Procedures for DNA Extraction from Opium Poppy (Papaver somniferum L.) and Poppy Seed-Containing Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Food Material
2.2. DNA Extraction from Seeds
2.3. DNA Extraction from Ground Seeds
2.4. DNA Extraction from Pollen Grains
2.5. DNA Extraction from Poppy Seed Filling
2.6. DNA Extraction from Poppy Oil
2.7. Qualitative and Quantitative Analysis of Extracted DNA
2.8. PCR Amplification
3. Results and Discussion
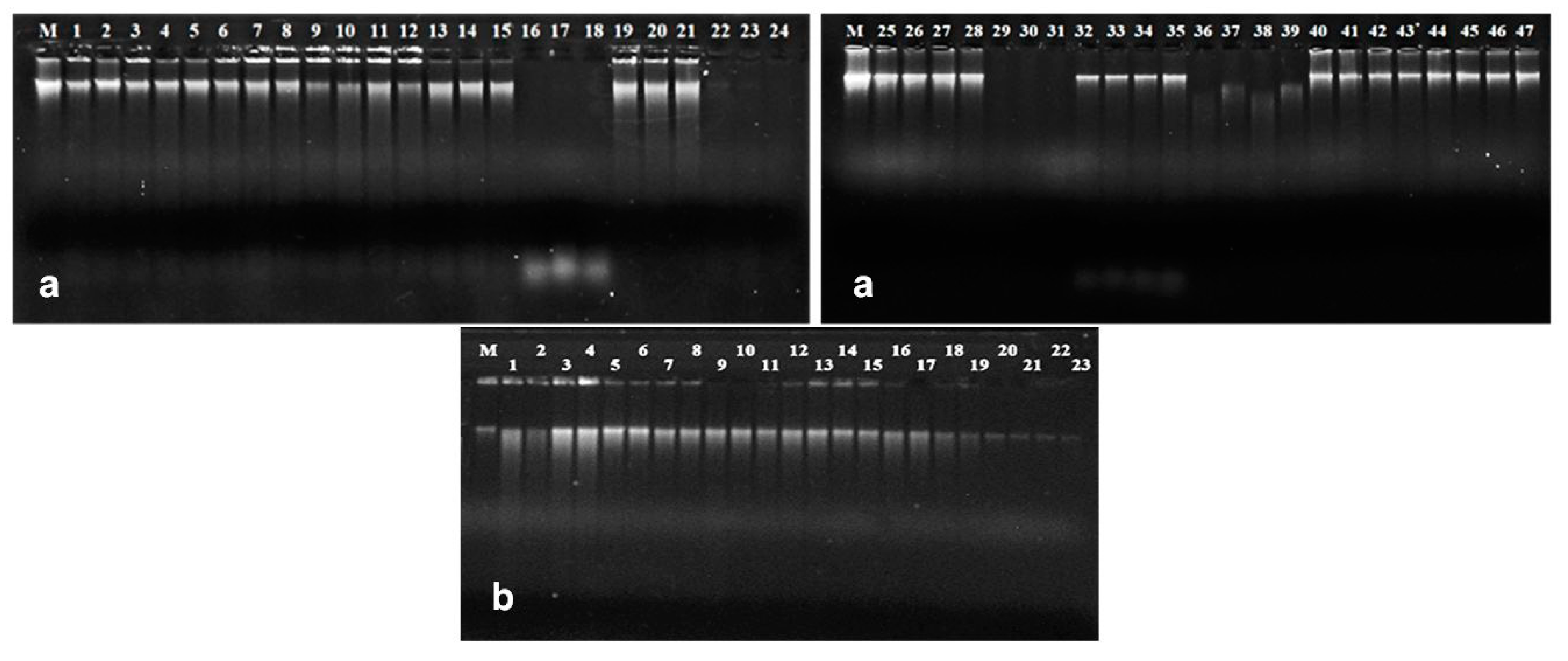
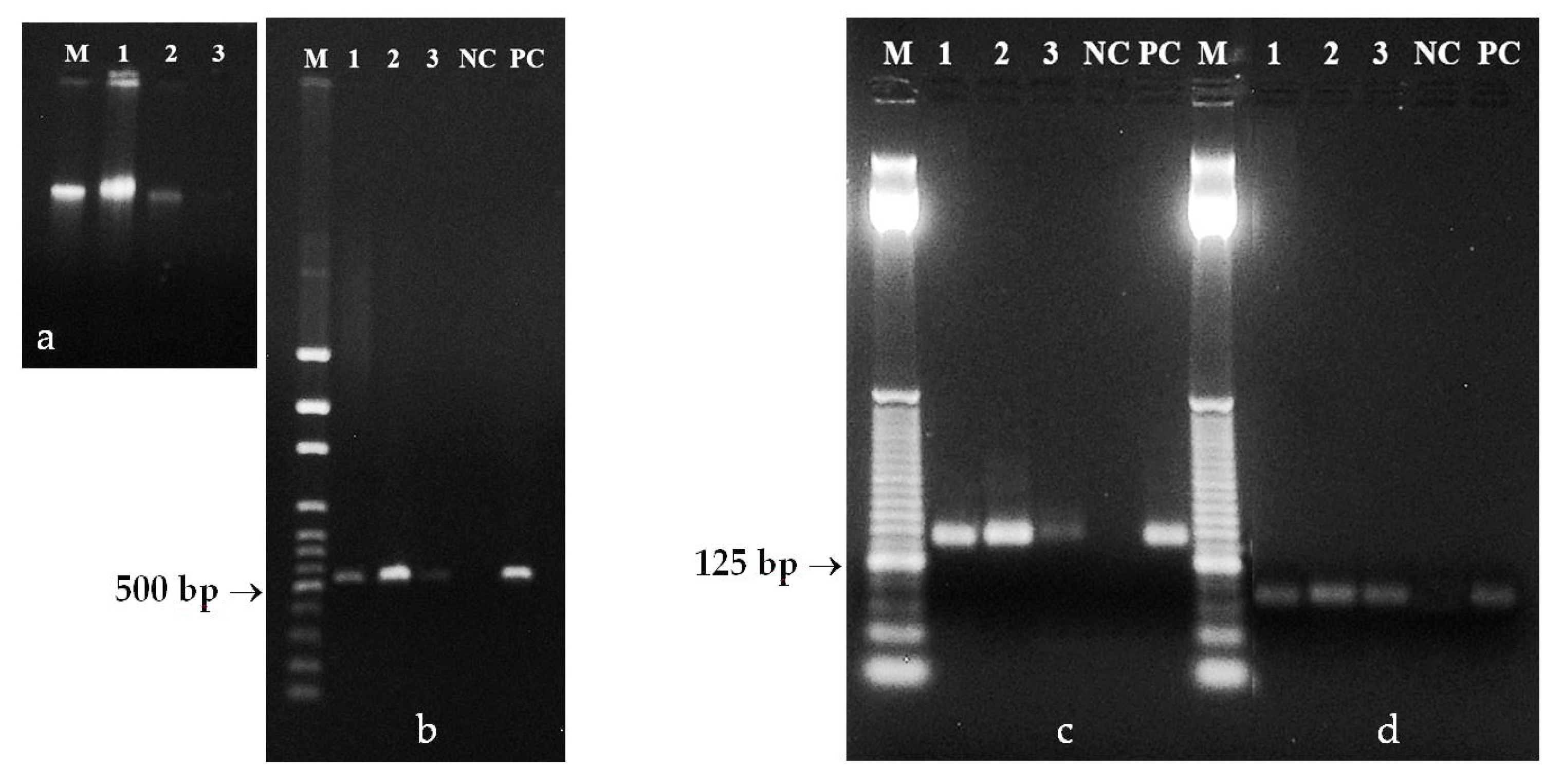
3.1. DNA from Mature Seeds
3.2. DNA from Ground Seeds
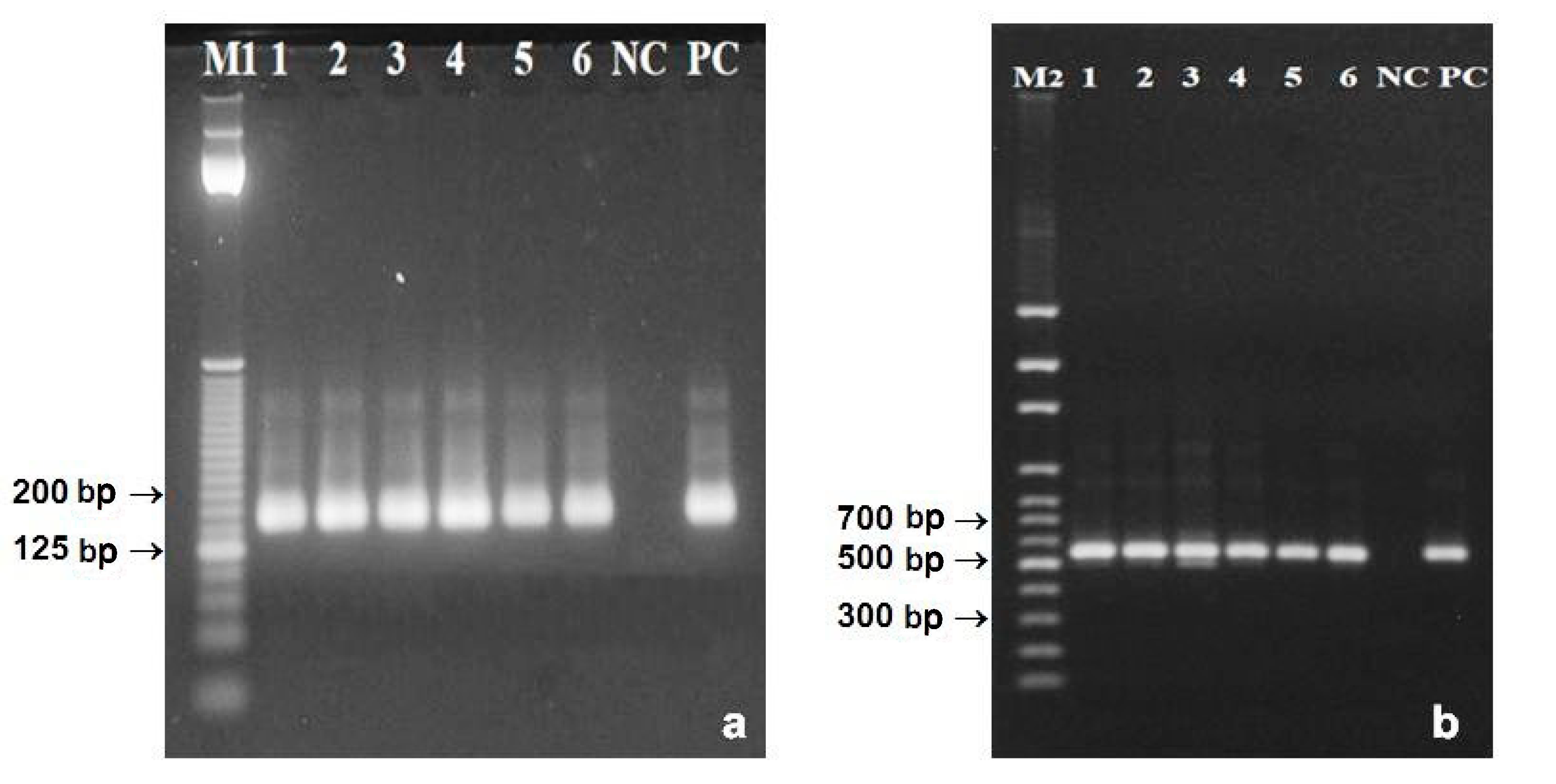
3.3. DNA from Poppy Pollen Grains
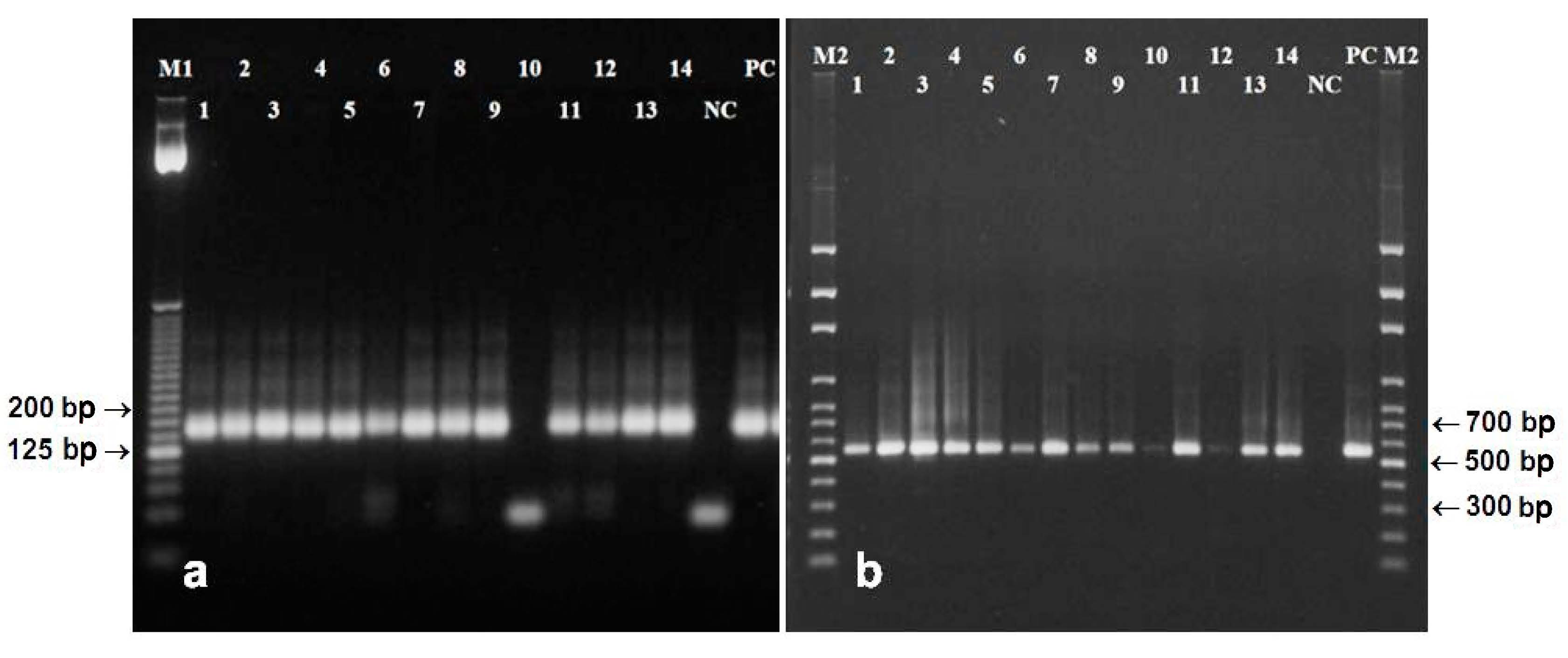
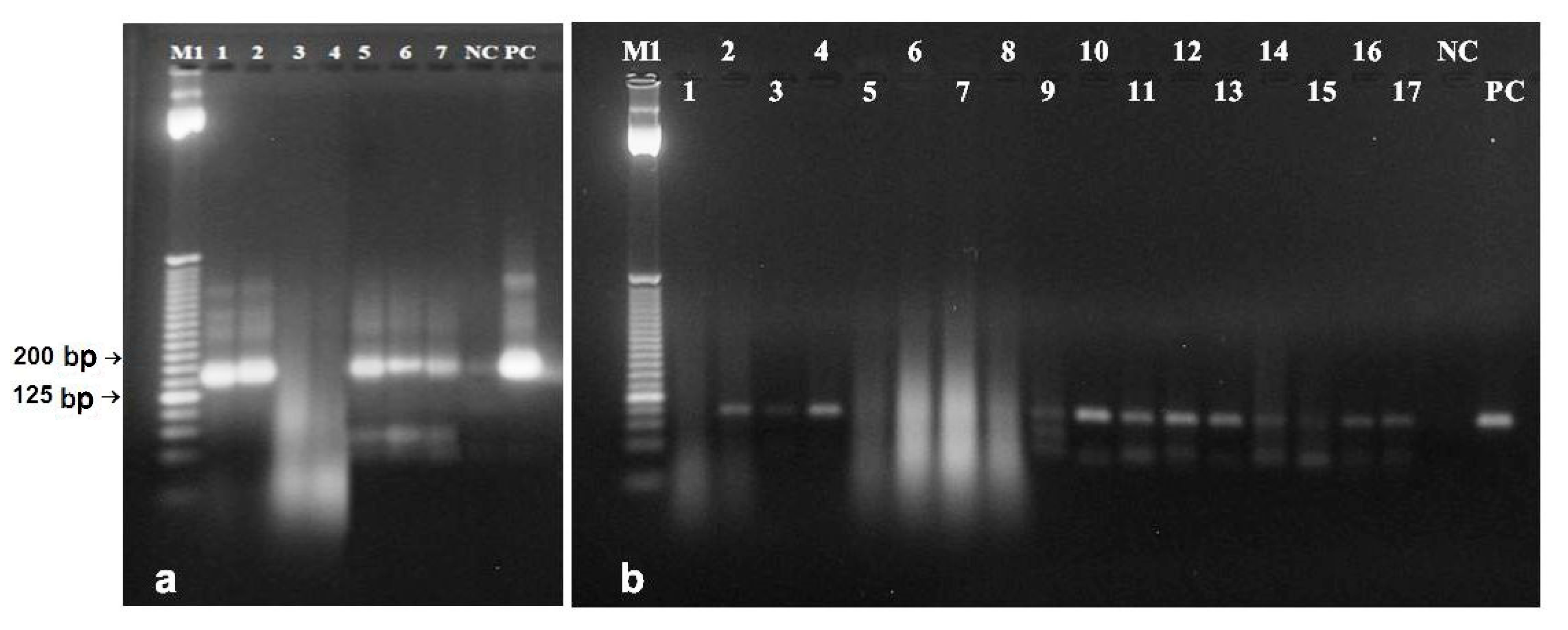
3.4. DNA from Poppy Seed Filling
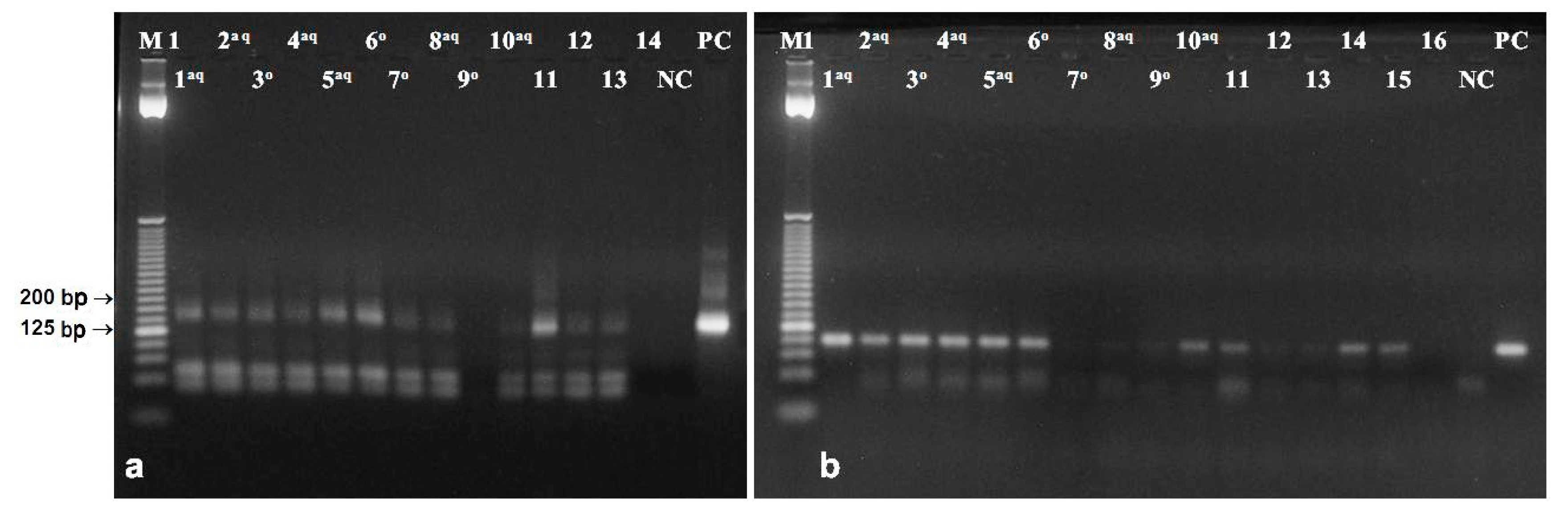
3.5. DNA from Poppy Oil
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ali, N.; de Cássia Pontello Rampazzo, R.; Dias Tavares Costa, A.; Krieger, M.A. Current nucleic acid extraction methods and their implications to point-of-care diagnostics. Biomed Res. Int. 2017, 2017, 9306564. