]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Phenolic–protein interactions: Effects on food properties and health benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Lee, S.K.; Kim, E.O.; Oh, J.H.; Yoon, K.S.; Parris, N.; Hicks, K.B.; Moreau, R.A. Antioxidant and antimelanogenic activities of polyamine conjugates from corn bran and related hydroxycinnamic acids. J. Agric. Food Chem. 2007, 55, 3920–3925. [Google Scholar] [CrossRef] [PubMed]
- Collison, A.; Yang, L.; Dykes, L.; Murray, S.; Awika, J.M. Influence of genetic background on anthocyanin and copigment composition and behavior during thermoalkaline processing of maize. J. Agric. Food Chem. 2015, 63, 5528–5538. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Macoy, D.M.; Kim, W.-Y.; Lee, S.Y.; Kim, M.G. Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant Biotechnol. Rep. 2015, 9, 269–278. [Google Scholar] [CrossRef]
- Majumdar, R.; Minocha, R.; Lebar, M.D.; Rajasekaran, K.; Long, S.; Carter-Wientjes, C.; Minocha, S.C.; Cary, J.W. Contribution of maize polyamine and amino acid metabolism towards resistance against Aspergillus flavus infection and aflatoxin production. Front. Plant Sci. 2019, 10, 692. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Estrada, B.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Chemopreventive effects of feruloyl putrescines from wastewater (Nejayote) of lime-cooked white maize (Zea mays). J. Cereal Sci. 2015, 64, 23–28. [Google Scholar] [CrossRef]
- Kim, E.-O.; Kwon, T.-K.; Choi, S.-W. Diferuloylputrescine, a predominant phenolic amide in corn bran, potently induces apoptosis in human leukemia U937 cells. J. Med. Food 2014, 17, 519–526. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, C.; Ou, S. Derivatives of ferulic acid: Structure, preparation and biological activities. Annu. Res. Rev. Biol. 2015, 512–528. [Google Scholar] [CrossRef]
- Wang, S.; Suh, J.H.; Zheng, X.; Wang, Y.; Ho, C.-T. Identification and quantification of potential anti-inflammatory hydroxycinnamic acid amides from wolfberry. J. Agric. Food Chem. 2017, 65, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; He, Y.; Lu, F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018, 269, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef] [Green Version]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [Green Version]
- Vaz Patto, M.; Moreira, P.; Carvalho, V.; Pego, S. Collecting maize (Zea mays L. convar. mays) with potential technological ability for bread making in Portugal. Genet. Resour. Crop Evol. 2007, 54, 1555–1563. [Google Scholar] [CrossRef]
- Brites, C.M.; Trigo, M.J.; Carrapiço, B.; Alviña, M.; Bessa, R.J. Maize and resistant starch enriched breads reduce postprandial glycemic responses in rats. Nutr. Res. 2011, 31, 302–308. [Google Scholar] [CrossRef]
