Detection of Lipid Oxidation in Infant Formulas: Application of Infrared Spectroscopy to Complex Food Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Infant Milk Formula Production and Oxidation Conditions
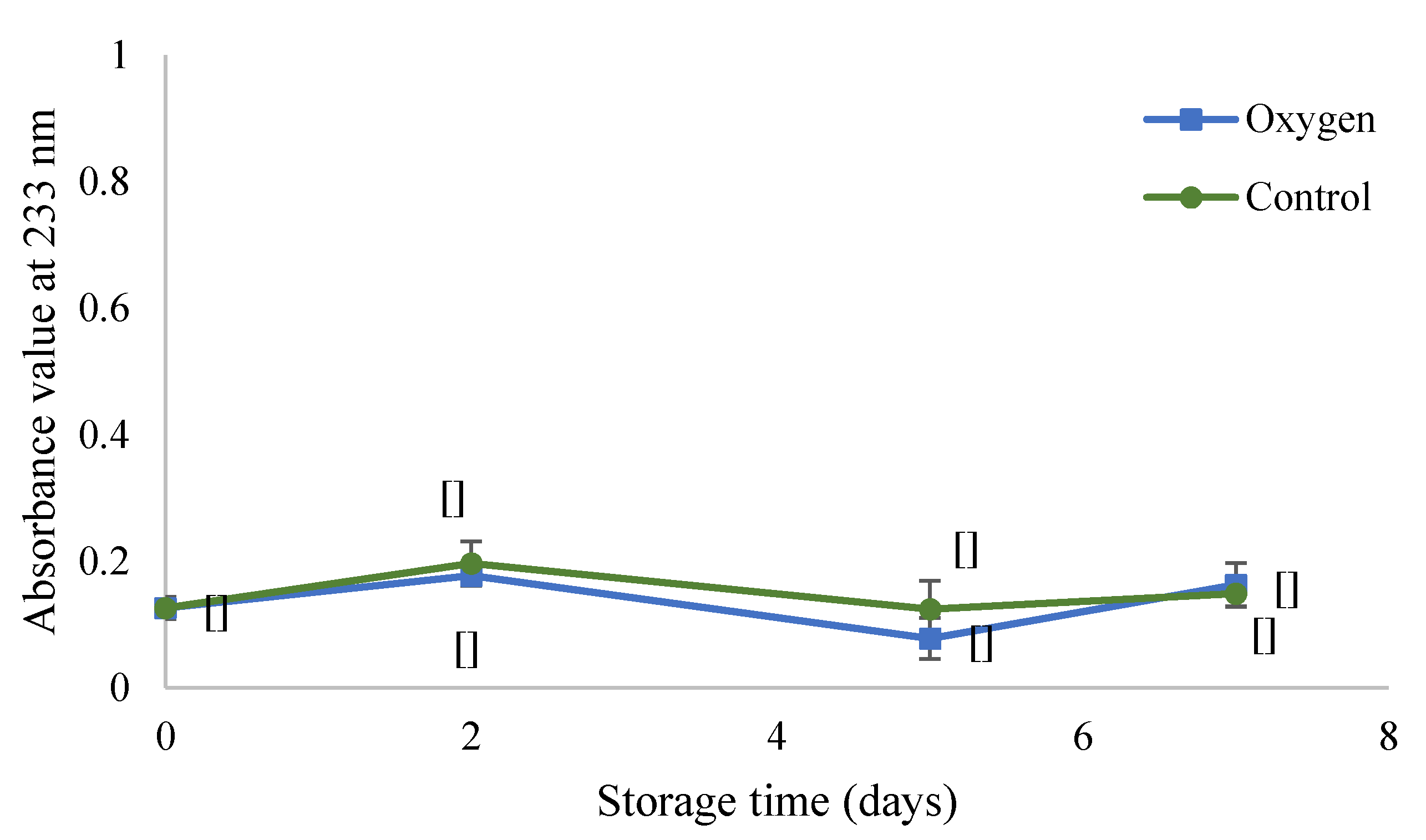
2.2. Conjugated Diene Determination (CD Values)
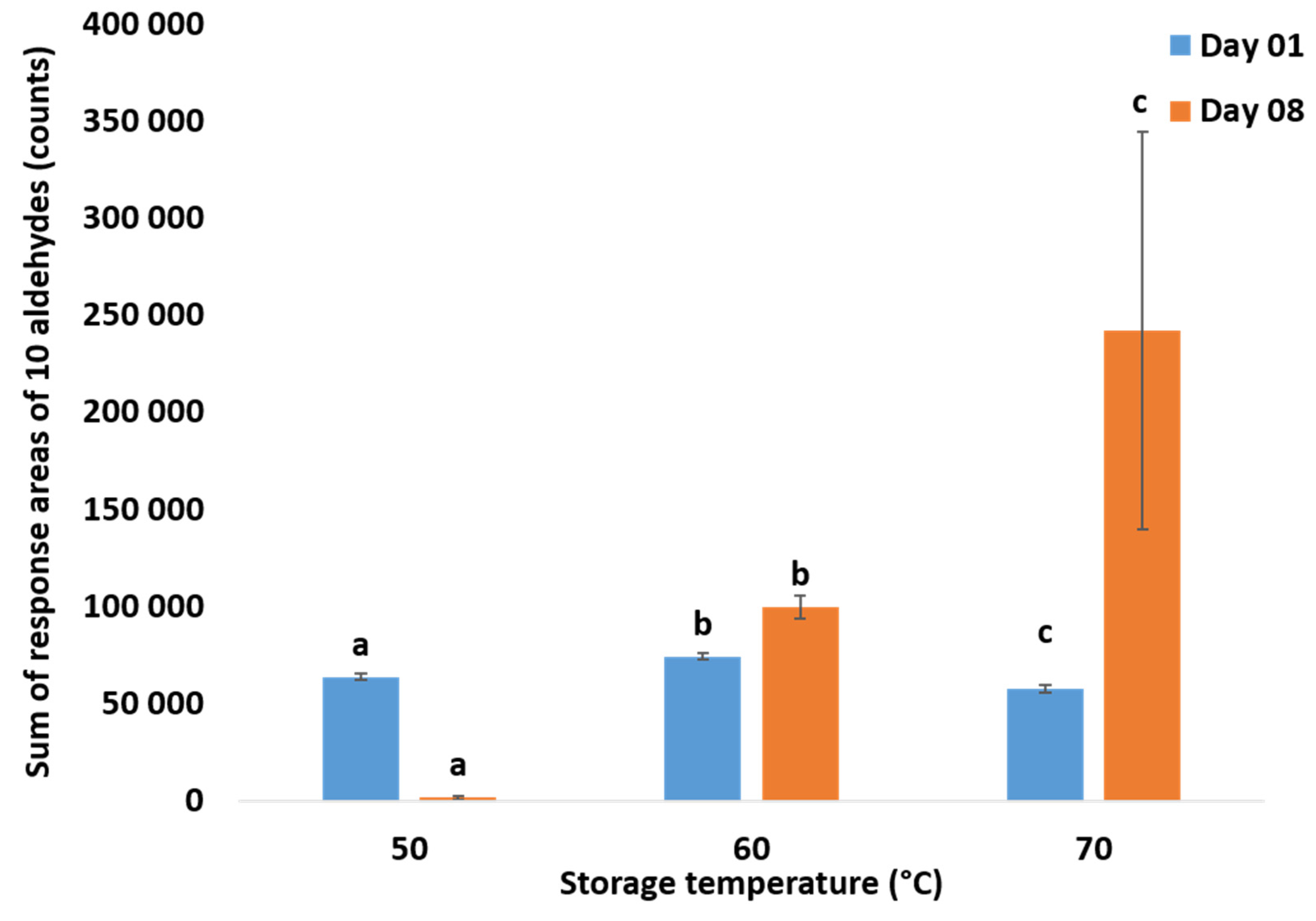
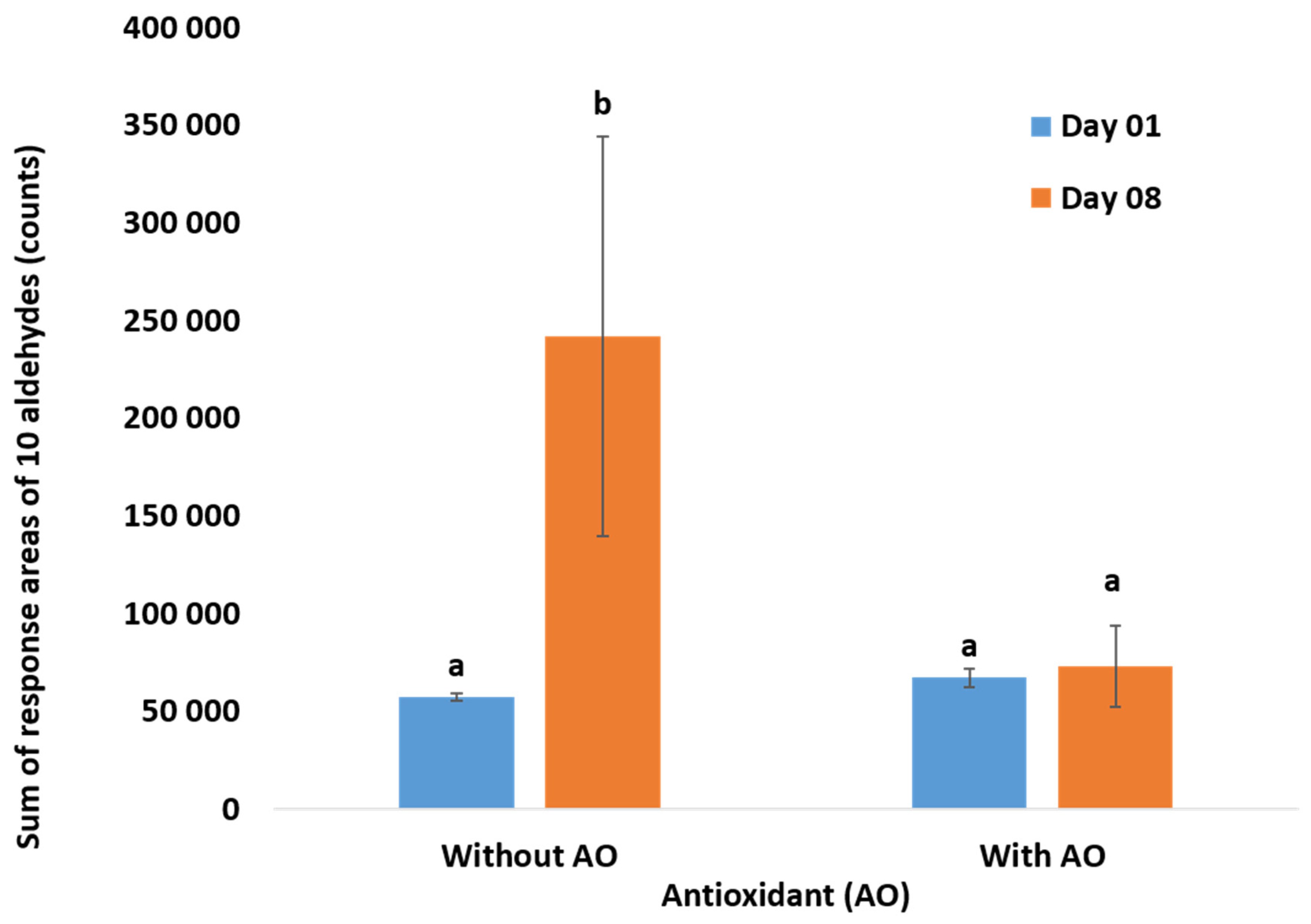
2.3. Total Volatile Measurements using GC-MS
2.4. Near- and Mid-Infrared Measurements
2.5. Pretreatment and Multivariate Data Analysis
3. Results and Discussion
3.1. Oxidation Monitoring by Reference Methods
3.1.1. Determination of Conjugated Diene Values
3.1.2. Measurement of Released Volatiles by HS-SPME/GC-MS
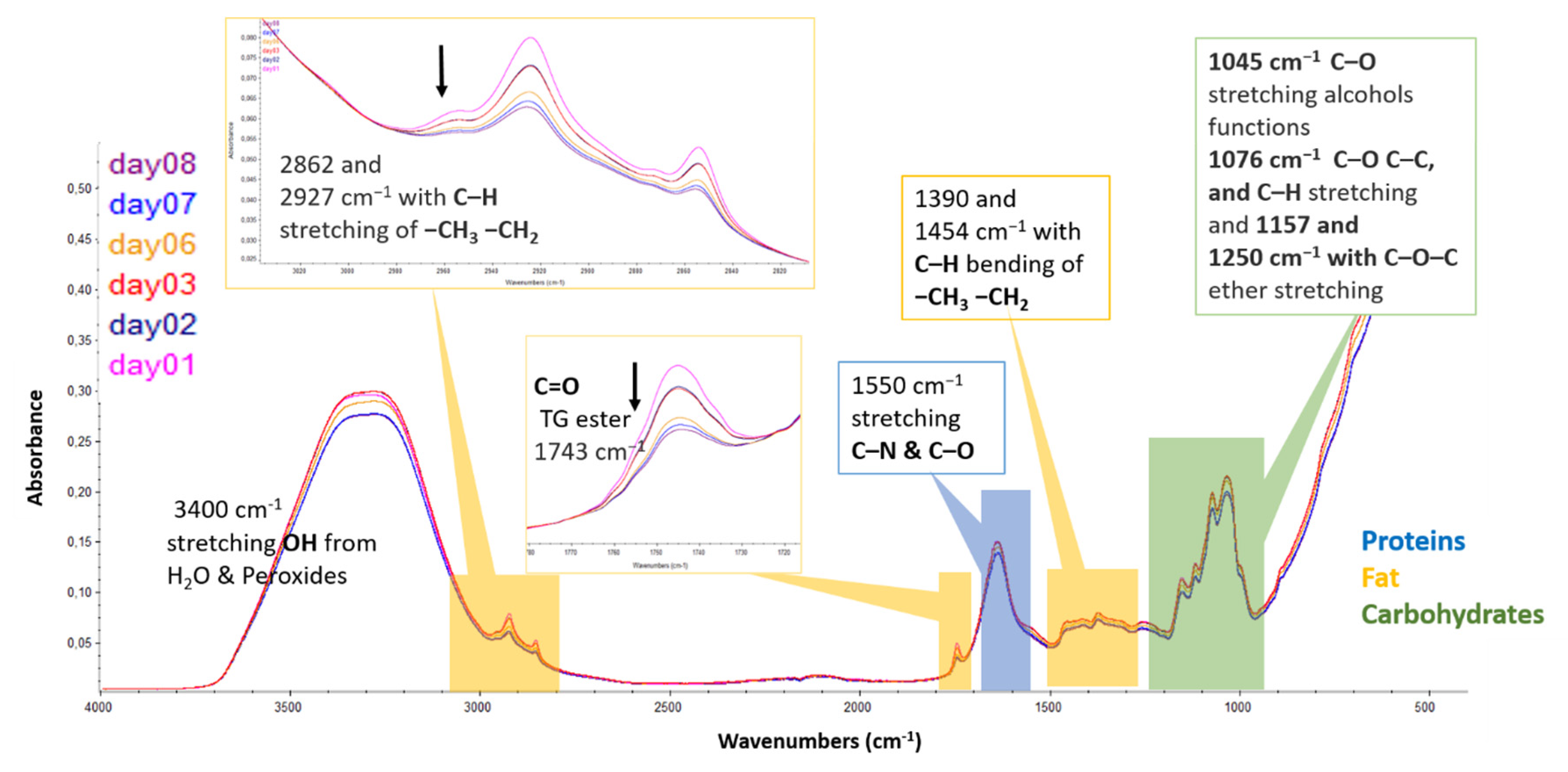
3.2. Infrared Spectroscopy in Mid-Region ATR-FTIR Applied to Infant Milk Formulas
3.2.1. Spectral Changes in Infant Milk Formulas
3.2.2. Prediction of the Oxidation Levels of Infant Milk Formula (IMF) using ATR-FTIR
3.3. Infrared Spectroscopy in the Near-Region (NIRS) Applied on Infant Milk Formulas
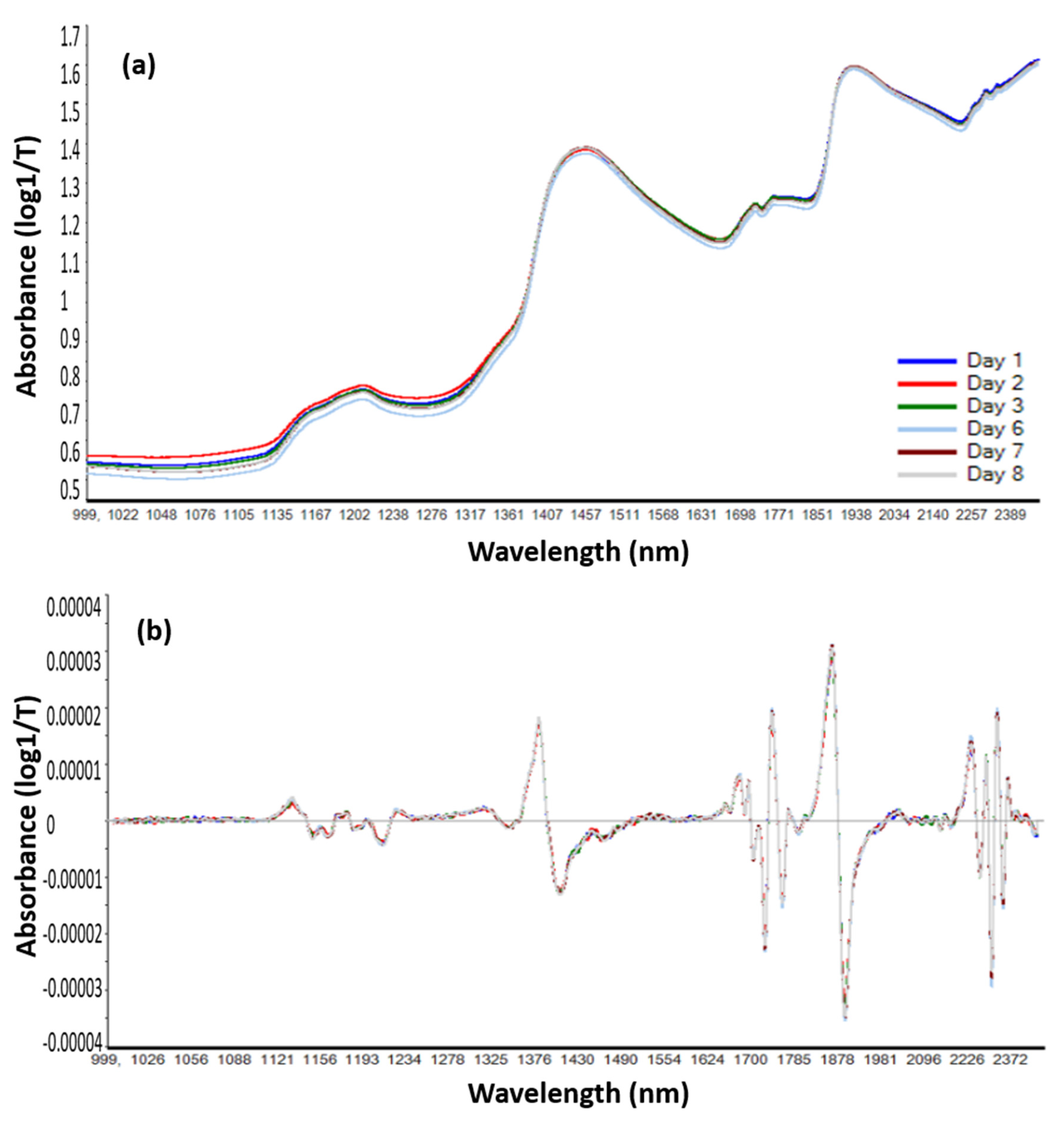
3.3.1. Spectral Changes of Infant Milk Formulas
3.3.2. Prediction of the Oxidation Level of IMF using NIRS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kerdiles, O.; Layé, S.; Calon, F. Omega-3 polyunsaturated fatty acids and brain health: Preclinical evidence for the prevention of neurodegenerative diseases. Trends Food Sci. Technol. 2017, 69, 203–213. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the substantiation of a health claim related to DHA and contribution to normal brain development pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3840. [CrossRef]
- Taneja, A.; Singh, H. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annu. Rev. Food Sci. Technol. 2012, 3, 105–123. [Google Scholar] [CrossRef]