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardo, G.; Del Gaudio, S.; Galderisi, U.; Cascino, A.; Cipollaro, M. Comparative evaluation of different DNA extraction procedures from food samples. Biotechnol. Prog. 2007, 23, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Jasbeer, K.; Son, R.; Mohamad Ghazali, F.; Cheah, Y. Real-time PCR evaluation of seven DNA extraction methods for the purpose of GMO analysis. Int. Food Res. J. 2009, 16, 329–341. [Google Scholar]
- Raieta, K.; Muccillo, L.; Colantuoni, V. A novel reliable method of DNA extraction from olive oil suitable for molecular traceability. Food Chem. 2015, 172, 596–602. [Google Scholar] [CrossRef]
- Manning, L.; Soon, J.M. Developing systems to control food adulteration. Food Policy 2014, 49, 23–32. [Google Scholar] [CrossRef]
- Böhme, K.; Calo-Mata, P.; Barros-Velazquez, J.; Ortea, I. Review of recent DNA-based methods for main food authentication topics. J. Agric. Food Chem. 2019, 67, 3854–3864. [Google Scholar] [CrossRef] [PubMed]
- Peano, C.; Samson, M.C.; Palmieri, L.; Gulli, M.; Marmiroli, N. Qualitative and quantitative evaluation of the genomic DNA extracted from GMO and non-GMO foodstuffs with four different extraction methods. J. Agric. Food Chem. 2004, 52, 6962–6968. [Google Scholar] [CrossRef]
- Smith, D.S.; Maxwell, P.W.; De Boer, S.H. Comparison of several methods for the extraction of DNA from potatoes and potato-derived products. J. Agric. Food Chem. 2005, 53, 9848–9859. [Google Scholar] [CrossRef]