- Alves, M.L.; Belo, M.; Carbas, B.; Brites, C.; Paulo, M.; Mendes-Moreira, P.; Brites, C.; Bronze, M.d.R.; Šatović, Z.; Vaz Patto, M.C. Long-term on-farm participatory maize breeding by stratified mass selection retains molecular diversity while improving agronomic performance. Evol. Appl. 2018, 11, 254–270. [Google Scholar] [CrossRef] [Green Version]
- Mussi de Mira, N.V.; Cerdeira Barros, R.M.; Schiocchet, M.A.; Noldin, J.A.; Lanfer-Marquez, U.M. Extração, análise e distribuição dos ácidos fenólicos em genótipos pigmentados e não pigmentados de arroz (Oryza sativa L.). Cienc. Tecnol. Aliment. 2008, 28, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Dobberstein, D.; Bunzel, M. Separation and detection of cell wall-bound ferulic acid dehydrodimers and dehydrotrimers in cereals and other plant materials by reversed phase high-performance liquid chromatography with ultraviolet detection. J. Agric. Food Chem. 2010, 58, 8927–8935. [Google Scholar] [CrossRef]
- Zaupa, M.; Calani, L.; Del Rio, D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly) phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef]
- Callipo, L.; Cavaliere, C.; Fuscoletti, V.; Gubbiotti, R.; Samperi, R.; Laganà, A. Phenilpropanoate identification in young wheat plants by liquid chromatography/tandem mass spectrometry: Monomeric and dimeric compounds. J. Mass Spectrom. 2010, 45, 1026–1040. [Google Scholar] [CrossRef]
- Arranz, S.; Calixto, F.S. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Chiremba, C.; Taylor, J.R.; Rooney, L.W.; Beta, T. Phenolic acid content of sorghum and maize cultivars varying in hardness. Food Chem. 2012, 134, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Das, A.K.; Singh, V. Antioxidative free and bound phenolic constituents in botanical fractions of Indian specialty maize (Zea mays L.) genotypes. Food Chem. 2016, 201, 298–306. [Google Scholar] [CrossRef]
- Ndolo, V.U.; Beta, T. Comparative studies on composition and distribution of phenolic acids in cereal grain botanical fractions. Cereal Chem. 2014, 91, 522–530. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, C.; Zhao, X.; Xia, Y.; Sun, X.; Xie, W.; Ye, Y.; Lu, X.; Xu, G. Deep Annotation of Hydroxycinnamic Acid Amides in Plants Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry and Its In Silico Database. Anal. Chem. 2018, 90, 14321–14330. [Google Scholar] [CrossRef] [Green Version]
- Burt, A.J.; Arnason, J.T.; García-Lara, S. Natural variation of hydroxycinnamic acid amides in maize landraces. J. Cereal Sci. 2019, 88, 145–149. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019, 295, 214–223. [Google Scholar] [CrossRef]
- Guo, W.; Beta, T. Phenolic acid composition and antioxidant potential of insoluble and soluble dietary fibre extracts derived from select whole-grain cereals. Food Res. Int. 2013, 51, 518–525. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.-S.M. The impact of milling and thermal processing on phenolic compounds in cereal grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Niu, R.; Minami, E.; Saka, S. Characterization of three tissue fractions in corn (Zea mays) cob. Biomass Bioenergy 2018, 115, 130–135. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Q.; Beta, T. Antioxidant properties of commercial wild rice and analysis of soluble and insoluble phenolic acids. Food Chem. 2010, 121, 140–147. [Google Scholar] [CrossRef]