- European Union. Public Office of the European Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding (Text with EEA relevance). Off. J. Eur. Union 2015. [Google Scholar]
- Innis, S.M. Human milk: Maternal dietary lipids and infant development. Proc. Nutr. Soc. 2007, 66, 397–404. [Google Scholar] [CrossRef]
- Venkataraman, S.; Schafer, F.Q.; Buettner, G.R. Detection of lipid radicals using EPR. Antioxid. Redox Signal. 2004, 6, 631–638. [Google Scholar] [CrossRef]
- Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Correlating volatile lipid oxidation compounds with consumer sensory data in dairy based powders during storage. Antioxidants 2020, 9, 338. [Google Scholar] [CrossRef]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef]
- Siefarth, C.; Serfert, Y.; Drusch, S.; Buettner, A. Comparative evaluation of diagnostic tools for oxidative deterioration of polyunsaturated fatty acid-enriched infant formulas during storage. Foods 2014, 3, 30–65. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation, 2nd ed.; Woodhead Publishing: Oakland, CA, USA, 2015; ISBN 978-0-85709-792-7. [Google Scholar]
- Liao, H.; Zhu, M.; Chen, Y. 4-Hydroxy-2-nonenal in food products: A review of the toxicity, occurrence, mitigation strategies and analysis methods. Trends Food Sci. Technol. 2020, 96, 188–198. [Google Scholar] [CrossRef]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1700047. [Google Scholar] [CrossRef]
- Let, M.B.; Jacobsen, C.; Frankel, E.N.; Meyer, A.S. Oxidative flavour deterioration of fish oil enriched milk. Eur. J. Lipid Sci. Technol. 2003, 105, 518–528. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat Rodas, S.; Holgado, F.; Márquez-Ruiz, G.; Dobarganes, M. Influence of two lipid extraction procedures on the peroxide value in powdered infant formulas. Eur. Food Res. Technol. 