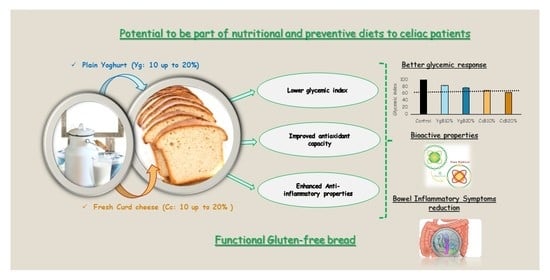
Glycemic Response and Bioactive Properties of Gluten-Free Bread with Yoghurt or Curd-Cheese Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Bread Dough Preparation
2.3. Pasting Properties of Gluten-Free Dough
2.4. In Vitro Starch Hydrolysis
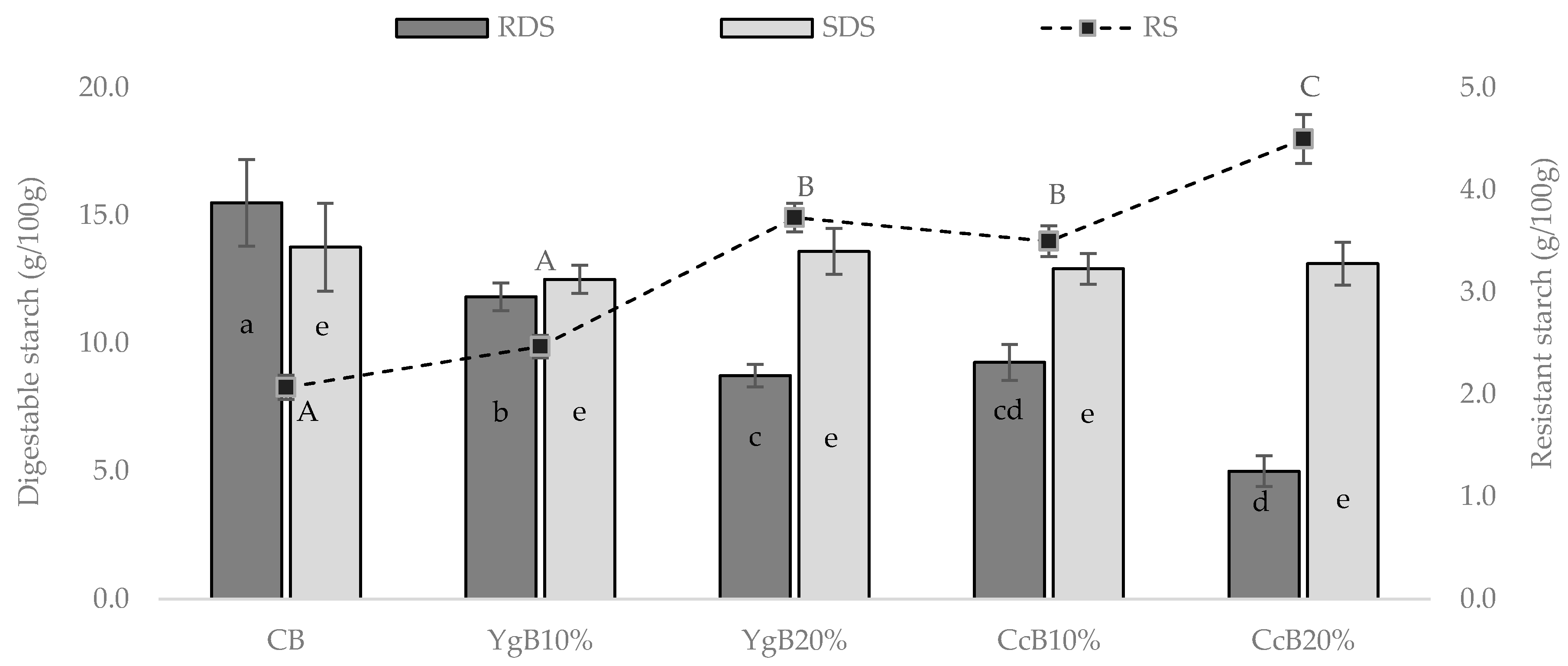
2.4.1. Digestible Starch Fraction
2.4.2. Resistant Starch
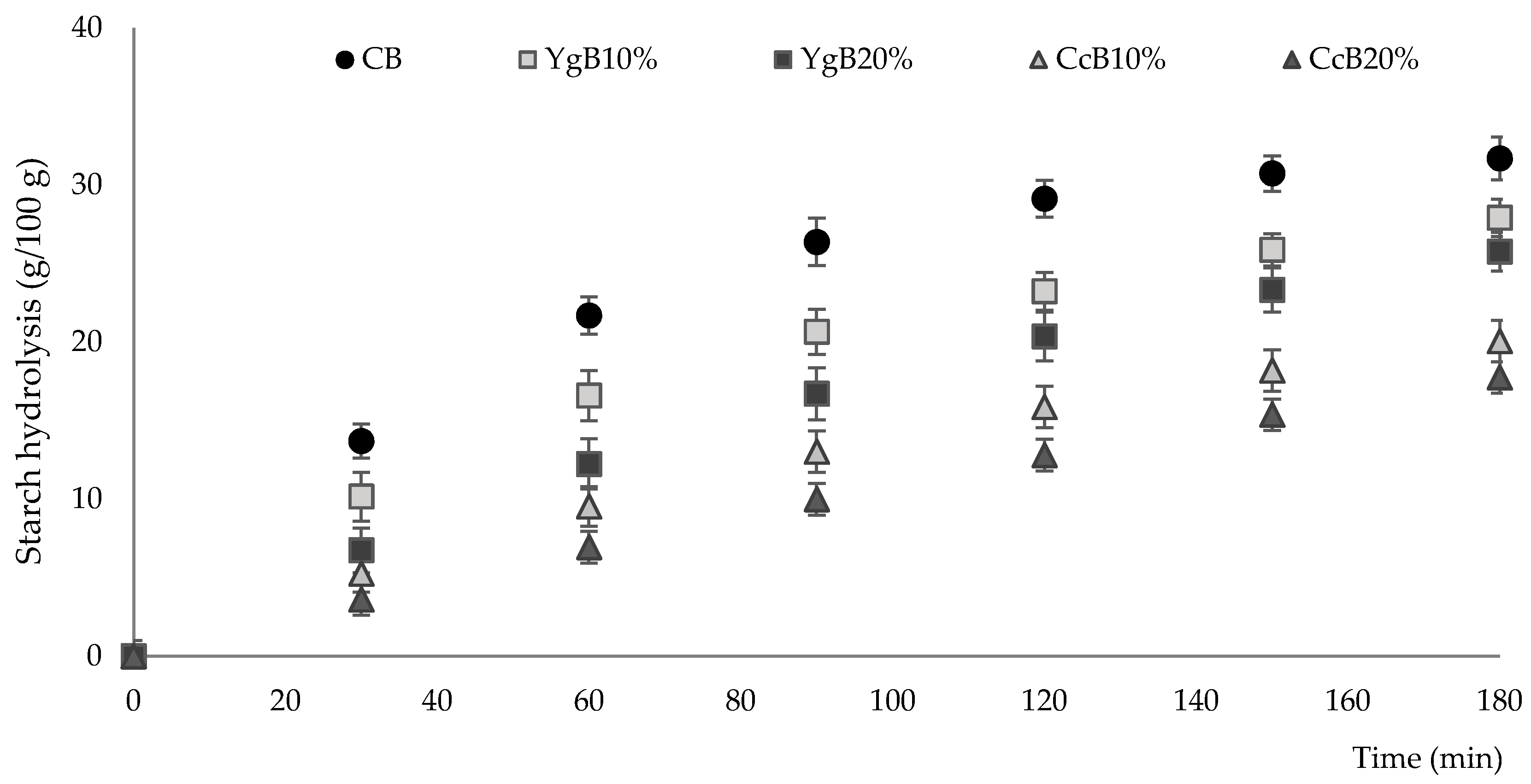
2.4.3. In Vitro Starch Digestion and Estimation of Gluten-Free Bread Glycemic Index
2.5. Antioxidant and Anti-Inflammatory Activities of the Gluten-Free Bread
2.5.1. Antioxidant Activity
2.5.2. MMP-9 Inhibition Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Pasting Properties
- (i)
- Insufficient hydration of starch granules resulting from the competition for free water by denatured whey proteins (derived from curd cheese additions [40]);
- (ii)
- Starch-protein interactions, reducing the accessibility of enzymatic attack to starch granules, thus limiting the degree of starch hydrolysis [8];
- (iii)
- Amylose-lipid complexes formed during cooling, lowering the final peak consistency [41].