- Pedersen, M.B.; Bunzel, M.; Schäfer, J.; Knudsen, K.E.B.; Sørensen, J.F.; Yu, S.; Lærke, H.N. Ferulic acid dehydrodimer and dehydrotrimer profiles of distiller’s dried grains with solubles from different cereal species. J. Agric. Food Chem. 2015, 63, 2006–2012. [Google Scholar] [CrossRef]
- Vismeh, R.; Lu, F.; Chundawat, S.P.; Humpula, J.F.; Azarpira, A.; Balan, V.; Dale, B.E.; Ralph, J.; Jones, A.D. Profiling of diferulates (plant cell wall cross-linkers) using ultrahigh-performance liquid chromatography-tandem mass spectrometry. Analyst 2013, 138, 6683–6692. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.-T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef]
- Waterstraat, M.; Bunzel, M. A multi-step chromatographic approach to purify radically generated ferulate oligomers reveals naturally occurring 5-5/8-8 (cyclic)-, 8-8 (noncyclic)/8-O-4-, and 5-5/8-8 (noncyclic)-coupled dehydrotriferulic acids. Front. Chem. 2018, 6, 190. [Google Scholar] [CrossRef]
- Sen, A.; Bergvinson, D.; Miller, S.S.; Atkinson, J.; Fulcher, R.G.; Arnason, J.T. Distribution and microchemical detection of phenolic acids, flavonoids, and phenolic acid amides in maize kernels. J. Agric. Food Chem. 1994, 42, 1879–1883. [Google Scholar] [CrossRef]
- Kim, E.O.; Min, K.J.; Kwon, T.K.; Um, B.H.; Moreau, R.A.; Choi, S.W. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem. Toxicol. 2012, 50, 1309–1316. [Google Scholar] [CrossRef]



| Variety/Flour | Description |
|---|---|
| Broa-213 | Yellow grain. Early intermediate type. Collect from the farmer in the 2005 expedition to the Central Northern region of Portugal [20]. |
| Pigarro | White grain. Flint type FAO 300 with strong fasciation expression, used in the best soils for human consumption. Participatory improved population [20]. |
| Castro Verde | Yellow grain. Late flint type FAO 600, with big grain row number and large ear size. Participatory improved population [20]. |
| Verdeal de Aperrela | White grain. Late flint type FAO 600 used for breadmaking. Participatory improved population [20]. |
| Fandango | Yellow grain. Synthetic open-pollinated variety, dent type FAO 600, big grain row number and large ear size. Participatory improved population [20]. |
| Commercial | White colour. Nacional Type 175, wholegrain flour. |
| # | λmax | RT | Putative Identification | m/z | MS/MS Ions | SF | IF |
|---|---|---|---|---|---|---|---|
| Small Phenolic Compounds | |||||||
| 3 | 260, 294 | 30.96 | Protocatechuic acid (−) Ұ | 153 | 109, 108 | - | B, M, R, W |
| 4 | 286 | 39.65 | Ferulic acid hexoside 1 (+) | 357 | 195 | B | - |