2008, 226, 1159–1166. [Google Scholar] [CrossRef]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Influence of fat extraction methods on the peroxide value in infant formulas. Food Res. Int. 2012, 48, 584–591. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Biasioli, F.; Cosio, M.S.; Schampicchio, M. Hexanal as biomarker for milk oxidative stress induced by copper ions. J. Dairy Sci. 2017, 100, 1650–1656. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, R.-G.; Erichsen, H.; Soerensen, J.; Petersen, M.A.; Skibsted, L.H. High temperature storage of infant formula milk powder for prediction of storage stability at ambient conditions. Int. Dairy J. 2017, 73, 166–174. [Google Scholar] [CrossRef]
- Cen, H.; He, Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007, 18, 72–83. [Google Scholar] [CrossRef]
- Xu, J.-L.; Riccioli, C.; Sun, D.-W. An overview on nondestructive spectroscopic techniques for lipid and lipid oxidation analysis in fish and fish products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 466–477. [Google Scholar] [CrossRef]
- Christy, A.A.; Kasemsumran, S.; Du, Y.; Ozaki, Y. The detection and quantification of adulteration in olive oil by near-infrared spectroscopy and chemometrics. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2004, 20, 935–940. [Google Scholar] [CrossRef]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Kays, S.E.; Barton, F.E.; Windham, W.R. Predicting Protein Content by near infrared reflectance spectroscopy in diverse cereal food products. J. Infrared Spectrosc. 2000, 8, 35–43. [Google Scholar] [CrossRef]
- Moh, M.H.; Che Man, Y.B.; van de Voort, F.R.; Abdullah, W.J.W. Determination of peroxide value in thermally oxidized crude palm oil by near infrared spectroscopy. J. Am. Oil Chem. Soc. 1999, 76, 19–23. [Google Scholar] [CrossRef]
