Physicochemical, Sensory, and Cooking Qualities of Pasta Enriched with Oat β-Glucans, Xanthan Gum, and Vital Gluten
Abstract
1. Introduction
2. Material and Methods
2.1. Characteristics of Raw Materials
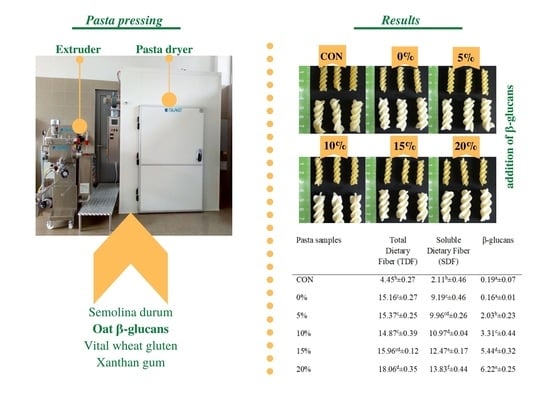
2.2. Pasta Preparation
2.3. Chemical Analysis
2.4. Physical Properties
2.5. Cooking Quality of Pasta Samples
2.6. Color of Pasta
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Pasta Processing
3.2. Chemical Analysis
3.3. Physical Properties
3.4. Cooking Quality
3.5. Color of Pasta
3.6. Sensory Quality
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santeramo, F.G.; Carlucci, D.; De Devitiis, B.; Seccia, A.; Stasi, A.; Viscecchia, R.; Nardone, G. Emerging trends in European food, diets and food industry. Food Res. Int. 2018, 104, 39–47. [Google Scholar] [CrossRef]
- Crowe, K.M.; Francis, C. Position of the Academy of Nutrition and Dietetics: Functional Foods. J. Acad. Nutr. Diet. 2013, 113, 1096–1103. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Rodriguez, O.; Mata, F.; Verdejo-Román, J.; Ramírez-Bernabé, R.; Moreno, D.; Vilar-Lopez, R.; Soriano-Mas, C.; Verdejo-García, A. Neural-based valuation of functional foods among lean and obese individuals. Nutr. Res. 2020, 78, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Riemenschneider, H.; Müller, G.; Levin-Zamir, D.; Van den Broucke, S.; Schwarz, P.E.H. Comparative analysis of diabetes self-management education programs in the European Union Member States. Prim. Care Diabetes 2017, 11, 529–537. [Google Scholar] [CrossRef]
- Hammad, M.A.; Syed Sulaiman, S.A.; Abubakar, U.; Mohamed Noor, D.A. The effects of glycemic control on morbidity and survival among diabetic patients. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1035–1040. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Is Beneficial for the Prevention of Cardiovascular Disease: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2017, 16, 289–299. [Google Scholar] [CrossRef]
- Xi, P.; Liu, R.H. Whole food approach for type 2 diabetes prevention. Mol. Nutr. Food Res. 2016, 60, 1819–1836. [Google Scholar] [CrossRef]
- Krawȩcka, A.; Sobota, A.; Sykut-Domańska, E. Functional Cereal Products in the Diet for Type 2 Diabetes Patients. Int. J. Food Sci. 2019, 2019, 4012450. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and Magnesium Intake and Incidence of Type 2 Diabetes. Arch. Intern. Med. 2007, 167, 956. [Google Scholar] [CrossRef]
- Davison, K.M.; Temple, N.J. Cereal fiber, fruit fiber, and type 2 diabetes: Explaining the paradox. J. Diabetes Complications 2018, 32, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Othman, R.A.; Moghadasian, M.H.; Jones, P.J. Cholesterol-lowering effects of oat β-glucan. Nutr. Rev. 2011, 69, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oat β-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Ciccoritti, R.; Nocente, F.; Sgrulletta, D.; Gazza, L. Cooking quality, biochemical and technological characteristics of bran-enriched pasta obtained by a novel pasta-making process. LWT Food Sci. Technol. 2019, 101, 10–16. [Google Scholar] [CrossRef]