3.2. In Vitro Starch Digestion of the Gluten-Free Bread
3.3. Hydrolysis Kinetics and Estimated Glycemic Index of Gluten-Free Bread
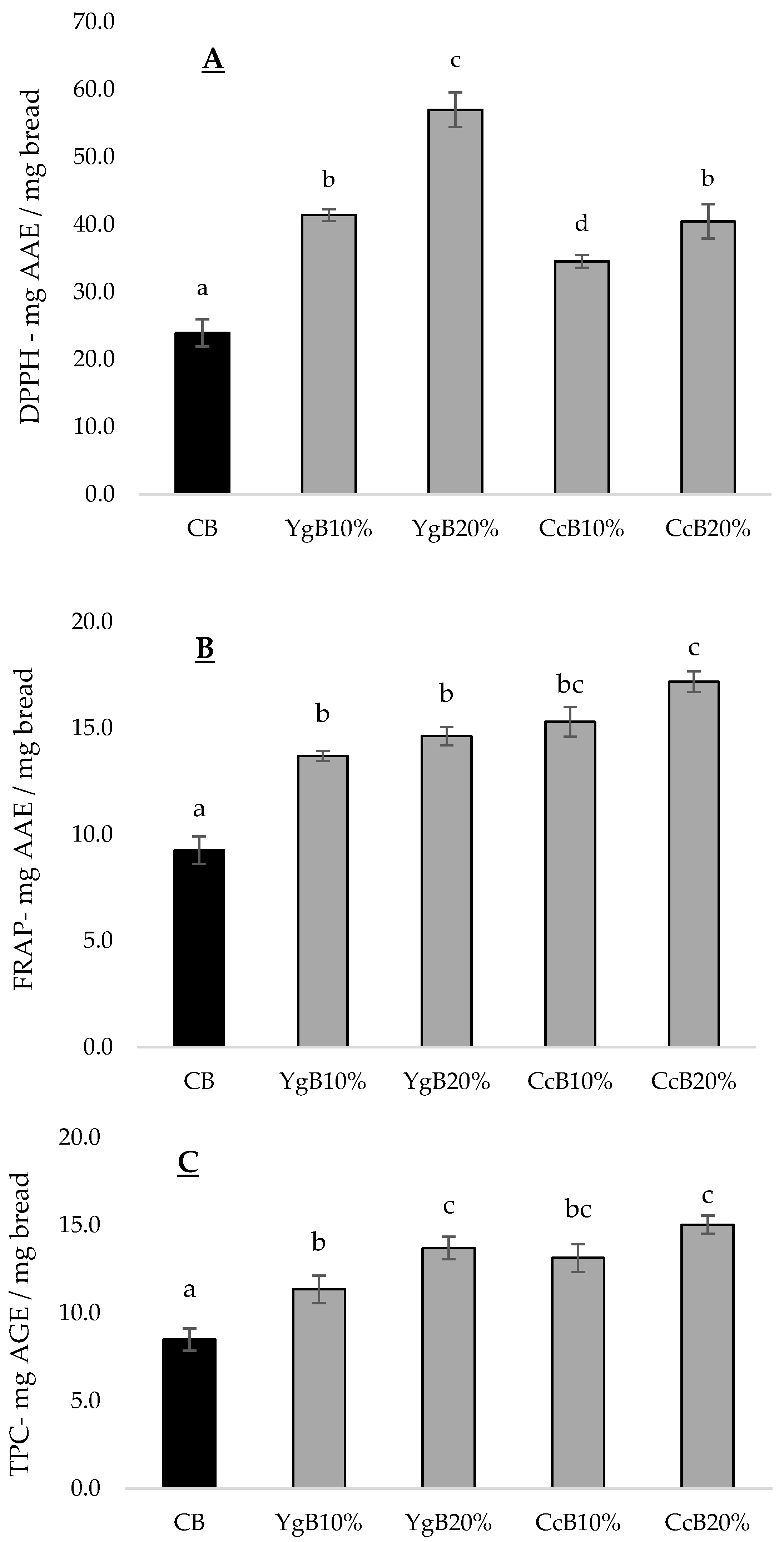
3.4. Effect of Yoghurt and Curd Cheese Enrichment on Gluten-Free Bread Bioactive Properties
3.4.1. Antioxidant Activity
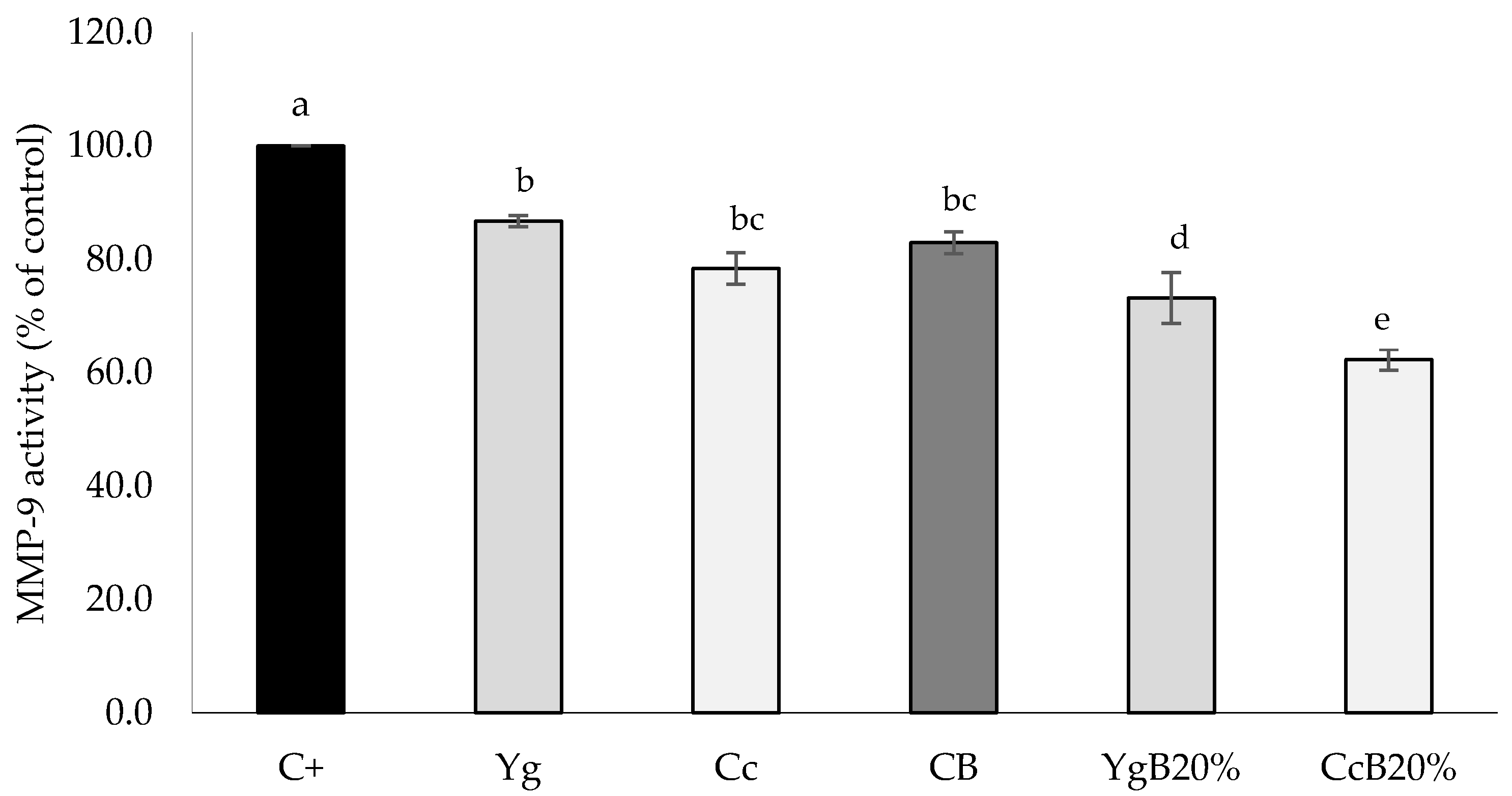
3.4.2. MMP-9 Inhibition Activity
3.5. Correlations between Starch Behavior, Glycemic Index, and Bread Bioactivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muir, J.G.; Varney, J.E.; Ajamian, M.; Gibson, P.R. Gluten-free and low-FODMAP sourdoughs for patients with coeliac disease and irritable bowel syndrome: A clinical perspective. Inter. J. Food Microb. 2019, 290, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiological approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Green, P.H.; Jabri, B. Coeliac disease. Lancet 2003, 362, 383–391. [Google Scholar] [CrossRef]
- Haines, M.L.; Anderson, R.P.; Gibson, P.R. Systematic review: The evidence base for long-term management of coeliac disease. Aliment. Pharmacol. Therap. 2008, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J. Food Eng. 2007, 79, 1033–1047. [Google Scholar] [CrossRef]
- Marco, C.; Rosell, C.M. Functional and rheological properties of protein enriched gluten-free composite flours. J. Food Eng. 2008, 88, 94–103. [Google Scholar] [CrossRef]
- Segura, M.E.; Rosell, C.M. Chemical composition and starch digestibility of different gluten-free breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef]
- Fardet, A.; Leenhardt, F.; Lioger, D.; Scalbert, A.; Rémésy, C. Parameters controlling the glycemic response to breads. Nutr. Res. Rev. 2006, 19, 18–25. [Google Scholar] [CrossRef]
- de la Hera, E.; Rosell, C.M.; Gomez, M. Effect of water content and flour particle size on gluten-free bread quality and digestibility. Food Chem. 2014, 151, 526–531. [Google Scholar] [CrossRef]
- Sahagún, M.; Benavent-Gi, Y.; Rosell, C.; Gómez, M. Modulation of in vitro digestibility and physical characteristics of protein enriched gluten free breads by defining hydration. LWT Food Sci. Technol. 2020, 117, 108642. [Google Scholar] [CrossRef]