- Wójcicki, K.; Khmelinskii, I.; Sikorski, M.; Sikorska, E. Near and mid infrared spectroscopy and multivariate data analysis in studies of oxidation of edible oils. Food Chem. 2015, 187, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Wehling, R.L.; Cuppett, S.L. Method for determining oxidation of vegetable oils by near-infrared spectroscopy. J. Am. Oil Chem. Soc. 2001, 78, 495–502. [Google Scholar] [CrossRef]
- Borchman, D.; Sinha, S. Determination of products of lipid oxidation by infrared spectroscopy. In Oxidative Stress Biomarkers and Antioxidant Protocols; Armstrong, D., Ed.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2002; pp. 21–28. ISBN 978-1-59259-173-2. [Google Scholar]
- Klaypradit, W.; Kerdpiboon, S.; Singh, R. Application of artificial neural networks to predict the oxidation of menhaden fish oil obtained from fourier transform infrared spectroscopy method. Food Bioprocess. Technol. 2011, 4, 475–480. [Google Scholar] [CrossRef]
- Lerma-García, M.J.; Simó-Alfonso, E.F.; Bendini, A.; Cerretani, L. Rapid evaluation of oxidised fatty acid concentration in virgin olive oil using Fourier-transform infrared spectroscopy and multiple linear regression. Food Chem. 2011, 124, 679–684. [Google Scholar] [CrossRef]
- Setiowaty, G.; Che Man, Y.B. A rapid Fourier transform infrared spectroscopic method for the determination of 2-TBARS in palm olein. Food Chem. 2003, 81, 147–154. [Google Scholar] [CrossRef]
- Daoud, S.; Bou-maroun, E.; Dujourdy, L.; Waschatko, G.; Billecke, N.; Cayot, P. Fast and direct analysis of oxidation levels of oil-in-water emulsions using ATR-FTIR. Food Chem. 2019, 293, 307–314. [Google Scholar] [CrossRef]
