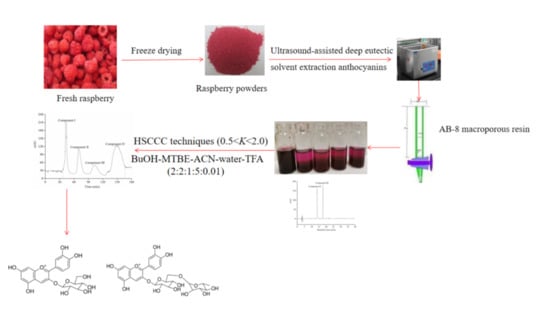
Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Reagents and Solvents
2.3. Preparation of DESs
2.4. UADESE Procedures
2.5. Determination of the Anthocyanin Yield
2.6. Experimental Design
2.6.1. Screening of Variables
2.6.2. Box-Behnken Design (BBD)
2.6.3. Genetic Algorithm (GA)
2.7. Purification of Anthocyanins Using Macroporous Adsorption Resin
2.8. High-Speed Counter Current Chromatography Isolation of High-Purity Anthocyanins
2.8.1. Measurement of the Partition Coefficient (K)
2.8.2. Preparation of High-Speed Counter Current Chromatography Solvent System and Sample Solution
2.8.3. High-Speed Counter Current Chromatography Separation Procedure
2.9. High-Speed Counter Current Chromatography Peaks Identification
2.9.1. UV-Visible Spectrophotometer (UV-Vis) Analysis