- Chung, H.J.; Lin, Q.; Hoover, R.; Warkentin, T.D.; Vandenberg, B. In-vitro starch digestibility expected glycemic index and thermal and pasting properties of flours from pea, lentil and chickpea cultivars. Food Chem. 2008, 111, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Guo, Z.; Sun, B.; Zhao, Y. Identification of anthocyanins from four kinds of berries and their inhibition activity to alpha-glycosidase and protein tyrosine phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017, 65, 6211–6221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, X.; Yu, H.; Zhao, X.; Zhong, X.; Li, Q.; Tang, J.; Zhao, Y. Effect of liquid fermentation on bread fortified with Lycium ruthenicum: A quality attribute and in vitro digestibility study. Food Chem. 2019, 299, 125131. [Google Scholar] [CrossRef] [PubMed]
- Markle, B.; May, E.J.; Majumdar, A.P.N. Do nutraceutics play a role in the prevention and treatment of colorectal cancer? Cancer Metastasis Rev. 2010, 29, 395–404. [Google Scholar] [CrossRef]
- Malla, N.; Sjøli, S.; Winberg, J.O.; Hadler-Olsen, E.; Uhlin-Hansen, L. Biological and pathobiological functions of gelatinase dimers and complexes. Connect. Tissue Res. 2008, 49, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Vansaun, M.N.; Shim, J.H.; Matrisian, L.M.; Gorden, D.L. Increased metastases are associated with inflammation and matrix metalloproteinase-9 activity at incision sites in a murine model of peritoneal dissemination of colorectal cancer. J. Surg. Res. 2013, 180, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Tijerina-Sáenz, A.; Innis, S.M.; Kitts, D.D. Antioxidant capacity of human milk and its association with vitamins A and E and fatty acid composition. Acta Pediatric ISSN 2009, 98, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hosseinian, F.S.; Tsopmo, A.; Friel, J.K.; Beta, T. Evaluation of antioxidant capacity and aroma quality of breast milk. Nutrit. J. 2009, 25, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Poppi, F.A.; Costa, M.R.; Rensis, C.M.V.B.; Silvieri, K. Whey and its proteins: Composition and functional activity. UNOPAR Scientific. Biolog. Health Sci. 2010, 12, 31–37. [Google Scholar]