| 5 | n/d | 46.16 | Ferulic acid hexoside 2 (+) | 357 | 195, 149, 185 | B | - |
| 6 | 325, 298 | 46.93 | Vanillic acid (−) Ұ | 169 | 93, 123, 65 | B, M, R, W | B, M, R, W |
| 7 | 294 | 47.83 | Ferulic acid hexoside 3 (+) | 357 | 195, 149, 185 | B, W | - |
| 8 | 324, 295 | 51.99 | Caffeic acid (−) Ұ | 179 | 135 | B | B, M, R, W |
| 9 | 275 | 53.60 | Syringic acid (+) Ұ | 199 | 140, 155, 123 | B, M, R, W | B, M, R, W |
| 10 | 286 | 55.08 | p-Hydroxybenzaldehyde (−) | 121 | 39, 92 | B, M, R, W | B, M, R, W |
| 11 | 279, 309 | 65.88 | Vanillin (+) Ұ | 153 | 93, 125, 65 | B, M, R, W | B, M, R, W |
| 12 | 296 | 69.60 | Coumaroyl glycerol (−) | 237 | 145, 119, 163 | B, M, R | - |
| 13 | 310 | 70.91 | p-Coumaric acid (trans) (−) Ұ | 163 | 119, 93 | B, M, R, W | B, M, R, W |
| 14 | 297 | 71.31 | Syringaldehyde (+) Ұ | 183 | 123, 95, 155, 140 | B, M, R | B, M, R, W |
| 15 | n/d | 73.00 | p-Coumaric acid (cis) (−) | 163 | 119, 93 | B, M, R, W | B, M, R, W |
| 16 | 322, 295 | 78.97 | Ferulic acid (trans) (−) Ұ | 193 | 134, 149, 178 | B, M, R, W | B, M, R, W |
| 17 | 312 | 81.11 | Ferulic acid (cis) (−) | 193 | 134, 149, 178 | B, M, R, W | B, M, R, W |
| Ferulic Acid Dehydrodimers | |||||||
| 18 | 333 | 82.86 | 8-8′-DFAc(−) | 385 | 267, 158, 173 | - | B, M, R, W |
| 20 | 318, 284 | 85.29 | DFA, hydrated 1 (−) | 403 | 178, 148, 193, 134 | - | B, M, R, W |
| 22 | 325 | 86.84 | 8-8′-DFA (−) | 385 | 282, 173, 123 | - | B, M, R |
| 23 | n/d | 87.23 | DFA, hydrated 2 (−) | 403 | 239, 279, 265, 134, 148 | - | B, M |
| 26 | 284, 318 | 88.91 | 8-8′-DFAf(−) | 403 | 151, 148, 233, 163 | - | B, M, R |
| 28 | 322 | 89.64 | 8-5′-DFA (−) | 385 | 282, 267, 326, 297, 323, 341 | - | B, M, R, W |
| 29 | n/d | 90.88 | DFA, hydrated 3 (−) | 403 | 193, 308, 149, 164 | - | B, M, R |
| 49 | 284, 318 | 97.33 | DFA 1 (−) | 385 | 173, 123, 282 | - | B, M |
| 52 | 322 | 98.64 | 4-O-5′-DFA (−) | 385 | 139, 193, 267, 329 | - | B, M |
| 62 | 318 | 101.78 | DFA 2 (−) | 385 | 267, 382 | - | B, M, R, W |
| 70 | 322 | 104.50 | 5-5-DFA (−) | 385 | 282, 326, 341, 267 | - | B, M, R, W |
| 84 | 319 | 109.19 | 8-5′-DFAf (−) | 385 | 282, 326, 341, 267 | - | B, M, R, W |
| 89 | 322, 294 | 110.85 | 8-O-4-DFA (trans/trans) (−) | 385 | 134, 178, 149, 193 | - | B, M, R, W |
| 93 | 318, 289 | 113.35 | 8-O-4-DFA (trans/cis) (−) | 385 | 134, 178, 149, 193 | - | B, M |
| 98 | 318 | 115.83 | DFA 3 (−) | 385 | - | - | B, M, R |
| 107 | 318 | 121.94 | DFA 4 (−) | 385 | - | - | B, M |
| 122 | 284 | 129.81 | 8-5′-DFAdc (trans) (−) | 341 | - | - | B, M |
| 123 | n/d | 131.10 | 8-5′-DFAdc (cis) (−) | 341 | - | - | B, M |
| Ferulic Acid Dehydrotrimers and Tetramers | |||||||
| 31 | n/d | 91.54 | TFA 1 (−) | 577 | 435, 508, 178 | - | B, M, R |
| 48 | n/d | 96.94 | TFA, hydrated 1 (−) | 595 | - | - | B, M |
| 53 | 322 | 98.91 | 8-O-4′/5-8″-TFA (−) | 577 | 193, 355 | - | B, M, R |
| 56 | 318 | 100.12 | TFA, hydrated 2 (−) | 595 | 317, 545, 367 | - | B, M |