- Daoud, S.; Waschatko, G.; Bou-Maroun, E.; Cayot, P. Fast, direct and in situ monitoring of lipid oxidation in an oil-in-water emulsion by near infrared spectroscopy. Anal. Methods 2020, 12, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- BELHAJ, N.; Arab-Tehrany, E.; Linder, M. Oxidative kinetics of salmon oil in bulk and in nanoemulsion stabilized by marine lecithin. Process. Biochem. 2010, 45, 187–195. [Google Scholar] [CrossRef]
- Guzun-Cojocaru, T.; Koev, C.; Yordanov, M.; Karbowiak, T.; Cases, E.; Cayot, P. Oxidative stability of oil-in-water emulsions containing iron chelates: Transfer of iron from chelates to milk proteins at interface. Food Chem. 2011, 125, 326–333. [Google Scholar] [CrossRef]
- Hayati, I.N.; Man, Y.B.C.; Tan, C.P.; Aini, I.N. Monitoring peroxide value in oxidized emulsions by Fourier transform infrared spectroscopy. Eur. J. Lipid Sci. Technol. 2005, 107, 886–895. [Google Scholar] [CrossRef]
- Nalur, S.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar]
- Jensen, P.N.; Sørensen, G.; Engelsen, S.B.; Bertelsen, G. Evaluation of quality changes in walnut kernels (Juglans regia L.) by Vis/NIR spectroscopy. J. Agric. Food Chem. 2001, 49, 5790–5796. [Google Scholar] [CrossRef]
- Kaddour, A.A.; Grand, E.; Barouh, N.; Baréa, B.; Villeneuve, P.; Cuq, B. Near-infrared spectroscopy for the determination of lipid oxidation in cereal food products. Eur. J. Lipid Sci. Technol. 2006, 108, 1037–1046. [Google Scholar] [CrossRef]
- Ni, Y.; Mei, M.; Kokot, S. Analysis of complex, processed substances with the use of NIR spectroscopy and chemometrics: Classification and prediction of properties—The potato crisps example. Chemom. Intell. Lab. Syst. 2011, 105, 147–156. [Google Scholar] [CrossRef]
- Wang, X.; Esquerre, C.; Downey, G.; Henihan, L.; O’Callaghan, D.; O’Donnell, C. Assessment of infant formula quality and composition using Vis-NIR, MIR and Raman process analytical technologies. Talanta 2018, 183, 320–328. [Google Scholar] [CrossRef]