- Augustin, M.A.; Udabage, P. Influence of processing on functionality of milk and dairy proteins. Adv. Food Nutr. Res. 2007, 53, 1–38. [Google Scholar] [PubMed]
- Ceapa, C.; Wopereis, H.; Rezaiki, L.; Kleerebezem, M.; Knol, J.; Oozeer, R. Influence of fermented milk products, prebiotics and probiotics on microbiota composition and health. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Labonte, M.E.; Couture, P.; Richard, C.; Desroches, S.; Lamarche, B. Impact of dairy products on biomarkers of inflammation: A systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am. J. Clin. Nutr. 2013, 97, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Danesi, F.; Dardevet, D.; Dupont, D.; Fernandez, A.S.; Gille, D.; Shahar, D.R. Dairy products and inflammation: A review of the clinical evidence. Crit. Rev. Food Sci. Nutr. 2017, 57, 2497–2525. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, S.; Yousefi, S.; Faraji, A. Developing an innovative textural structure for semi-volume breads based on interaction of spray-dried yogurt powder and jujube polysaccharide. Inter. J. Biol. Macromol. 2017, 104, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Graça, C.; Raymundo, A.; Sousa, I. Wheat bread with dairy products-technology, nutritional, and sensory properties. Appl. Sci. J. Eco Nov. Foods Spec. Issues 2019, 9, 4101. [Google Scholar] [CrossRef]
- Morens, C.; Bos, C.; Pueyo, M.E.; Benamouzig, R.; Gausseres, N.; Luengo, C. Increasing habitual protein intake accentuates differences in postprandial dietary nitrogen utilization between protein sources in humans. J. Nutr. 2003, 133, 2733–2740. [Google Scholar] [CrossRef]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant activity of whey protein hydrolysates in milk beverage system. J. Food Sci. Technol. 2015, 52, 3235–3241. [Google Scholar] [CrossRef]