| 61 | 318 | 101.70 | TFA 2 (−) | 577 | 146 | - | B, M |
| 64 | 316 | 102.67 | TFA, hydrated 3 (−) | 595 | - | - | B, M |
| 71 | n/d | 104.91 | 8-8′c-/4-O-8″-TFA (−) | 577 | 341, 533, 489 | - | B, M |
| 94 | 318 | 113.54 | 4-O-8′/5′-5″/8″-5″′-TeFA (−) | 769 | 274 | - | B, M |
| 101 | 319 | 117.17 | 8-O-4′-5-5″-TFA (−) | 577 | 355, 533, 193, 489 | - | B, M, R, W |
| 109 | 318 | 122.74 | TFA 3 (−) | 577 | 355 | - | B, M |
| 113 | 316 | 124.73 | TFA 4 (−) | 577 | 355 | - | B, M |
| 126 | 319, 291 | 131.98 | 8-O-4′/4-O-8″-TFA (−) | 577 | 193 | - | B, M, R |
| 130 | 318 | 135.24 | 4-O-8′/5′-5″/8″-O-4-TeFA (−) | 769 | 193 | - | B, M |
| Soluble Hydroxycinnamic Acid Amides | |||||||
| 21 | 290 | 85.88 | N-Coumaroyl spermidine (+) | 292 | 147, 204 | B | - |
| 25 | n/d | 88.74 | N,N′-Dicoumaroyl spermidine (cis/cis) (+) | 438 | 147, 204, 292, 275, 72 | B, M | B, M |
| 32 | n/d | 91.75 | N,N′-Coumaroyl feruloyl spermidine (cis/cis) (+) | 468 | 177, 234, 204, 292, 147, 322, 145 | B, M | - |
| 36 | 290 | 93.05 | N,N′-Dicoumaroyl spermidine (cis/trans) (+) | 438 | 147, 204, 292, 275, 72 | B, M | B, M |
| 39 | n/d | 94.40 | N,N′-Diferuloyl spermidine (cis/cis) (+) | 498 | 177, 234, 322, 145 | B, M, W | - |
| 40 | n/d | 94.60 | N,N′-Coumaroyl feruloyl spermidine (cis/trans) 1 (+) | 468 | 177, 204, 292, 147, 322, 234, 275, 145 | B, M, W, R | - |
| 44 | n/d | 96.13 | N,N′-Dicoumaroyl putrescine (cis/cis) (+) | 381 | 189, 145, 147, 101, 277, 177, 321 | B, M | - |
| 45 | n/d | 96.42 | N,N′-Diferuloyl spermidine (cis/trans) (+) | 498 | 177, 234, 322, 305, 145 | B, M | - |
| 46 | n/d | 96.59 | N,N′-Coumaroyl feruloyl spermidine (cis/trans) 2 (+) | 468 | 177, 204, 292, 147, 322, 234, 275, 145 | B, M, W, R | - |
| 47 | 294 | 96.62 | N,N′-Dicoumaroyl spermidine (trans/trans) (+) | 438 | 147, 204, 292, 275, 72, 221 | B, M | B, M |
| 51 | n/d | 98.52 | N,N′-Coumaroyl feruloyl putrescine (cis/cis) (+) | 411 | 177, 147, 235 | B, M | B, M |
| 54 | n/d | 99.44 | N,N′-Coumaroyl feruloyl spermidine (trans/trans) (+) | 468 | 177, 234, 204, 292, 322, 147, 145, 305, 275 | B, M | - |
| 57 | n/d | 100.61 | N,N′-Diferuloyl spermidine (trans/trans) | 498 | 177, 322, 234, 145, | B, M, W | - |
| 58 | 290 | 100.83 | N,N′-Diferuloyl putrescine (cis/cis) (+) | 441 | 177, 265, 145, 89, 117, 248 | B, M | B, M |
| 60 | n/d | 101.43 | N,N′-Dicoumaroyl putrescine (cis/trans) (+) | 381 | 147, 235, 218 | B, M | - |
| 67 | 291 | 103.47 | N,N′-Coumaroyl feruloyl putrescine (cis/trans) 1 (+) | 411 | 177, 147, 145, 265, 235, 218 | B, M | B, M |
| 69 | 292 | 104.09 | N,N′-Coumaroyl feruloyl putrescine (cis/trans) 2 (+) | 411 | 177, 147, 145, 265, 235, 218 | B, M | B, M |
| 73 | n/d | 105.30 | N,N′-Dicoumaroyl putrescine (trans/trans) (+) | 381 | 147, 235, 218, 89, 72 | B, M | - |
| 74 | 293 | 105.88 | N,N′-Diferuloyl putrescine (cis/trans) (+) | 441 | 177, 145, 265, 248 | B, M | B, M |