- Cleary, L.; Brennan, C. The influence of a (1→3)(1→4)-β-D-glucan rich fraction from barley on the physico-chemical properties and in vitro reducing sugars release of durum wheat pasta. Int. J. Food Sci. Technol. 2006, 41, 910–918. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→3)(1→4)-β-d-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Jenkins, D.J.A.; Zdravkovic, U.; Würsch, P.; Vuksan, V. Depression of the glycemic index by high levels of β-glucan fiber in two functional foods tested in type 2 diabetes. Eur. J. Clin. Nutr. 2002, 56, 622–628. [Google Scholar] [CrossRef]
- Thomas, A.; Schönauer, M.; Achermann, F.; Schnell, O.; Hanefeld, M.; Ziegelasch, H.J.; Mastrototaro, J.; Heinemann, L. The “glucose pentagon”: Assessing glycemic control of patients with diabetes mellitus by a model integrating different parameters from glucose profiles. Diabetes Technol. Ther. 2009, 11, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, S.W.; Taghrid, L.; Laromiguiere, M.; Huet, D.; Boillot, J.; Rigoir, A.; Elgrably, F.; Slama, G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: A randomized controlled trial. Diabetes Care 2004, 27, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- European Council. Official Journal of the European Union; EU Publications: Luxemburg, 2012; Volume 55. [Google Scholar]
- Piwińska, M.; Wyrwisz, J.; Kurek, M.; Wierzbicka, A. Effect of oat β-glucan fiber powder and vacuum-drying on cooking quality and physical properties of pasta. CyTA J. Food 2016, 14, 101–108. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, M.A.; Brennan, C.S. How combinations of dietary fibres can affect physicochemical characteristics of pasta. Starch/Staerke 2015, 66, 41–46. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Fellows, C.M. Effect of soluble fibre (guar gum and carboxymethylcellulose) addition on technological, sensory and structural properties of durum wheat spaghetti. Food Chem. 2012, 131, 893–900. [Google Scholar] [CrossRef]
- Aravind, N.; Sissons, M.; Egan, N.; Fellows, C. Effect of insoluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 130, 299–309. [Google Scholar] [CrossRef]
- Chillo, S.; Ranawana, D.V.; Henry, C.J.K. Effect of two barley β-glucan concentrates on in vitro glycaemic impact and cooking quality of spaghetti. LWT Food Sci. Technol. 2011, 44, 940–948. [Google Scholar] [CrossRef]
- Brennan, C.S.; Kuri, V.; Tudorica, C.M. Inulin-enriched pasta: Effects on textural properties and starch degradation. Food Chem. 2004, 86, 189–193. [Google Scholar] [CrossRef]