- Graça, C.; Raymundo, A.; Sousa, I. Yogurt as an alternative ingredient to improve the functional and nutritional properties of gluten-free breads. Foods J. 2020, 9, 111. [Google Scholar] [CrossRef]
- Graça, C.; Raymundo, A.; Sousa, I. Yoghurt and curd cheese addition to wheat bread dough: Impact on in vitro starch digestibility and estimated glycemic index. Food Chem. 2021, 339, 127887. [Google Scholar] [CrossRef]
- Goni, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Reshmi, S.K.; Sudha, M.L.; Shashirekha, M.N. Starch digestibility and predicted glycemic index in the bread fortified with pomelo (Citrus maxima) fruit segments. Food Chem. 2017, 237, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Oktay, I.; Gülçin, Ö.; Küfrevioğlu, I. Determination of in Vitro Antioxidant Activity of fennel (Foeniculum vulgare) Seed Extracts. Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Lima, A.I.G.; Mota, J.; Monteiro, S.A.V.S.; Ferreira, R.M.S.B. Legume seeds and colorectal cancer revisited: Protease inhibitors reduce MMP-9 activity and colon cancer cell migration. Food Chem. 2016, 197, 30–38. [Google Scholar] [CrossRef]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharides producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2017, 110, 52–61. [Google Scholar] [CrossRef]
- Rosell, C.M.; Collar, C.; Haros, M. Assessment of hydrocolloid effects on the thermo-mechanical properties of wheat using the Mixolab. Food Hydrocoll. 2007, 21, 452–462. [Google Scholar] [CrossRef]
- Rosell, C.M.; Foegeding, A. Interaction of hydroxypropylmethylcellulose with gluten proteins: Small deformation properties during thermal treatment. Food Hydrocoll. 2007, 21, 1092–1100. [Google Scholar] [CrossRef]