| 78 | 292, 308 | 107.56 | N,N′-Coumaroyl feruloyl putrescine (trans/trans) (+) | 411 | 177, 147, 145, 235, 265, 89, 218 | B, M | B, M |
| 82 | 290 | 108.65 | bis-N,N′-Diferuloyl putrescine (−) | 877 | 439 | B, M | - |
| 86 | 317, 293 | 109.65 | N,N′-Diferuloyl putrescine (trans/trans) (+) | 441 | 177, 145, 265, 248, 89, 117, 72 | B, M | B, M |
| Insoluble Hydroxycinnamic Acid Amides | |||||||
| 19 | n/d | 84.03 | N,N′-Coumaroyl dehydrotriferuloyl putrescine 1 (+) | 795 | 409, 519, 719 | - | M |
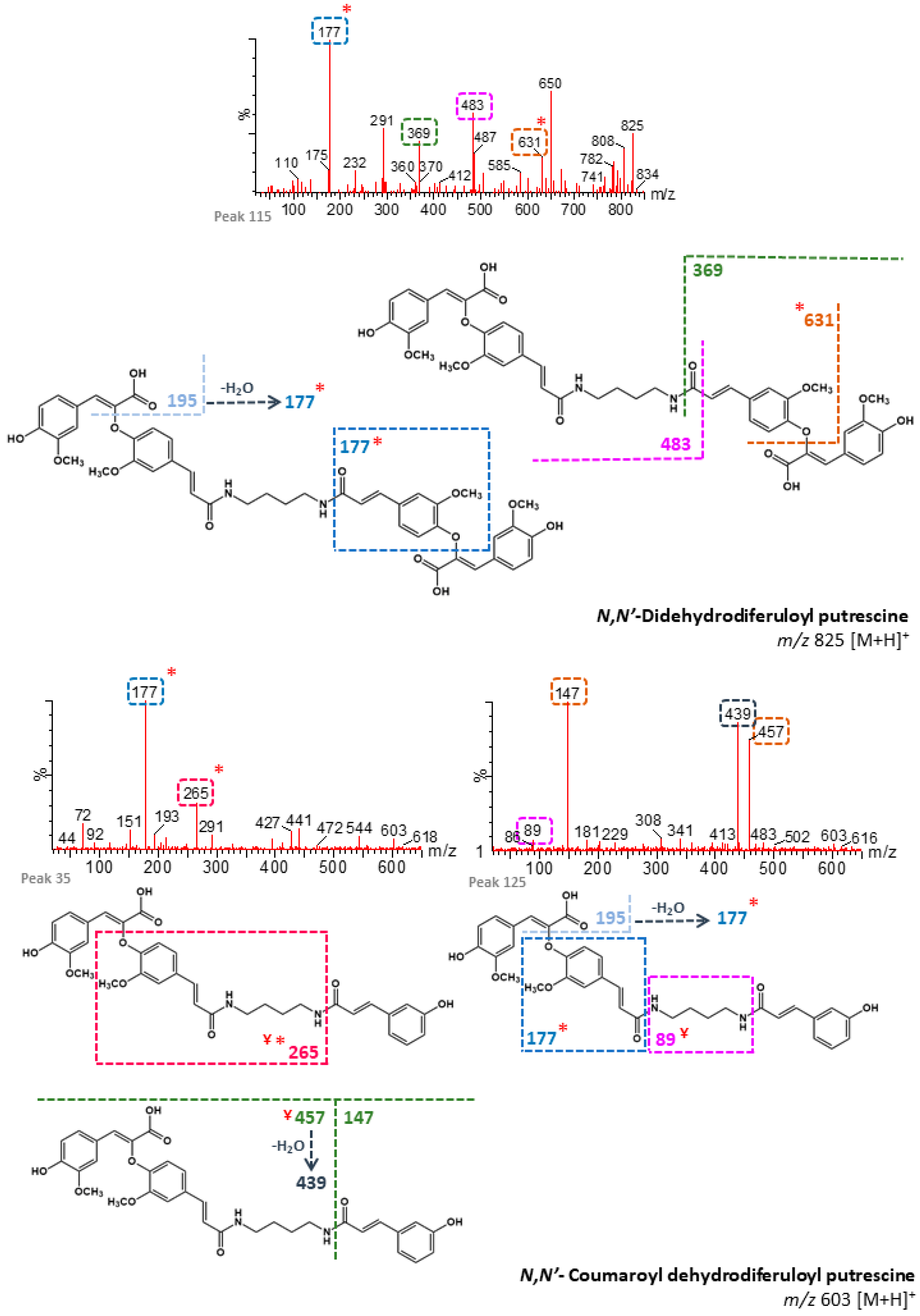
| 24 | n/d | 87.48 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 1 (+) | 603 | 177, 427, 265, 195 | - | B, M |
| 27 | n/d | 89.40 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 2 (+) | 603 | 177, 265 | - | B, M |
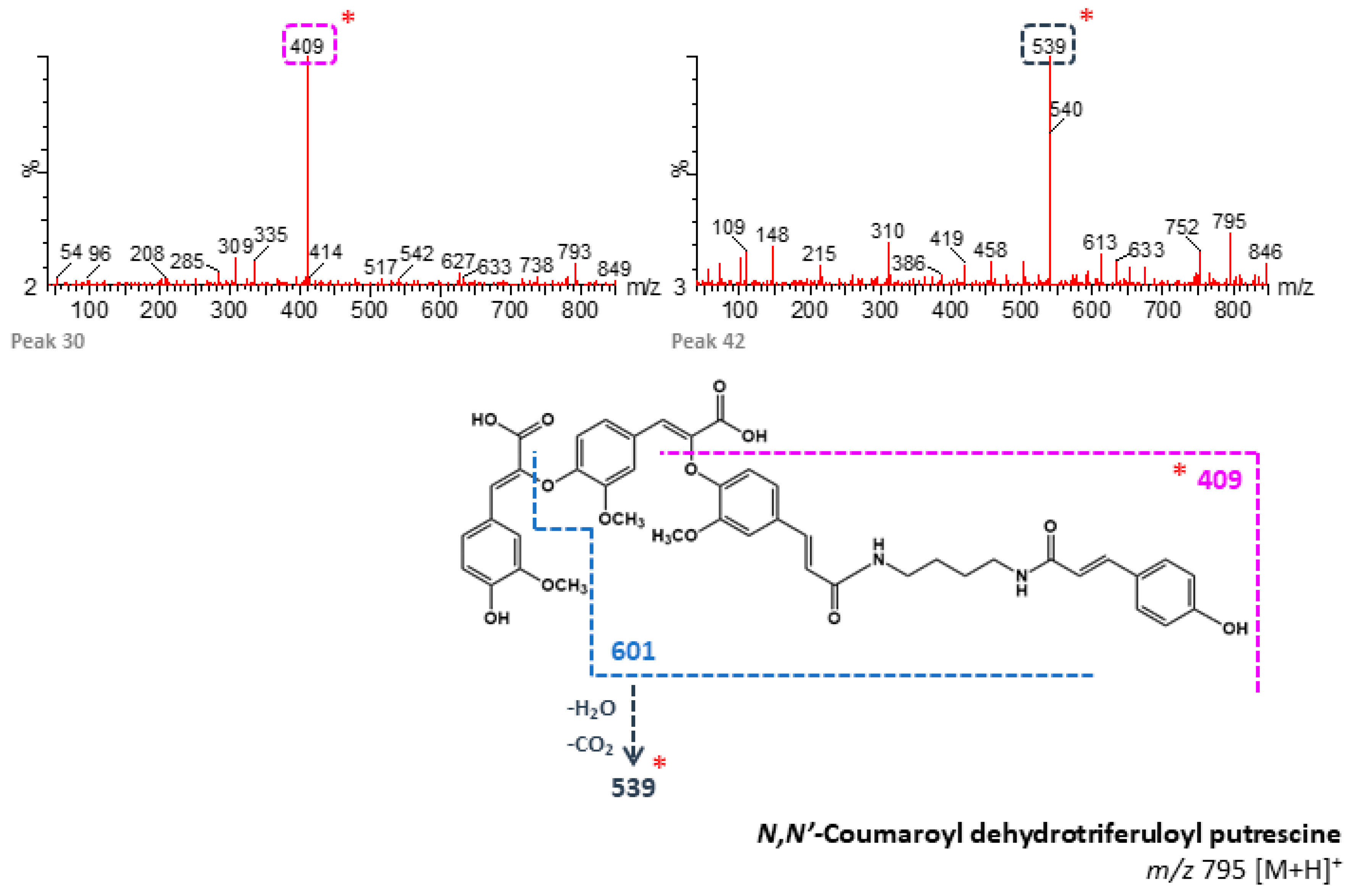
| 30 | n/d | 91.20 | N,N′-Coumaroyl dehydrotriferuloyl putrescine 2 (+) | 795 | 409 | - | B, M |
| 33 | n/d | 92.22 | N-Dehydrodiferuloyl putrescine 1 (+) | 457 | 89, 72, 115 | - | B, M |
| 34 | n/d | 92.24 | N,N′-Coumaroyl dehydrotriferuloyl putrescine 3 (+) | 795 | 409, 533 | - | B, M |
| 35 | n/d | 92.44 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 3 (+) | 603 | 177, 265, 72 | - | B, M |
| 37 | n/d | 93.35 | N-Dehydrodiferuloyl putrescine 2 (+) | 457 | 115, 265, 72 | - | B, M |
| 38 | n/d | 93.59 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 4 (+) | 603 | 415, 148 | - | B, M |
| 41 | n/d | 95.02 | N-Dehydrodiferuloyl putrescine 3 (+) | 457 | 115, 298, 72 | - | B, M |
| 42 | n/d | 95.61 | N,N′-Coumaroyl dehydrotriferuloyl putrescine 4 (+) | 795 | 539, 148 | - | B, M |
| 43 | n/d | 95.68 | N-Dehydrodiferuloyl putrescine 4 (+) | 457 | 351, 319, 277, 115 | - | B, M |
| 50 | n/d | 97.44 | N,N′-Coumaroyl dehydrotriferuloyl putrescine 5 (+) | 795 | 539, 135, 195 | - | B, M |
| 55 | n/d | 99.70 | N-Dehydrodiferuloyl putrescine 5 (+) | 457 | 115, 98, 177, 244, 365 | - | B, M |
| 59 | n/d | 101.10 | N-Dehydrodiferuloyl putrescine 6 (+) | 457 | 351, 440, 115, 72, 369 | - | B, M |
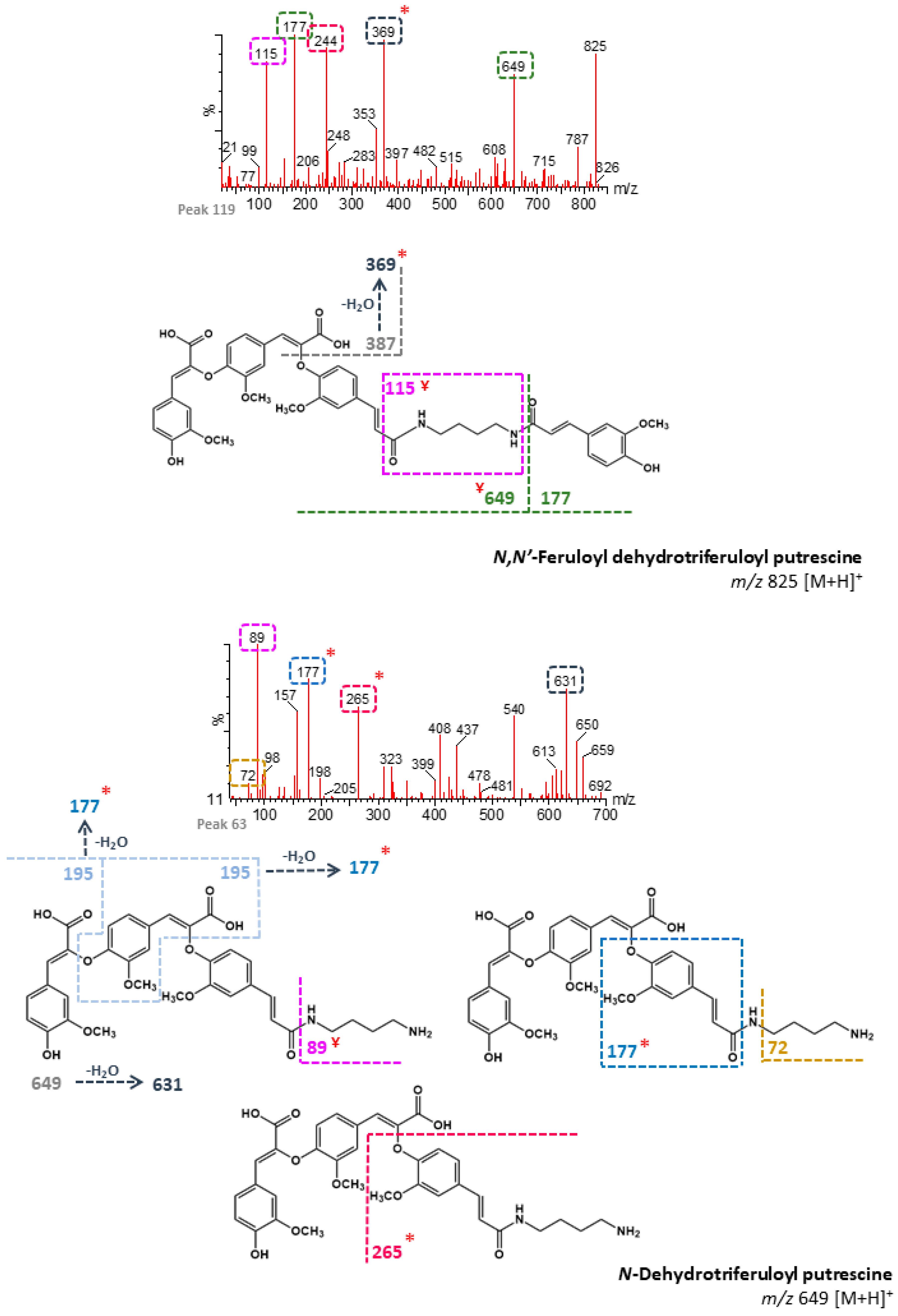
| 63 | n/d | 101.80 | N-Dehydrotriferuloyl putrescine 1 (+) | 649 | 89, 177, 631, 265, 72 | - | B, M |
| 65 | n/d | 103.11 | N-Dehydrodiferuloyl putrescine 7 (+) | 457 | 351, 177, 175, 440, 72, 115, 263 | - | B, M |
| 66 | n/d | 103.33 | N,N′-Coumaroyl dehydrotriferuloyl putrescine 6 (+) | 795 | 539, 394, 435 | B, M | |
| 68 | n/d | 103.87 | N,N′-Feruloyl dehydrodiferuloyl putrescine 1 (+) | 633 | 177, 432, 465, 387 | - | B, M |
| 72 | n/d | 105.27 | N,N′-Feruloyl dehydrodiferuloyl putrescine 2 (+) | 633 | 177, 457, 369, 341, 72 | - | B, M |
| 75 | n/d | 106.33 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 5 (+) | 603 | 457, 369, 72, 83, 369, 411 | - | B, M |
| 76 | n/d | 106.82 | N,N′-Feruloyl dehydrodiferuloyl putrescine 3 (+) | 633 | 177, 265, 439, 457 | - | B, M |
| 77 | n/d | 107.45 | N,N′-Feruloyl dehydrodiferuloyl putrescine 4 (+) | 633 | 177, 341, 439, 589 | - | B, M |