- Gryson, N. Effect of food processing on plant DNA degradation and PCR-based GMO analysis: A review. Anal. Bioanal. Chem. 2010, 396, 2003–2022. [Google Scholar] [CrossRef]
- Weighardt, F. GMO quantification in processed food and feed. Nat. Biotechnol. 2007, 25, 1213–1214. [Google Scholar] [CrossRef]
- Demeke, T.; Jenkins, G.R. Influence of DNA extraction methods, PCR inhibitors and quantification methods on real-time PCR assay of biotechnology-derived traits. Anal. Bioanal. Chem. 2010, 396, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Y.; Ge, Y.; Wang, J.; Xu, B.; Huang, W.; Yuan, F. Detection of olive oil using the Evagreen real-time PCR method. Eur. Food. Res. Technol. 2008, 227, 1117–1124. [Google Scholar] [CrossRef]
- Ramos-Gómez, S.; Busto, M.D.; Perez-Mateos, M.; Ortega, N. Development of a method to recovery and amplification DNA by real-time PCR from commercial vegetable oils. Food Chem. 2014, 158, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Piarulli, L.; Savoia, M.; Taranto, F.; D’Agostino, N.; Sardaro, R.; D’Agostino, N.; Gadaleta, S.; Fucilli, V.; De Giovanni, C.; Montemurro, C.; et al. A Robust DNA Isolation Protocol from Filtered Commercial Olive Oil for PCR-Based Fingerprinting. Foods 2019, 8, 462. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Arslan, N. Opium poppy (Papaver somniferum). In Medicinal and Aromatic Plants of the Middle-East. Medicinal and Aromatic Plants of the World; Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 305–332. [Google Scholar]
- Bail, S.; Majchrzak, D.; Krist, S.; Elmadfa, I.; Buchbauer, G. Sensory evaluation of poppy oil blended with sunflower seed oil. Nahrung 2008, 32, 8–15. [Google Scholar]
- Krist, S.; Stuebiger, G.; Bail, S.; Unterweger, H. Detection of adulteration of poppy seed oil with sunflower oil based on volatiles and triacylglycerol composition. J. Agric. Food Chem. 2006, 84, 6385–6389. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Gultekin, V.; Allmer, J.; Doganlar, S.; Frary, A. Development of genomic simple sequence repeat markers in opium poppy by next-generation sequencing. Mol. Breed. 2014, 34, 323–334. [Google Scholar] [CrossRef]
- Mičianová, V.; Ondreičková, K.; Muchová, D.; Klčová, L.; Hudcovicová, M.; Havrlentová, M.; Mihálik, D.; Kraic, J. Forensic application of EST-derived STR markers in opium poppy. Biologia 2017, 7, 587–594. [Google Scholar] [CrossRef]
- Vašek, J.; Čílová, D.; Melounová, M.; Svoboda, P.; Vejl, P.; Štikarová, R.; Vostrý, L.; Kuchtová, P.; Ovesná, J. New EST-SSR markers for individual genotyping of opium poppy cultivars (Papaver somniferum L.). Plants 2020, 9, 10. [Google Scholar] [CrossRef]
- Sangwan, R.S.; Yadav, U.S.; Sangwan, N.S. Isolation of genomic DNA from defatted oil seed residue of opium poppy (Papaver somniferum). Plant. Mol. Biol. Rep. 2000, 18, 265–270. [Google Scholar] [CrossRef]
- Marciano, M.A.; Panicker, S.X.; Liddil, G.D.; Lindgren, D.; Sweder, K.S. Development of a method to extract opium poppy (Papaver somniferum L.) DNA from heroin. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Roman, M.G.; LaRue, B.; Gangitano, D.; Houston, R. Evaluation of 19 short tandem repeat markers for individualization of Papaver somniferum. Sci. Justice 2020, 60, 253–262. [Google Scholar] [CrossRef]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA mini preparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Community Reference Laboratory for GM Food and Feed. Seeds Sampling and DNA Extraction of Oilseed Rape. Report on the Validation of an Oilseed Rape DNA Extraction Method from Seeds: Bayer BioScience N.V. Available online: http://gmo-crl.jrc.ec.europa.eu/summaries/T45_DNAExtr_sampl_correctedversion1.pdf (accessed on 24 July 2020).