- Noisuwan, A.; Broland, J.; Wilkinson, B.; Hemar, Y. Effect of milk protein products on the rheological and thermal (DSC) properties of normal rice starch and waxy rice starch. Food Hydrocoll. 2008, 22, 174–183. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Yamauchi, H.; Kim, S.; Hashimotom, N.; Noda, T. RVA study of mixtures of wheat flour and potato starches with different phosphorus contents. Food Chem. 2007, 102, 1105–1111. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingmans, S.M.; Gummings, J.H. Classification and measurement of nutritionally important starch fraction. Europ. J. Clin. Nutr. 1996, 46, 335–505. [Google Scholar]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef]
- Cavallero, A.; Empilli, S.; Brighenti, F.; Stanca, A.M. High (1→3, 1→4)-β- glucan barley fractions in bread making and their effects on human glycemic response. J. Cereal Sci. 2002, 36, 59–66. [Google Scholar] [CrossRef]
- Barine, K.K.D.; Yorte, G.S. In vitro starch hydrolysis and prediction of glycemic index (PGI) in ‘‘Amala” and plantain based baked products. J. Food Res. 2016, 5, 73–80. [Google Scholar] [CrossRef]
- Bae, M.J.; Karadeniz, F.; Lee, S.G.; Seo, Y.; Kong, C.S. Inhibition of MMP-2 and MMP-9 activities by Limonium tetragonum extract. Prev. Nutr. Food Sci. 2016, 21, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.; Oliveira, J.; Mota, J.; Ferreira, R.B. Proteins in soy might have a higher role in cancer prevention than previously expected: Soybean protein fractions are more effective MMP-9 inhibitors than non-protein fractions, even in cooked seeds. Nutrients 2017, 9, 201. [Google Scholar] [CrossRef]

| * Ingredients (% w/w) | CB | YgB10% | YgB20% | CcB10% | CcB20% |
|---|---|---|---|---|---|
| Buckwheat | 16.6 | 14.0 | 11.0 | 12.6 | 10.0 |
| Rice | 25.0 | 21.0 | 17.0 | 20.0 | 13.0 |
| Potato starch | 14.0 | 11.0 | 9.0 | 11.0 | 8.0 |
| Yogurt/Curd cheese | 0.0 | 10.0 | 20.0 | 10.0 | 20.0 |
| Total water absorption ** | 37.0 | 37.5 | 40.0 | 39.0 | 42.0 |
| Samples | C∞ * | K * | R2 | AUC 0–180 | HI (%) | IG ** |
|---|---|---|---|---|---|---|
| CB | 33.1 ± 0.9 a | 0.0179 ± 0.002 a | 0.915 | 4122.9 ± 61.2 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| YgB10% | 28.0 ± 0.8 b | 0.0153 ± 0.002 a | 0.972 | 3239.6 ± 36.0 b | 78.6 ± 1.3 b | 82.8 ± 0.7 b |