- Kudake, D.C.; Pawar, A.V.; Muley, A.B.; Parate, V.R.; Talib, M.I. Enrichment of Wheat Flour Noodles with Oat Flour: Effect on Physical, Nutritional, Antioxidant and Sensory Properties. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 204–213. [Google Scholar] [CrossRef]
- Jayasena, V.; Nasar-Abbas, S.M. Development and quality evaluation of high-protein and high-dietary-fiber pasta using lupin flour. J. Texture Stud. 2012, 43, 153–163. [Google Scholar] [CrossRef]
- Tolve, R.; Pasini, G.; Vignale, F.; Favati, F.; Simonato, B. Effect of grape pomace addition on the technological, sensory, and nutritional properties of durum wheat pasta. Foods 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Sykut-Domańska, E.; Zarzycki, P.; Sobota, A.; Teterycz, D.; Wirkijowska, A.; Blicharz-Kania, A.; Andrejko, D.; Mazurkiewicz, J. The potential use of by-products from coconut industry for production of pasta. J. Food Process. Preserv. 2020, 44, e14490. [Google Scholar] [CrossRef]
- Sobota, A.; Rzedzicki, Z.; Zarzycki, P.; Kuzawińska, E. Application of common wheat bran for the industrial production of high-fibre pasta. Int. J. Food Sci. Technol. 2015, 50, 111–119. [Google Scholar] [CrossRef]
- Milde, L.B.; Chigal, P.S.; Olivera, J.E.; González, K.G. Incorporation of xanthan gum to gluten-free pasta with cassava starch. Physical, textural and sensory attributes. LWT 2020, 131, 109674. [Google Scholar] [CrossRef]
- Raungrusmee, S.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of resistant starch, xanthan gum, inulin and defatted rice bran on the physicochemical, functional and sensory properties of low glycemic gluten-free noodles. LWT 2020, 126, 109279. [Google Scholar] [CrossRef]
- Brennan, C.S.; Tudorica, C.M. Fresh Pasta Quality as Affected by Enrichment of Nonstarch Polysaccharides. J. Food Sci. 2007, 72, S659–S665. [Google Scholar] [CrossRef]
- Villeneuve, S.; Des Marchais, L.-P.; Gauvreau, V.; Mercier, S.; Do, C.B.; Arcand, Y. Effect of flaxseed processing on engineering properties and fatty acids profiles of pasta. Food Bioprod. Process. 2013, 91, 183–191. [Google Scholar] [CrossRef]
- Mekuria, B.; Sa, E. Effects of Vital Gluten Enrichment on Qualities of Value Added Products. J. Food Process. Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Kaur, M.; Sandhu, K.S.; Arora, A.P.; Sharma, A. Gluten free biscuits prepared from buckwheat flour by incorporation of various gums: Physicochemical and sensory properties. LWT Food Sci. Technol. 2015, 62, 628–632. [Google Scholar] [CrossRef]
- Sobota, A.; Wirkijowska, A.; Zarzycki, P. Application of vegetable concentrates and powders in coloured pasta production. Int. J. Food Sci. Technol. 2020, 55, 2677–2687. [Google Scholar] [CrossRef]