- Community Reference Laboratory for GM Food and Feed. Seeds Sampling and DNA Extraction of Oilseed Rape. Report on the Validation of an Oilseed Rape DNA Extraction Method from Seeds: Monsanto Company. Available online: http://gmo-crl.jrc.ec.europa.eu/summaries/CRL-VL-26-04-XP-Corrected-version-1.pdf (accessed on 24 July 2020).
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Borodina, T.A.; Lehrach, H.; Soldatov, A.V. DNA purification on homemade silica spin-columns. Anal Biochem. 2003, 321, 135–137. [Google Scholar] [CrossRef]
- Doveri, S.; O’Sullivan, D.M.; Lee, D. Non-concordance between genetic profiles of olive oil and fruit: A cautionary note to use of DNA markers for provenance testing. J. Agric. Food Chem. 2006, 54, 9221–9226. [Google Scholar] [CrossRef]
- Consolandi, C.; Palmieri, L.; Severgnini, M.; Maestri, E.; Marmiroli, N.; Agrimonti, C.; Baldoni, L.; Donini, P.; De Bellis, G.; Castiglioni, B. A procedure for olive oil traceability and authenticity: DNA extraction, multiplex PCR and LDR-universal array analysis. Eur. Food Res. Technol. 2008, 277, 1429–1438. [Google Scholar] [CrossRef]
- Giménez, M.J.; Pistón, F.; Martín, A.; Atienza, S.G. Application of real-time PCR on the development of molecular markers and to evaluate critical aspects for olive oil authentication. Food Chem. 2010, 118, 482–487. [Google Scholar] [CrossRef]
- Şelale, H.; Çelik, I.; Gültekin, V.; Allmer, J.; Doganlar, S.; Frary, A. Development of EST-SSR markers for diversity and breeding studies in opium poppy. Plant Breed. 2013, 132, 344–351. [Google Scholar] [CrossRef]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef]
- Sharma, A.D.; Gill, P.K.; Singh, P. DNA isolation from dry and fresh samples of polysaccharide-rich plants. Plant Mol. Biol. Rep. 2002, 20, 415a–415f. [Google Scholar] [CrossRef]
- Ferdous, J.; Hanafi, M.M.; Rafii, M.Y.; Muhammad, K. A quick DNA extraction protocol: Without liquid nitrogen in ambient temperature. Afr. J. Biotechnol. 2012, 11, 6956–6964. [Google Scholar]
- Abdel-Latif, A.; Osman, G. Comparison of three genomic DNA extraction methods to obtain high DNA quality from maize. Plants Methods 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Olden, M.J.; Blasic, J.R.; Bussjaeger, L.; Kao, C.; Shokere, L.A.; Kendall, D.C.; Freese, L.; Jenkins, G.R. Evaluation of Extraction Methodologies for Corn Kernel (Zea mays) DNA for Detection of Trace Amounts of Biotechnology-Derived DNA. J. Agric. Food. Chem. 2003, 51, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Cankar, K.; Štebih, D.; Dreo, T.; Žel, J.; Gruden, K. Critical points of DNA quantification by real-time PCR —Effects of DNA extraction method and sample matrix on quantification of genetically modified organisms. BMC Biotechnol. 2006, 6, 37. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, F.; Du, Y.; Liu, C.; Bu, G.; Xin, Y.; Liu, B. A modified SDS-based DNA extraction method from raw soybean. Biosci. Rep. 2019, 39, BSR20182271. [Google Scholar] [CrossRef] [PubMed]
- Mafra, I.; Silva, S.A.; Moreira, E.J.M.O.; da Silva, C.S.F.; Beatriz, M.; Oliveira, P.P. Comparative study of DNA extraction methods for soybean derived food products. Food Control. 2008, 19, 1183–1190. [Google Scholar] [CrossRef]
- Chitti, J.A.; Allen, R.S.; First, A.J.; Larkin, P.J. Genetic transformation in commercial Tasmanian cultivars of opium poppy, Papaver somniferum, and movement of transgenic pollen in the field. Funct. Plant. Biol. 2003, 30, 1045–1058. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Liu, M.; Huang, W.; Wang, Y.; Wu, Y. A multiplex real/time PCR method applied to detect eight pollen species in food for the prevention of allergens. Eur. Food Res. Technol. 2019, 245, 2195–2207. [Google Scholar] [CrossRef]