| YgB20% | 27.0 ± 3.0 b | 0.0068 ± 0.001 b | 0.984 | 2766.2 ± 83.0 c | 67.1 ± 1.7 c | 76.5 ± 0.9 c |
| CcB10% | 20.0 ± 1.2 c | 0.0068 ± 0.003 b | 0.984 | 2185.1 ± 28.0 d | 53.0 ± 1.0 d | 68.0 ± 0.5 d |
| CcB20% | 18.0 ± 0.4 c | 0.0029 ± 0.001 c | 0.985 | 1728.3 ± 21.2 e | 42.0 ± 0.1 e | 62.7 ± 0.1 e |
| Parameters Correlated | Linear Equation | R2 |
|---|---|---|
| FC vs. DP | FC = 218.48 − 3.49 × DP | 0.922 |
| RDS vs. DP | RDS = 13.52 − 0.27 × DP | 0.910 |
| RDS vs. PBC | RDS = 29.3 − 2.58 × PBC | 0.993 |
| RS vs. DP | RS = 2.30 + 0.05 × DP | 0.940 |
| RS vs. PBC | RS = - 1.25 + 0.60 × PBC | 0.933 |
| RS vs. FC | RS = 7.52 − 0.02 × FC | 0.900 |
| GI vs. FC | GI = 23.14 + 0.27 × FC | 0.900 |
| GI vs. RDS | GI = 55.32 + 2.68 × RDS | 0.944 |
| GI vs. RS | GI = 121.60 − 12.91 × RS | 0.952 |
| GI vs. DP | GI = 89.88 − 0.99 × DP | 0.922 |
| FRAP vs. TPC | FRAP = 0.60 + 1.26 × TPC | 0.850 |
| GI vs. TPC | GI = 134.76 − 5.43 × FRAP | 0.900 |
| GI vs. FRAP | GI = 132.80 − 3.97 × FRAP | 0.920 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graça, C.; Mota, J.; Lima, A.; Boavida Ferreira, R.; Raymundo, A.; Sousa, I. Glycemic Response and Bioactive Properties of Gluten-Free Bread with Yoghurt or Curd-Cheese Addition. Foods 2020, 9, 1410. https://doi.org/10.3390/foods9101410
Graça C, Mota J, Lima A, Boavida Ferreira R, Raymundo A, Sousa I. Glycemic Response and Bioactive Properties of Gluten-Free Bread with Yoghurt or Curd-Cheese Addition. Foods. 2020; 9(10):1410. https://doi.org/10.3390/foods9101410
Chicago/Turabian StyleGraça, Carla, Joana Mota, Ana Lima, Ricardo Boavida Ferreira, Anabela Raymundo, and Isabel Sousa. 2020. "Glycemic Response and Bioactive Properties of Gluten-Free Bread with Yoghurt or Curd-Cheese Addition" Foods 9, no. 10: 1410. https://doi.org/10.3390/foods9101410
APA StyleGraça, C., Mota, J., Lima, A., Boavida Ferreira, R., Raymundo, A., & Sousa, I. (2020). Glycemic Response and Bioactive Properties of Gluten-Free Bread with Yoghurt or Curd-Cheese Addition. Foods, 9(10), 1410. https://doi.org/10.3390/foods9101410