- AACC. American Association of Cereal Chemistry Approved Methods, 10th ed.; St. Paul. Available online: http://methods.aaccnet.org/toc.aspx (accessed on 29 July 2020).
- AOAC. Association of Official Analytical Chemists International. Official Methods, 20th ed.; Rockville. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 29 July 2020).
- The Polish Committee for Standarization. PKN Polish Standard. Cereal Products. Wheat Flour for Alimentary Pasta. Available online: https://infostore.saiglobal.com/STORE/Details.aspx?ProductID=340448 (accessed on 29 July 2020).
- Zarzycki, P.; Sobota, A. Effect of storage temperature on falling number and apparent viscosity of gruels from wheat flours. J. Food Sci. Technol. 2015, 52, 437–443. [Google Scholar] [CrossRef]
- Obuchowski, W. Technologia Przemysłowej Produkcji Makaronu (Technology of Industrial Pasta Production); Wydawnictwo Akademii Rolniczej im. Augusta Cieszkowskiego: Poznań, Poland, 1997. [Google Scholar]
- Sozer, N.; Dalgiç, A.C.; Kaya, A. Thermal, textural and cooking properties of spaghetti enriched with resistant starch. J. Food Eng. 2007, 81, 476–484. [Google Scholar] [CrossRef]
- Tudoricǎ, C.M.; Kuri, V.; Brennan, C.S. Nutritional and physicochemical characteristics of dietary fiber enriched pasta. J. Agric. Food Chem. 2002, 50, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Patil, G.R. Soluble dietary fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- Sobota, A.; Dobosz, M. Quality of whole grain pasta available in market (in Polish). Food. Sci. Technol. Qual. 2010, 6, 83–99. [Google Scholar]
- Zarzycki, P.; Sobota, A.; Kuzawińska, E.; Wirkijowska, A.; Sykut-Domańska, E. Estimation of degree of starch gelatinisation in instant pasta using measurements of viscosity and water absorption of ground instant pasta dispersions. Acta Agrophys. 2017, 24, 625–632. [Google Scholar]
- Brennan, M.A.; Derbyshire, E.; Tiwari, B.K.; Brennan, C.S. Enrichment of extruded snack products with coproducts from chestnut mushroom (Agrocybe aegerita) production: Interactions between dietary fiber, physicochemical characteristics, and glycemic load. J. Agric. Food Chem. 2012, 60, 4396–4401. [Google Scholar] [CrossRef]
- Anttila, H.; Sontag-Strohm, T.; Salovaara, H. Viscosity of beta-glucan in oat products. Agric. Food Sci. 2004, 13, 80–87. [Google Scholar] [CrossRef]
- Mäkelä, N.; Brinck, O.; Sontag-Strohm, T. Viscosity of β-glucan from oat products at the intestinal phase of the gastrointestinal model. Food Hydrocoll. 2020, 100, 105422. [Google Scholar] [CrossRef]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.S.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, J.; Qin, Y.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Bian, X.; Shao, Z. Rheological and functional properties of oat β-glucan with different molecular weight. J. Mol. Struct. 2020, 1209, 127944. [Google Scholar] [CrossRef]