| 79 | n/d | 107.97 | N-Dehydrotriferuloyl putrescine 2 (+) | 649 | 265, 89, 177, 440 | - | B, M |
| 80 | n/d | 108.18 | N,N′-Feruloyl dehydrodiferuloyl putrescine 5 (+) | 633 | 177, 369, 457, 439, 574, 291, 145, 89 | - | B, M |
| 81 | n/d | 108.58 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 6 (+) | 603 | 457, 385 | - | B, M |
| 83 | n/d | 109.16 | N-Dehydrotriferuloyl putrescine 3 (+) | 649 | 89, 72, 148, 265 | - | B, M |
| 85 | n/d | 109.34 | N,N′-Feruloyl dehydrodiferuloyl putrescine 6 (+) | 633 | 177, 351, 245, 439 | - | B, M |
| 87 | n/d | 110.28 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 7 (+) | 603 | 365, 439, 351 | - | B, M |
| 88 | n/d | 110.34 | N,N′-Feruloyl dehydrodiferuloyl putrescine 7 (+) | 633 | 457, 177, 439, 369, 265 | - | B, M |
| 90 | n/d | 110.90 | N,N′-Coumaroyl dehydrotriferuloyl putrescine 7 (+) | 795 | 409 | - | B, M |
| 91 | n/d | 111.80 | N,N′-Feruloyl dehydrodiferuloyl putrescine 8 (+) | 633 | 177, 439, 457, 589 | - | B, M |
| 92 | n/d | 112.32 | N,N′-Feruloyl dehydrodiferuloyl putrescine 9 (+) | 633 | 265, 177, 351, 369, 439 | - | B, M |
| 95 | n/d | 113.60 | N-Dehydrotriferuloyl putrescine 4 (+) | 649 | 72, 89, 177, 631 | - | B, M |
| 96 | n/d | 114.02 | N,N′-Feruloyl dehydrodiferuloyl putrescine 10 (+) | 633 | 439, 457, 369, 277 | - | B, M |
| 97 | n/d | 115.02 | N,N′-Feruloyl dehydrodiferuloyl putrescine 11 (+) | 633 | 519, 439, 351, 145, 175, 177 | - | B, M |
| 99 | n/d | 115.88 | N-Dehydrotriferuloyl putrescine 5 (+) | 649 | 177, 115 | - | B, M |
| 100 | n/d | 116.69 | N,N′-Feruloyl dehydrodiferuloyl putrescine 12 (+) | 633 | 245, 151, 291, 351, 177, 439 | - | B, M |
| 102 | n/d | 117.21 | N-Dehydrotriferuloyl putrescine 6 (+) | 649 | 245, 177, 323 | - | B, M |
| 103 | n/d | 118.49 | N,N′-Feruloyl dehydrodiferuloyl putrescine 13 (+) | 633 | 439, 457, 177 | - | B, M |
| 104 | n/d | 118.97 | N,N′-Didehydrodiferuloyl putrescine 1 (+) | 825 | 367, 631 | - | B, M |
| 105 | n/d | 119.43 | N,N′-Feruloyl dehydrodiferuloyl putrescine 14 (+) | 633 | 177, 439, 351, 457, 115, 89 | - | B, M |
| 106 | n/d | 120.04 | N,N′-Feruloyl dehydrotriferuloyl putrescine 1 (+) | 825 | 631, 177, 265, 649 | - | B, M |
| 108 | n/d | 122.23 | N-Dehydrotriferuloyl putrescine 7 (+) | 649 | 382, 265, 72, 89, 439 | - | B, M |
| 110 | n/d | 122.80 | N,N′-Feruloyl dehydrodiferuloyl putrescine 15 (+) | 633 | 177, 439, 265, 369, 351, 457 | - | B, M |
| 111 | n/d | 123.55 | N-Dehydrotriferuloyl putrescine 8 (+) | 649 | 473, 145, 177 | - | B, M |
| 112 | n/d | 124.17 | N,N′-Feruloyl dehydrodiferuloyl putrescine 16 (+) | 633 | 351, 177, 265, 72 | - | B, M |
| 114 | n/d | 124.93 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 8 (+) | 603 | 457, 235, 147 | - | B, M |
| 115 | n/d | 125.38 | N,N′-Didehydrodiferuloyl putrescine 2 (+) | 825 | 177, 650, 483, 369, 631 | - | B, M |
| 116 | n/d | 126.08 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 9 (+) | 603 | 439, 369, 147, 457 | - | B, M |