2.9.2. High-Performance Liquid Chromatography-Diode Array Detector (HPLC-DAD) and High-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (HPLC-ESI-MS/MS) Analysis
2.9.3. Nuclear Magnetic Resonance (NMR) Analysis
2.10. Statistical Analysis
3. Results and Discussions
3.1. Screening of DESs System for the Extraction of Anthocyanins
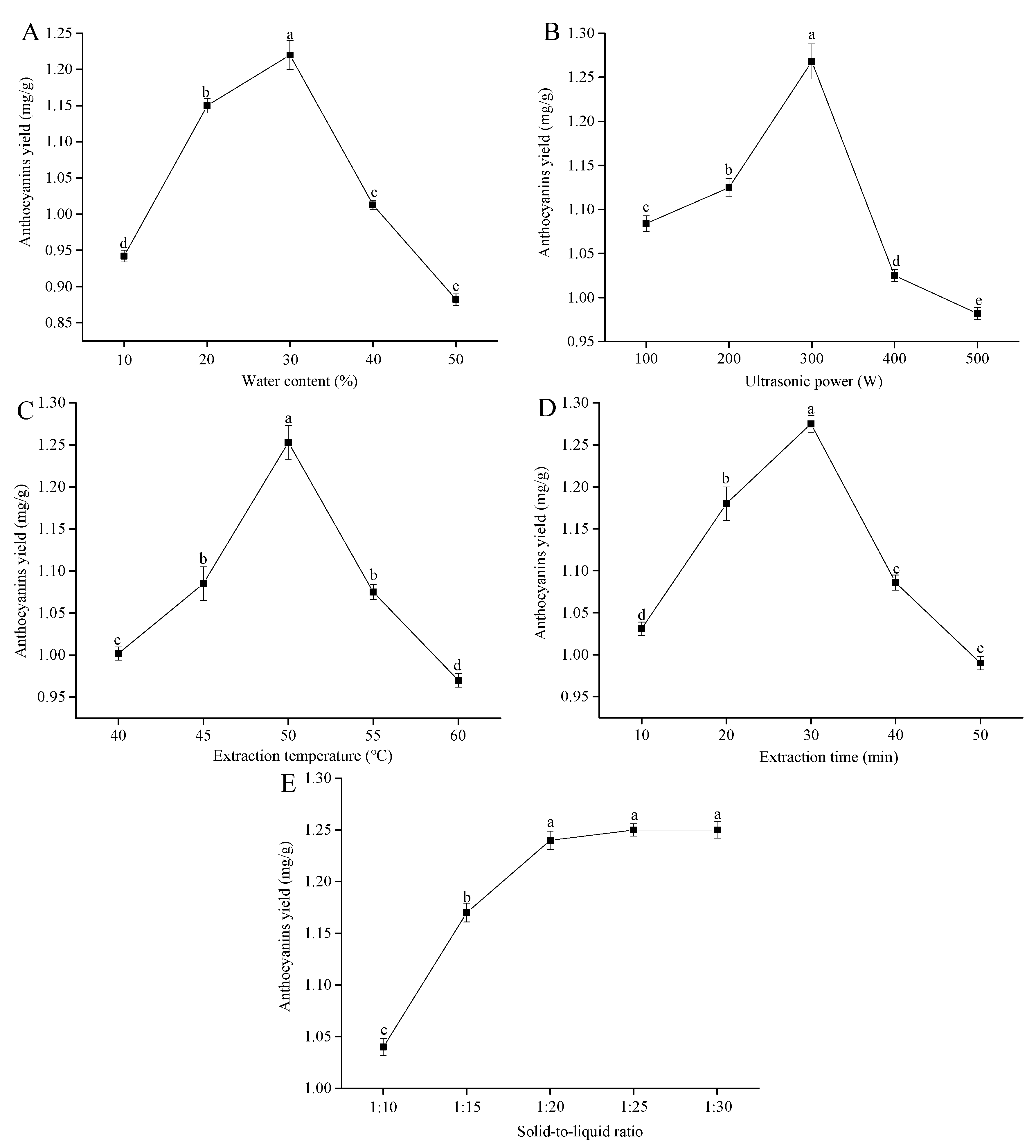
3.2. Single-Factor Experiments for Anthocyanin Extractions
3.3. Modeling of the Extraction Process