- Zarzycki, P.; Sykut-Domańska, E.; Sobota, A.; Teterycz, D.; Krawęcka, A.; Blicharz-Kania, A.; Andrejko, D.; Zdybel, B. Flaxseed Enriched Pasta—Chemical Composition and Cooking Quality. Foods 2020, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Sobota, A.; Zarzycki, P. Effect of Pasta Cooking Time on the Content and Fractional Composition of Dietary Fiber. J. Food Qual. 2013, 36, 127–132. [Google Scholar] [CrossRef]
- Hajji, T.; Sfayhi-Terras, D.; El Felah, M.; Rezgui, S.; Ferchichi, A. Incorporation of β-glucans into pasta extracted from two tunisian barley cultivars. Int. J. Food Eng. 2016, 12, 701–710. [Google Scholar] [CrossRef]
- Cassidy, Y.M.; McSorley, E.M.; Allsopp, P.J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. [Google Scholar] [CrossRef]
- Jaworska, D.; Królak, M.; Przybylski, W.; Jezewska-Zychowicz, M. Acceptance of Fresh Pasta with β-Glucan Addition: Expected Versus Perceived Liking. Foods 2020, 9, 869. [Google Scholar] [CrossRef]

| Samples | Raw Materials (%) | Water Addition (g kg−1) | Pressure (MPa) | Barrel Temp. (°C) | Extruder Output (kg h−1) | |||
|---|---|---|---|---|---|---|---|---|
| Semolina Durum | Oat β-Glucans | Vital Wheat Gluten | Xanthan Gum | |||||
| CON | 100 | - | - | - | 305.2 | 8.5 | 28.9 | 34.68 |
| BG0 | 90 | 0 | 5 | 5 | 305.6 | 13 | 29.6 | 29.88 |
| BG5 | 85 | 5 | 5 | 5 | 309 | 13 | 29.8 | 31.56 |
| BG10 | 80 | 10 | 5 | 5 | 312.4 | 13 | 29.8 | 32.40 |
| BG15 | 75 | 15 | 5 | 5 | 315.8 | 13.5 | 29.8 | 32.40 |
| BG20 | 70 | 20 | 5 | 5 | 319.4 | 13.5 | 29.8 | 32.38 |
| Samples | Moisture | Protein | Fat | Ash | TDF | IDF | SDF | β-Glucans | Digestible Carbohydrate |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (g/100 g d.m.) | ||||||||
| Raw materials | |||||||||
| Semolina durum | 9.50 e ± 0.04 | 13.24 c ± 0.71 | 1.1 a ± 0.04 | 0.76 b ± 0.01 | 3.89 abc ± 0.15 | 2.07 b ± 0.09 | 1.83 ab ± 0.06 | 0.18 a ± 0.05 | 81.01 e ± 1.14 |
| Vital wheat gluten | 7.31 b ± 0.05 | 70.99 g ± 3.89 | 1.48 b ± 0.03 | 0.57 a ± 0.01 | 23.81 e ± 0.74 | 22.69 g ± 0.96 | 1.12 a ± 0.22 | nd | 3.15 b ± 0.55 |
| Xanthan gum | 11.17 h ± 0.13 | 5.97 a ± 0.38 | 2.03 de ± 0.16 | 8.36 g ± 0.09 | 83.36 k ± 4.9 | 3.17 b ± 0.28 | 80.19 f ± 1.86 | nd | 0.28 a ± 0.25 |
| Oat β-glucans | 4.19 a ± 0.07 | 8.45 b ± 0.06 | 3.56 g ± 0.08 | 1.99 f ± 0.02 | 33.48 f ± 1.35 | 0.64 a ± 0.2 | 32.85 e ± 1.15 | 27.58 f ± 0.03 | 52.52 c ± 1.51 |
| Pasta samples | |||||||||
| CON | 8.83 c ± 0.16 | 13.57 cd ± 0.1 | 1.67 bc ± 0.05 | 1.17 c ± 0.03 | 4.45 b ± 0.27 | 2.34 a ± 0.19 | 2.11 b ± 0.46 | 0.19 a ± 0.07 | 79.14 e ± 0.37 |
| BG0 | 9.05 cd ± 0.10 | 17.17 f ± 0.43 | 1.84 cd ± 0.07 | 1.38 d ± 0.02 | 15.16 c ± 0.27 | 5.97 d ± 0.19 | 9.19 c ± 0.46 | 0.16 a ± 0.01 | 64.45 d ± 1.47 |