- Gredilla, A.; Fdez-Ortiz de Vallejuelo, S.; Elejoste, N.; de Diego, A.; Madariaga, J.M. Non-destructive Spectroscopy combined with chemometrics as a tool for Green Chemical Analysis of environmental samples: A review. TrAC Trends Anal. Chem. 2016, 76, 30–39. [Google Scholar] [CrossRef]
- He, Y.; Tang, L.; Wu, X.; Hou, X.; Lee, Y.-I. Spectroscopy: The best way toward green analytical chemistry? Appl. Spectrosc. Rev. 2007, 42, 119–138. [Google Scholar] [CrossRef]
- AOCS Spectrophotometric Determination of Conjugated Dienoic Acid- Ti 1a-64. Off. Methods Recomm. Pr. Am. Oil Chem. Soc. 2009. Available online: https://www.aocs.org/attain-lab-services/methods/methods/search-results?method=111546 (accessed on 1 October 2020).
- Tsenkova, R.; Atanassova, S.; Itoh, K.; Ozaki, Y.; Toyoda, K. Near infrared spectroscopy for biomonitoring: Cow milk composition measurement in a spectral region from 1100 to 2400 nanometers. J. Anim. Sci. 2000, 78, 515. [Google Scholar] [CrossRef]
- Esbensen, K.H. Multivariate Data Analysis—In Practice: An Introduction to Multivariate Data Analysis and Experimental Design, 5th ed.; CAMO: Oslo, Norway, 2012; ISBN 978-82-993330-3-0. [Google Scholar]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; Wiley: Chichester, UK, 2006; ISBN 978-0-471-48977-1. [Google Scholar]
- Cebi, N.; Yilmaz, M.T.; Sagdic, O.; Yuce, H.; Yelboga, E. Prediction of peroxide value in omega-3 rich microalgae oil by ATR-FTIR spectroscopy combined with chemometrics. Food Chem. 2017, 225, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Westad, F.; Marini, F. Validation of chemometric models—A tutorial. Anal. Chim. Acta 2015, 893, 14–24. [Google Scholar] [CrossRef] [PubMed]
- AACC Near-Infrared Methods—Guidelines for Model Development and Maintenance. AACC Int. Approv. Methods 2009. Available online: http://methods.aaccnet.org/summaries/39-00-01.aspx (accessed on 1 October 2020).