| 117 | n/d | 126.54 | N,N′-Feruloyl dehydrodiferuloyl putrescine 17 (+) | 633 | 177, 457, 439 | - | B, M |
| 118 | n/d | 127.85 | N,N′-Feruloyl dehydrodiferuloyl putrescine 18 (+) | 633 | 177, 369, 265, 115, 439 | - | B, M |
| 119 | n/d | 127.97 | N,N′-Feruloyl dehydrotriferuloyl putrescine 2 (+) | 825 | 177, 369, 244, 115, 649 | - | B, M |
| 120 | n/d | 129.06 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 10 (+) | 603 | 457, 369, 86, 175, 219, 147 | - | B, M |
| 121 | n/d | 129.76 | N,N′-Feruloyl dehydrodiferuloyl putrescine 19 (+) | 633 | 177, 439, 457, 589, 115, 145 | - | B, M |
| 124 | n/d | 131.12 | N-Dehydrotriferuloyl putrescine 9 (+) | 649 | 351, 177 | - | B, M |
| 125 | n/d | 131.19 | N,N′-Coumaroyl dehydrodiferuloyl putrescine 11 (+) | 603 | 147, 439, 457, 89 | - | B, M |
| 127 | n/d | 132.59 | N,N′-Didehydrodiferuloyl putrescine 3 (+) | 825 | 177, 631 | - | B, M |
| 128 | n/d | 133.20 | N,N′-Feruloyl dehydrodiferuloyl putrescine 20 (+) | 633 | 177, 369, 457, 265, 291, 439, 145, 72, 89 | - | B, M |
| 129 | n/d | 134.97 | N,N′-Feruloyl dehydrodiferuloyl putrescine 21 (+) | 633 | 177, 457, 439, 291, 145, 351, 589 | - | B, M |
| 131 | n/d | 139.79 | N,N′-Feruloyl dehydrodiferuloyl putrescine 22 (+) | 633 | 177, 457, 439 | - | B, M |
| 132 | n/d | 144.90 | N,N′-Didehydrodiferuloyl putrescine 4 (+) | 825 | 177, 439 | - | B, M |
| 133 | n/d | 145.78 | N,N′-Feruloyl dehydrodiferuloyl putrescine 23 (+) | 633 | 177, 457, 439, 135 | - | B, M |
| 134 | n/d | 150.15 | N,N′-Feruloyl dehydrodiferuloyl putrescine 24 (+) | 633 | 177, 457 | - | B, M |
| 135 | n/d | 153.38 | N,N′-Feruloyl dehydrodiferuloyl putrescine 25 (+) | 633 | 177 | - | B, M |
| Other Compounds | |||||||
| 1 | 282 | 11.50 | Citric acid (−) Ұ | 191 | 87, 111, 85, 67 | B, M, R, W | B, M |
| 2 | n/d | 28.45 | Tyrosyl-tryptophan (-) | 366 | 160 | B, M | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento-Silva, A.; Duarte, N.; Mecha, E.; Belo, M.; Vaz Patto, M.C.; Bronze, M.d.R. Hydroxycinnamic Acids and Their Derivatives in Broa, a Traditional Ethnic Maize Bread. Foods 2020, 9, 1471. https://doi.org/10.3390/foods9101471
Bento-Silva A, Duarte N, Mecha E, Belo M, Vaz Patto MC, Bronze MdR. Hydroxycinnamic Acids and Their Derivatives in Broa, a Traditional Ethnic Maize Bread. Foods. 2020; 9(10):1471. https://doi.org/10.3390/foods9101471
Chicago/Turabian StyleBento-Silva, Andreia, Noélia Duarte, Elsa Mecha, Maria Belo, Maria Carlota Vaz Patto, and Maria do Rosário Bronze. 2020. "Hydroxycinnamic Acids and Their Derivatives in Broa, a Traditional Ethnic Maize Bread" Foods 9, no. 10: 1471. https://doi.org/10.3390/foods9101471
APA StyleBento-Silva, A., Duarte, N., Mecha, E., Belo, M., Vaz Patto, M. C., & Bronze, M. d. R. (2020). Hydroxycinnamic Acids and Their Derivatives in Broa, a Traditional Ethnic Maize Bread. Foods, 9(10), 1471. https://doi.org/10.3390/foods9101471