| BG5 | 10.33 g ± 0.06 | 15.7 ef ± 0.6 | 2.23 e ± 0.08 | 1.39 d ± 0.02 | 15.37 c ± 0.25 | 5.41 cd ± 1.59 | 9.96 cd ± 0.26 | 2.03 b ± 0.23 | 65.31 d ± 2.84 |
| BG10 | 9.93 f ± 0.18 | 15.9 ef ± 0.78 | 2.55 f ± 0.09 | 1.38 d ± 0.05 | 14.87 c ± 0.39 | 3.9 abc ± 0.34 | 10.97 d ± 0.04 | 3.31 c ± 0.44 | 65.30 d ± 1.54 |
| BG15 | 9.32 de ± 0.15 | 15.2 de ± 0.86 | 2.7 f ± 0.09 | 1.49 de ± 0.05 | 15.96 cd ± 0.12 | 3.5 ab ± 0.29 | 12.47 e ± 0.17 | 5.44 d ± 0.32 | 64.65 d ± 1.49 |
| BG20 | 9.17 d ± 0.03 | 15.33 de ± 0.76 | 2.74 f ± 0.09 | 1.55 e ± 0.05 | 18.06 d ± 0.35 | 4.23 bcd ± 0.09 | 13.83 f ± 0.44 | 6.22 e ± 0.25 | 62.32 d ± 1.27 |
| Samples | Equivalent Diameter (µm) | WAI | WSI |
|---|---|---|---|
| (%) | |||
| Raw materials | |||
| Semolina durum | 286.37 b ± 34 | 84.7 a ± 3.7 | 5.18 a ± 0.23 |
| Vital wheat gluten | 52 a ± 32 | 150.3 a ± 2.9 | 8.18 bc ± 0.13 |
| Xanthan gum | 25 a ± 16 | 2319.3 d ± 94.7 | 24.69 f ± 2.28 |
| Oat β-glucans | 83 a ± 20 | 900.9 c ± 17.2 | 66.26 g ± 1.9 |
| Pasta samples | |||
| CON | - | 100.4 a ± 2.3 | 9.5 bcd ± 0.1 |
| BG0 | - | 361 b ± 14.0 | 7.0 ab ± 0.1 |
| BG5 | - | 371.7 b ± 23.6 | 9.5 bcd ± 0.3 |
| BG10 | - | 376.9 b ± 4.7 | 10.9 cde ± 0.1 |
| BG15 | - | 377.6 b ± 4.5 | 12.2 de ± 0.1 |
| BG20 | - | 393.3 b ± 15.4 | 12.3 e ± 0.2 |
| Samples | Heating | Cooling | |||||
|---|---|---|---|---|---|---|---|
| Temp. 65 °C | Temp. 75 °C | Temp. 85 °C | Temp. 95 °C | Temp. 95 °C * | Temp. 50 °C | Temp. 50 °C ** | |
| Con | 0.006 aA ± 0 | 0.007 aB ± 0 | 0.010 aC ± 0 | 0.012 aD ± 0.001 | 0.014 aF ± 0.001 | 0.015 aG ± 0 | 0.013 aE ± 0 |
| BG0 | 0.012 bA ± 0 | 0.023 bB ± 0 | 0.028 bC ± 0 | 0.031 cE ± 0 | 0.030 cD ± 0 | 0.039 bG ± 0 | 0.035 bF ± 0 |
| BG5 | 0.013 cA ± 0 | 0.034 dE ± 0.001 | 0.031 cC ± 0 | 0.032 dD ± 0 | 0.030 cB ± 0 | 0.040 cG ± 0 | 0.036 cF ± 0 |
| BG10 | 0.014 dA ± 0 | 0.029 cB ± 0 | 0.032 dC ± 0.001 | 0.029 bB ± 0 | 0.029 bB ± 0.001 | 0.039 bE ± 0 | 0.035 bD ± 0 |
| BG15 | 0.017 eA ± 0 | 0.039 eD ± 0 | 0.038 eC ± 0 | 0.038 fC ± 0 | 0.036 dB ± 0 | 0.045 eF ± 0.001 | 0.044 dE ± 0 |
| BG20 | 0.025 fA ± 0.001 | 0.045 fE ± 0.001 | 0.041 fC ± 0.001 | 0.037 eB ± 0 | 0.037 eB ± 0 | 0.048 dE ± 0 | 0.044 dD ± 0 |
| Pasta Samples | Optimum Cooking Time (min) | Cooking Loss (% d.m.) | Cooking Weight Increase | Cooking Volume Increase |
|---|---|---|---|---|
| CON | 9 a ± 0.0 | 4.52 c ± 0.42 | 2.54 a ± 0.04 | 3.16 a ± 0.12 |
| BG0 | 9 a ± 0.0 | 2.95 a ± 0.17 | 2.73 b ± 0.02 | 4.29 d ± 0.18 |
| BG5 | 9 a ± 0.5 | 3.58 b ± 0.13 | 2.77 b ± 0.04 | 3.88 c ± 0.13 |
| BG10 | 9 a ± 0.5 | 3.82 b ± 0.11 | 2.74 b ± 0.02 | 3.46 b ± 0.16 |
| BG15 | 10 b ± 0.0 | 5.03 d ± 0.09 | 2.88 c ± 0.01 | 3.46 b ± 0 |
| BG20 | 11.5 c ± 0.5 | 5.14 d ± 0.09 | 3.09 d ± 0 | 3.38 b ± 0.18 |
| Pasta Samples | Uncooked | Cooked | ||||