- Mendonça, M.A.; Araújo, W.M.C.; Borgo, L.A.; De Rodrigues Alencar, E. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Volatile lipid oxidation products. Prog. Lipid Res. 1983, 22, 1–33. [Google Scholar] [CrossRef]
- Shi, H.; Ho, C.-T. The flavour of poultry meat. In Flavor of Meat and Meat Products; Shahidi, F., Ed.; Springer US: Boston, MA, USA, 1994; pp. 52–70. ISBN 978-1-4613-5911-1. [Google Scholar]
- Choe, J.; Oh, B.; Choe, E. Effect of soybean lecithin on iron-catalyzed or chlorophyll-photosensitized oxidation of canola oil emulsion. J. Food Sci. 2014, 79, C2203–C2208. [Google Scholar] [CrossRef]
- Doert, M.; Jaworska, K.; Moersel, J.-T.; Kroh, L.W. Synergistic effect of lecithins for tocopherols: Lecithin-based regeneration of α-tocopherol. Eur. Food Res. Technol. 2012, 235, 915–928. [Google Scholar] [CrossRef]
- Samdani, G.K.; McClements, D.J.; Decker, E.A. Impact of phospholipids and tocopherols on the oxidative stability of soybean oil-in-water emulsions. J. Agric. Food Chem. 2018, 66, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Alamed, J.; McClements, D.J.; Decker, E.A. Ability of chelators to alter the physical location and prooxidant activity of iron in oil-in-water emulsions. J. Food Sci. 2003, 68, 1952–1957. [Google Scholar] [CrossRef]
- Kristinova, V.; Aaneby, J.; Mozuraityte, R.; Storrø, I.; Rustad, T. The effect of dietary antioxidants on iron-mediated lipid peroxidation in marine emulsions studied by measurement of dissolved oxygen consumption. Eur. J. Lipid Sci. Technol. 2014, 116, 857–871. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, Q.; Zhang, Y.; Chen, J.; Sun, S.; Noda, I. Analysis of crystallized lactose in milk powder by Fourier-transform infrared spectroscopy combined with two-dimensional correlation infrared spectroscopy. J. Mol. Struct. 2010, 974, 88–93. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J. Rapid determination of tetracycline in milk by FT-MIR and FT-NIR spectroscopy. J. Dairy Sci. 2002, 85, 487–493. [Google Scholar] [CrossRef]
- Guillen, M.; Cabo, N. Some of the most significant changes in the Fourier transform infrared spectra of edible oils under oxidative conditions. J. Sci. Food Agric. 2000, 80, 2028–2036. [Google Scholar] [CrossRef]
- Kim, Y.; Himmelsbach, D.S.; Kays, S.E. ATR-Fourier transform mid-infrared spectroscopy for determination of trans fatty acids in ground cereal products without oil extraction. J. Agric. Food Chem. 2007, 55, 4327–4333. [Google Scholar] [CrossRef]
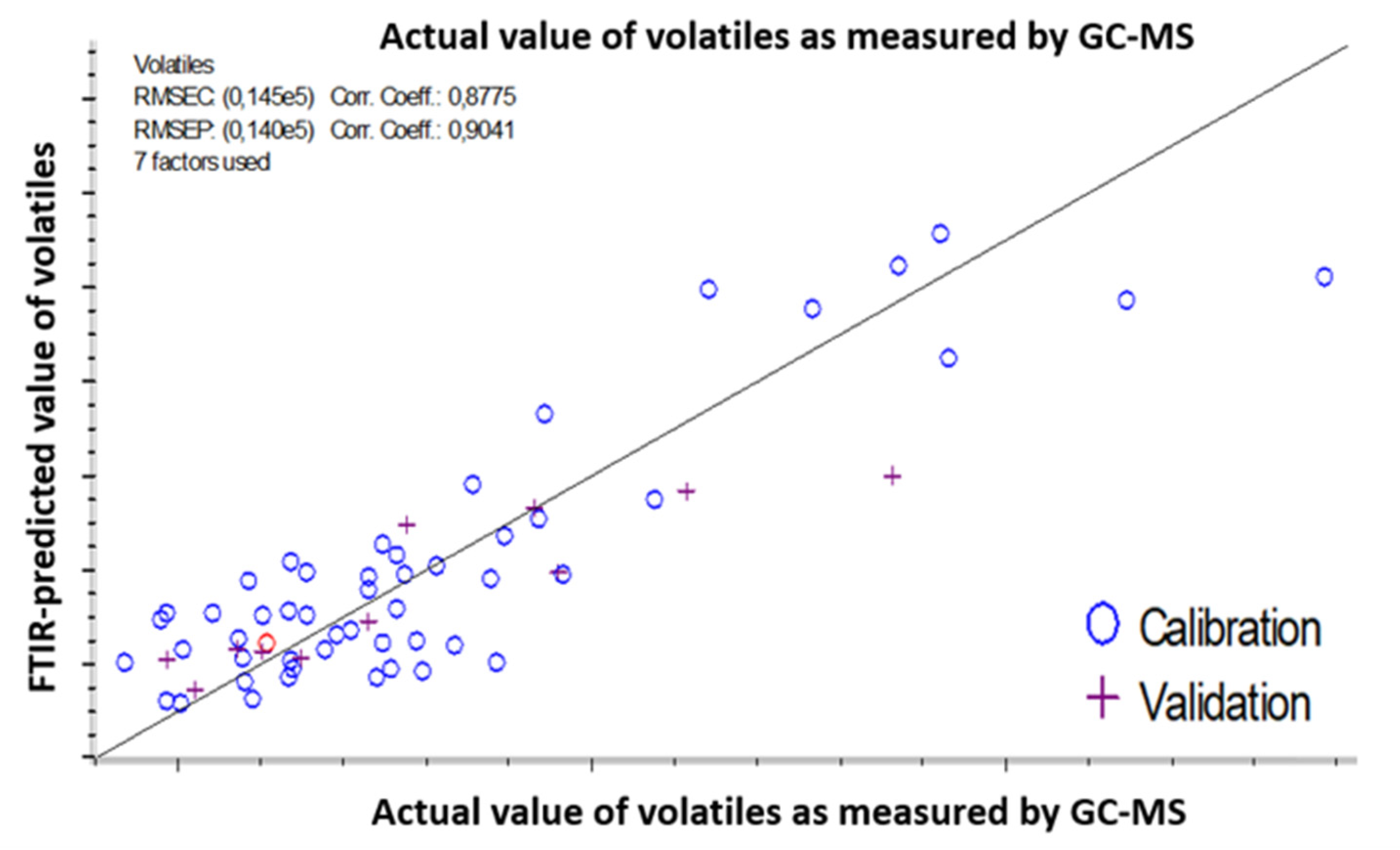