- Bell, K.L.; Burgess, K.S.; Okamoto, K.C.; Aranda, R.; Brosi, B.J. Review and future prospects for DNA barcoding methods in forensic palynology. Forensic Sci. Int. Genet. 2016, 21, 110–116. [Google Scholar] [CrossRef]
- Nikolić, Z.; Petrović, G.; Panković, D.; Ignjatov, M.; Marinković, D.; Stojanović, M.; Đorđević, V. Threshold level and traceability of Roundup Ready® soybeans in tofu production. Food Technol. Biotechnol. 2017, 44, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors—Occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Lipp, M.; Shillito, R.; Giroux, R.; Spiegelhalter, F.; Charlton, S.; Pinero, D.; Song, P. Polymerase chain reaction technology as analytical tool in agricultural biotechnology. J. AOAC Int. 2005, 88, 136–155. [Google Scholar] [CrossRef] [PubMed]
- Muzzalupo, I.; Perri, E. Recovery and characterisation of DNA from virgin olive oil. Eur. Food Res. Technol. 2002, 214, 528–531. [Google Scholar] [CrossRef]
- Pafundo, S.; Busconi, M.; Agrimonti, C.; Fogher, C.; Marmiroli, N. Storage-time effects on olive oil DNA assessed by Amplified Fragments Length Polymorphisms. Food Chem. 2010, 123, 787–793. [Google Scholar] [CrossRef]
- Piskata, Z.; Servusova, E.; Babak, V.; Nesvadbova, B.; Borilova, G. The quality of DNA isolated from processed food and feed via different extraction procedures. Molecules 2019, 24, 1188. [Google Scholar] [CrossRef]
- Murray, S.R.; Butler, R.C.; Hardacre, A.K.; Timmerman-Vaughan, G.M. Use of quantitative real-time PCR to estimate maize endogenous DNA degradation after cooking and extrusion or in food products. J. Agric. Food Chem. 2007, 55, 2231–2239. [Google Scholar] [CrossRef]
- Yoshimura, T.; Kuribara, H.; Matsuoka, T.; Kodama, T.; Iida, M.; Watanabe, T.; Akiyama, H.; Maitani, T.; Furui, S.; Hino, A. Applicability of the quantification of genetically modified organisms to foods processed from maize and soy. J. Agric. Food Chem. 2005, 53, 2052–2059. [Google Scholar] [CrossRef]
- Varma, A.; Padh, H.; Shrivastava, N. Plant genomic DNA isolation: An art or a science. Biotechnol. J. 2007, 2, 386–392. [Google Scholar] [CrossRef]
| Primer Name | Sequences of Primers | Tm (°C) | PCR Product Size (bp) |
|---|---|---|---|
| psSSR69-F | 5′-ATAGATTTATTTTGGCCACCT-3′ | 54.6 | 156 |
| psSSR69-R | 5′-CACCTATTGATTGAGGATGAA-3 | 55.2 | |
| tubulin beta-7 chain I-F | 5′-CGTGGGTCACAGCAATACAG-3′ | 59.4 | 96 |
| tubulin beta-7 chain I-R | 5′-ATGCCTAGGATCAGCAGCAC-3′ | 59.4 | |
| tubulin beta-7 chain II-F | 5′-AATCGGTGCAAAGTTCTGG-3′ | 54.5 | 553 |
| tubulin beta-7 chain II-R | 5′-GTTCCCATCCCAGATCCTG-3′ | 58.8 |
| P. somniferum L. Matrix | DNA Extraction Method | DNA Yield (μg) | DNA Concentration (ng/μL) | A260/280 | A260/230 | Target Locus | |
|---|---|---|---|---|---|---|---|
| psSSR69 | Tubulin II | ||||||
| Native seeds | Dellaporta et al. [24] | 82.76 * | 413.78 * | 1.66 * | 0.87 * | + | + |
| 90.68 | 453.39 | 1.62 | 0.84 | + | + | ||
| Dellaporta et al. [24] with CTAB | 53.32 * | 266.62 * | 1.62 * | 0.83 * | + | + | |
| 73.19 | 365.94 | 1.55 | 0.81 | + | + | ||
| Bayer BioScience N.V. [25] | 115.13 | 575.63 | 1.81 | 1.84 | + | + | |
| Murray, Thompson [27] | 289.84 | 483.07 | 2.10 | 2.37 | + | + | |
| Monsanto Company [26] | 15.52 | 62.06 | 1.77 | 2.21 | + | + | |
| Sagwan et al. [21] | 108.21 | 1082.14 | 1.96 | 2.51 | + | + | |
| Newly developed protocol | 248.30 | 703.85 | 1.91 | 2.19 | + | + | |
| DNeasy® Plant Maxi Kit | 108.83 | 145.11 | 1.96 | 2.18 | + | + | |
| Plant DNAzol® Reagent | 137.40 | 114.50 | 1.54 | 0.28 | - | - | |