|---|---|---|---|---|---|---|
| L * | a * | b * | L * | a * | b * | |
| CON | 51.52 c ± 1.41 | 2.11 d ± 0.15 | 16.22 c ± 0.96 | 76.67 a ± 0.80 | −0.43 b ± 0.11 | 17.95 c ± 1.07 |
| BG0 | 48.44 a ± 1.90 | 1.4 a ± 0.15 | 14.49 b ± 1.36 | 79.14 b ± 1.42 | −0.66 a ± 0.09 | 13.93 a ± 0.61 |
| BG5 | 49.78 ab ± 0.94 | 1.45 a ± 0.16 | 13.84 ab ± 0.56 | 79.11 b ± 1.18 | −0.73 a ± 0.10 | 13.82 a ± 0.91 |
| BG10 | 48.98 a ± 2.13 | 1.62 b ± 0.20 | 13.58 a ± 0.88 | 79.11 b ± 1.40 | −0.43 b ± 0.12 | 14.52 ab ± 0.96 |
| BG15 | 50.24 b ± 0.73 | 1.8 c ± 0.16 | 13.81 ab ± 0.84 | 76.14 a ± 1.07 | −0.30 b ± 0.09 | 14.11 ab ± 0.59 |
| BG20 | 51.10 bc ± 0.70 | 1.84 c ± 0.10 | 13.7 ab ± 0.34 | 75.95 a ± 1.59 | −0.31 b ± 0.08 | 14.92 b ± 0.92 |
| Pasta Samples | Uncooked | Cooked | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apparance | Color | Odor | Apparance | Color | Taste | Odor | Hardness | Adhesiveness | Springness | |
| CON | 4.6 a ± 0.55 | 4.2 a ± 0.45 | 4.8 a ± 0.45 | 3.6 a ± 0.45 | 4.2 b ± 0.45 | 5 b ± 0 | 4.8 a ± 0.45 | 4.6 a ± 0.55 | 5 c ± 0 | 4.8 b ± 0.45 |
| BG0 | 4.8 a ± 0.45 | 4.4 a ± 0.45 | 4.8 a ± 0.45 | 5 c ± 0 | 4.8 c ± 0 | 5 b ± 0 | 5 a ± 0 | 5 a ± 0 | 5 c ± 0 | 5 b ± 0 |
| BG5 | 5 a ± 0 | 4.6 a ± 0 | 5 a ± 0 | 4.6 bc ± 0.35 | 4.6 bc ± 0.55 | 4.6 ab ± 0.55 | 4.8 a ± 0.45 | 4.4 a ± 0.55 | 4.8 bc ± 0.45 | 4.6 b ± 0.55 |
| BG10 | 5 a ± 0 | 4.8 a ± 0.45 | 4.8 a ± 0.45 | 4.6 bc ± 0.35 | 4.6 bc ± 0.55 | 4.6 ab ± 0.55 | 4.8 a ± 0.45 | 4.8 a ± 0.45 | 5 c ± 0 | 4.6 b ± 0.55 |
| BG15 | 5 a ± 0 | 4.83 a ± 0.45 | 4.8 a ± 0.45 | 4.0 ab ± 0.45 | 4.2 b ± 0.45 | 4.2 ab ± 0.45 | 4.4 a ± 0.55 | 4.8 a ± 0.45 | 4.4 b ± 0.15 | 4.2 ab ± 0.45 |
| BG20 | 4.83 a ± 0 | 4.75 a ± 0.45 | 4.83 a ± 0.45 | 3.8 a ± 0.35 | 3.2 a ± 0.45 | 4 a ± 1 | 4.6 a ± 0.55 | 4.4 a ± 0.55 | 3.8 a ± 0.35 | 3.6 a ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawęcka, A.; Sobota, A.; Sykut-Domańska, E. Physicochemical, Sensory, and Cooking Qualities of Pasta Enriched with Oat β-Glucans, Xanthan Gum, and Vital Gluten. Foods 2020, 9, 1412. https://doi.org/10.3390/foods9101412
Krawęcka A, Sobota A, Sykut-Domańska E. Physicochemical, Sensory, and Cooking Qualities of Pasta Enriched with Oat β-Glucans, Xanthan Gum, and Vital Gluten. Foods. 2020; 9(10):1412. https://doi.org/10.3390/foods9101412
Chicago/Turabian StyleKrawęcka, Ada, Aldona Sobota, and Emilia Sykut-Domańska. 2020. "Physicochemical, Sensory, and Cooking Qualities of Pasta Enriched with Oat β-Glucans, Xanthan Gum, and Vital Gluten" Foods 9, no. 10: 1412. https://doi.org/10.3390/foods9101412
APA StyleKrawęcka, A., Sobota, A., & Sykut-Domańska, E. (2020). Physicochemical, Sensory, and Cooking Qualities of Pasta Enriched with Oat β-Glucans, Xanthan Gum, and Vital Gluten. Foods, 9(10), 1412. https://doi.org/10.3390/foods9101412