| Prediction Model | ATR-FTIR | NIRS |
|---|---|---|
| Spectral range | 4000–600 cm−1 | 800–2400 nm |
| Pretreatment | Standard normal variate (SNV)–baseline correction | Second derivative (gap 23 points, segment 19 points) |
| Calibration set (samples) | 52 | 50 |
| Validation set (samples) | 11 | 10 |
| Volatiles content range (counts) | 43,634–188,407 | 43,634–142,015 |
| Number of latent variables | 7 | 3 |
| Coefficient of correlation for calibration (R2) | 0.877 | 0.921 |
| Coefficient of correlation for prediction (R2) | 0.904 | 0.919 |
| Error of calibration (RMSEC) | 14,500 counts (18%) | 8180 counts (11.4%) |
| Error of prediction (RMSEP) | 14,000 counts (17.5%) | 6400 counts (9%) |
| RMSEC/RMSEP (<1.2) | 0.96 | 0.78 |
| Volatiles content/RMSEP | 10.34 | 15.37 |
| Range | Wavelength (nm) | Functional Group | Attribution |
|---|---|---|---|
| 1 | 1120–1280 | 1160/1210 sStretching C–H second overtone of CH3 and CH2 | Fat |
| 2 | 1280–1600 | 1440 O–H | Water |
| 1483 and 1570 N–H first overtone | Protein | ||
| C–H first overtone | Fat | ||
| 3 | 1630–1820 | 1726 and 1765 C–H first overtone for CH2 CH | Fat |
| 4 | 1820–2180 | 1950–deformation and stretching of O–H | Water |
| 1992/2110 N–H stretching | Protein | ||
| 5 | 2180–2500 | 2280 N–H/2308 C–H | Protein |
| 2310/2354 C–H | Fat | ||
| 2347 stretching CH2 and deformation of =CH2 2264 and 2494 | Carbohydrate |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daoud, S.; Bou-Maroun, E.; Waschatko, G.; Horemans, B.; Mestdagh, R.; Billecke, N.; Cayot, P. Detection of Lipid Oxidation in Infant Formulas: Application of Infrared Spectroscopy to Complex Food Systems. Foods 2020, 9, 1432. https://doi.org/10.3390/foods9101432
Daoud S, Bou-Maroun E, Waschatko G, Horemans B, Mestdagh R, Billecke N, Cayot P. Detection of Lipid Oxidation in Infant Formulas: Application of Infrared Spectroscopy to Complex Food Systems. Foods. 2020; 9(10):1432. https://doi.org/10.3390/foods9101432
Chicago/Turabian StyleDaoud, Samar, Elias Bou-Maroun, Gustav Waschatko, Benjamin Horemans, Renaud Mestdagh, Nils Billecke, and Philippe Cayot. 2020. "Detection of Lipid Oxidation in Infant Formulas: Application of Infrared Spectroscopy to Complex Food Systems" Foods 9, no. 10: 1432. https://doi.org/10.3390/foods9101432
APA StyleDaoud, S., Bou-Maroun, E., Waschatko, G., Horemans, B., Mestdagh, R., Billecke, N., & Cayot, P. (2020). Detection of Lipid Oxidation in Infant Formulas: Application of Infrared Spectroscopy to Complex Food Systems. Foods, 9(10), 1432. https://doi.org/10.3390/foods9101432