| QIAamp DNA Stool Mini Kit | 172.68 | 863.39 | 2.11 | 2.32 | + | + | |
| PowerSoil DNA Isolation Kit | 1.40 | 13.97 | 1.75 | 0.72 | + | - | |
| Ground seeds | Bayer BioScience N.V. [25] (1) | 18.43 | 92.17 | 1.79 | 1.12 | + | + |
| Bayer BioScience N.V. [25] (2) | 110.01 | 550.06 | 1.90 | 2.21 | + | + | |
| Monsanto Company [26] | 1.35 | 5.38 | 1.76 | 1.86 | + | + | |
| DNeasy® Plant Maxi Kit | 6.28 | 35.88 | 1.53 | 0.64 | + | + | |
| QIAamp DNA Stool Mini Kit | 37.96 | 189.79 | 2.13 | 2.25 | + | + | |
| Newly modified protocol | 175.11 | 437.77 | 1.90 | 2.19 | + | + | |
| Pollen grains | Newly developed protocol | 210.7 | 2107.0 | 1.95 | 2.17 | + | + |
| DNeasy® Plant Mini Kit | 2.22 | 22.2 | 1.92 | 1.1 | + | + | |
| QIAamp DNA Stool Mini Kit | 3.24 | 16.2 | 2.15 | 1.47 | + | + | |
| P. somniferum L. Matrix | DNA Extraction Method | Weight (g)/Volume (mL) of Sample | DNA Yield (μg) | DNA Concentration (ng/μL) | A260/280 | A260/230 | Target Locus | |
|---|---|---|---|---|---|---|---|---|
| psSSR69 | Tubulin I | |||||||
| Poppy seed filling | Bayer BioScience N.V. [25] | 1 | 79.91 | 399.55 | 1.94 | 1.92 | + | + |
| QIAamp DNA Stool Mini Kit | 0.5 | 7.16 | 35.82 | 1.96 | 0.90 | + | + | |
| Monsanto Company [26] | 1 | 241.26 | 1206.28 | 2 | 1.75 | - | - | |
| Monsanto Company [26] | 1 | 0.97 * | 32.41 * | 1.57 * | 0.40 * | + | + | |
| Newly developed protocol | 2 | 246.55 | 1232.75 | 2.04 | 1.84 | - | - | |
| Newly developed protocol | 2 | 0.81* | 26.91 * | 1.68 * | 0.59 * | + | + | |
| Poppy seed oil | Doveri et al. [29] | 1 | 0.43 | 7.1 | 2.53 | 0.01 | + | + |
| Consolandi et al. [30] aq | 3 | 3.02 | 60.3 | 1.62 | 0.52 | + | + | |
| Consolandi et al. [30] aq | 3 | 0.94 | 18.8 | 1.48 | 0.54 | + | + | |
| Consolandi et al. [30] oil | 3 | 0.14 | 2.8 | 1.44 | 0.56 | + | + | |
| Consolandi et al. [30] aq | 6 | 2.19 | 43.8 | 1.40 | 0.57 | + | + | |
| Consolandi et al. [30] aq | 6 | 1.20 | 23.9 | 1.36 | 0.45 | + | + | |
| Consolandi et al. [30] oil | 6 | 0.21 | 4.1 | 1.22 | 0.59 | + | + | |
| Giménez et al. [31] | 0.5 | 0.08 | 3.0 | 1.75 | 0.08 | + | - | |
| Giménez et al. [31] | 1.5 | 0.06 | 2.5 | 1.28 | 0.06 | + | - | |
| Raieta et al. [4] oil | 3 | 0.18 | 6.1 | 1.26 | 0.13 | + | - | |
| Raieta et al. [4] aq | 3 | 0.26 | 8.7 | 1.61 | 0.21 | + | - | |
| Raieta et al. [4] oil | 1 | 0.10 | 3.3 | 2.07 | 0.09 | - | - | |
| Raieta et al. [4] aq | 1 | 0.13 | 4.3 | 1.26 | 0.12 | + | + | |
| QIAamp DNA Stool Mini Kit | 0.2 | 1.05 | 20.9 | 1.84 | 0.34 | - | + | |
| QIAamp DNA Stool Mini Kit | 1 | 1.71 | 34.2 | 1.78 | 0.33 | - | + | |
| QIAamp DNA Stool Mini Kit | 15 | 1.83 | 36.6 | 1.96 | 0.09 | - | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaňuková, Š.; Mrkvová, M.; Mihálik, D.; Kraic, J. Procedures for DNA Extraction from Opium Poppy (Papaver somniferum L.) and Poppy Seed-Containing Products. Foods 2020, 9, 1429. https://doi.org/10.3390/foods9101429
Kaňuková Š, Mrkvová M, Mihálik D, Kraic J. Procedures for DNA Extraction from Opium Poppy (Papaver somniferum L.) and Poppy Seed-Containing Products. Foods. 2020; 9(10):1429. https://doi.org/10.3390/foods9101429
Chicago/Turabian StyleKaňuková, Šarlota, Michaela Mrkvová, Daniel Mihálik, and Ján Kraic. 2020. "Procedures for DNA Extraction from Opium Poppy (Papaver somniferum L.) and Poppy Seed-Containing Products" Foods 9, no. 10: 1429. https://doi.org/10.3390/foods9101429
APA StyleKaňuková, Š., Mrkvová, M., Mihálik, D., & Kraic, J. (2020). Procedures for DNA Extraction from Opium Poppy (Papaver somniferum L.) and Poppy Seed-Containing Products. Foods, 9(10), 1429. https://doi.org/10.3390/foods9101429