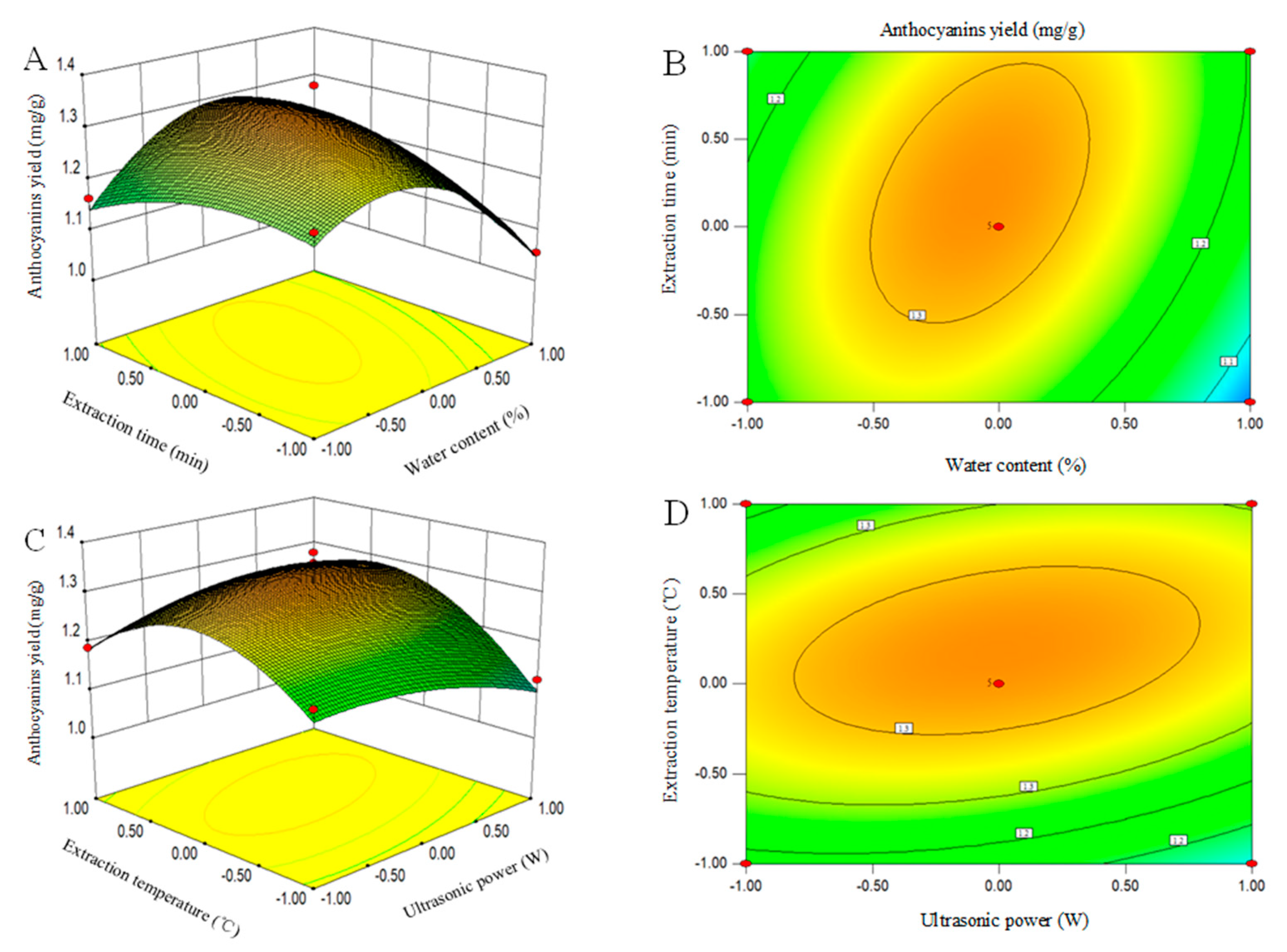
3.4. Interaction of Process Variables
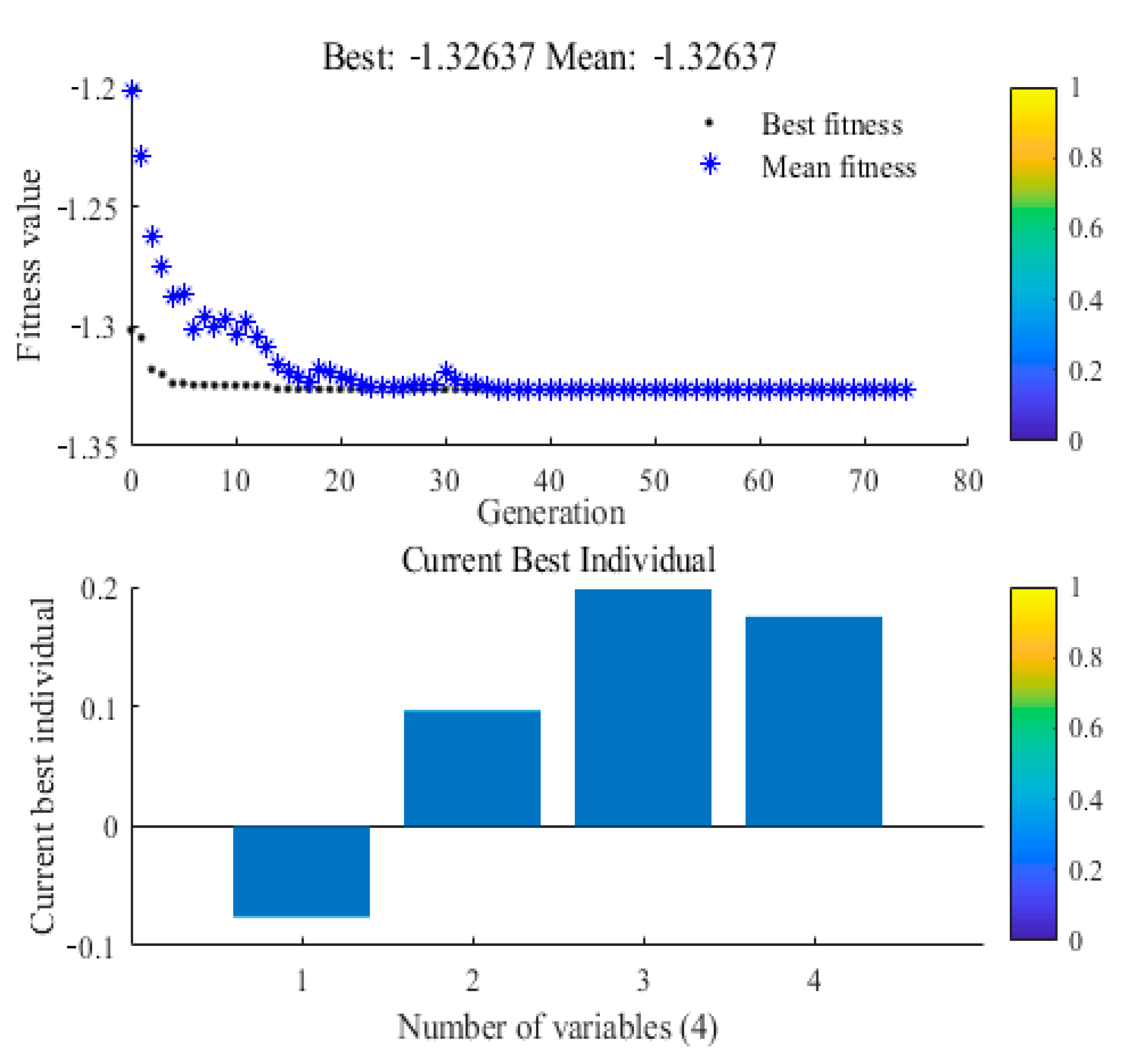
3.5. Optimal Values from the Genetic Algorithm and Confirmation
3.6. Selection of the Two-Phase Solvent System
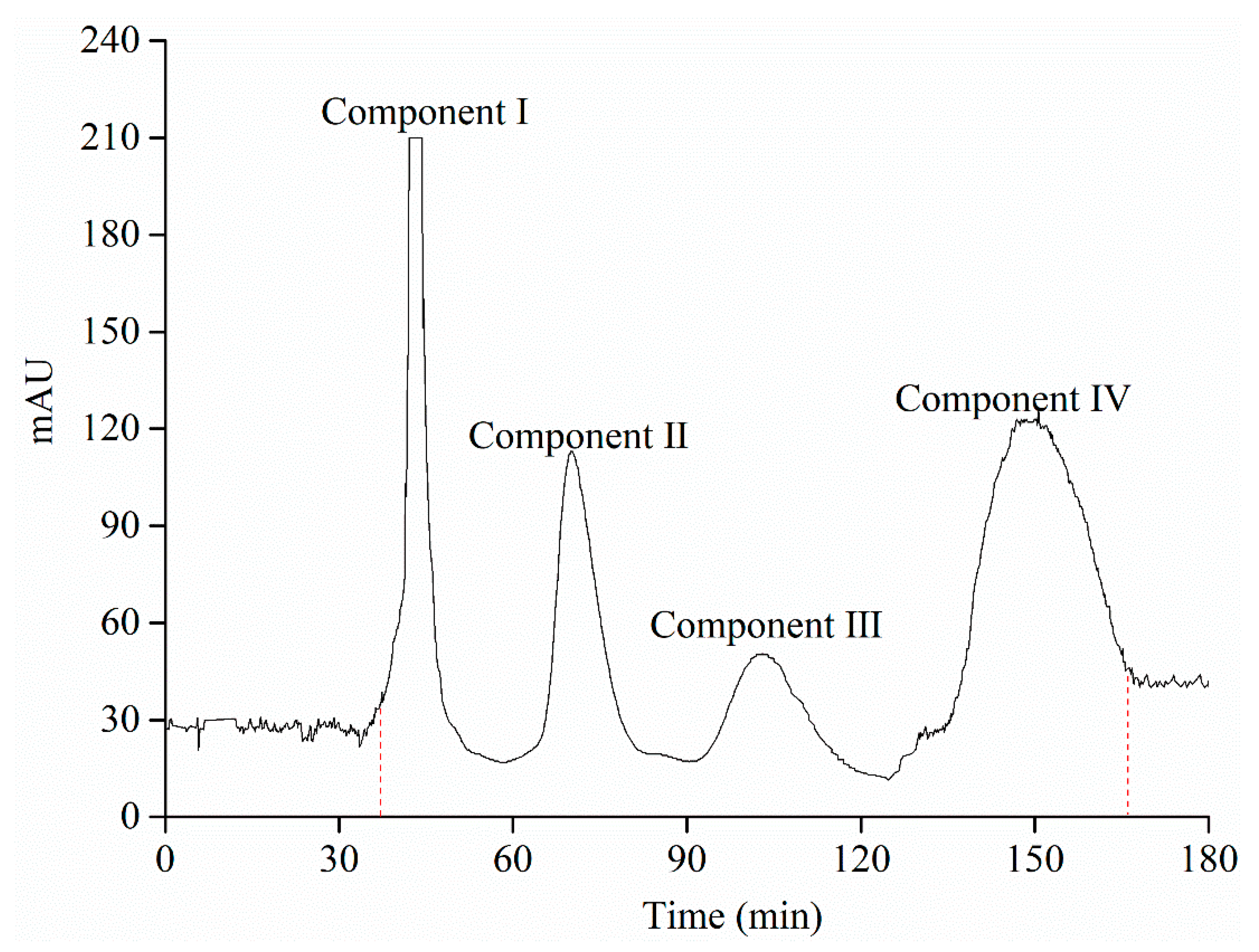
3.7. Isolation of the Purified Anthocyanin Extract Powders after AB-8 Macroporous Resin Column Chromatography by High-Speed Counter Current Chromatography
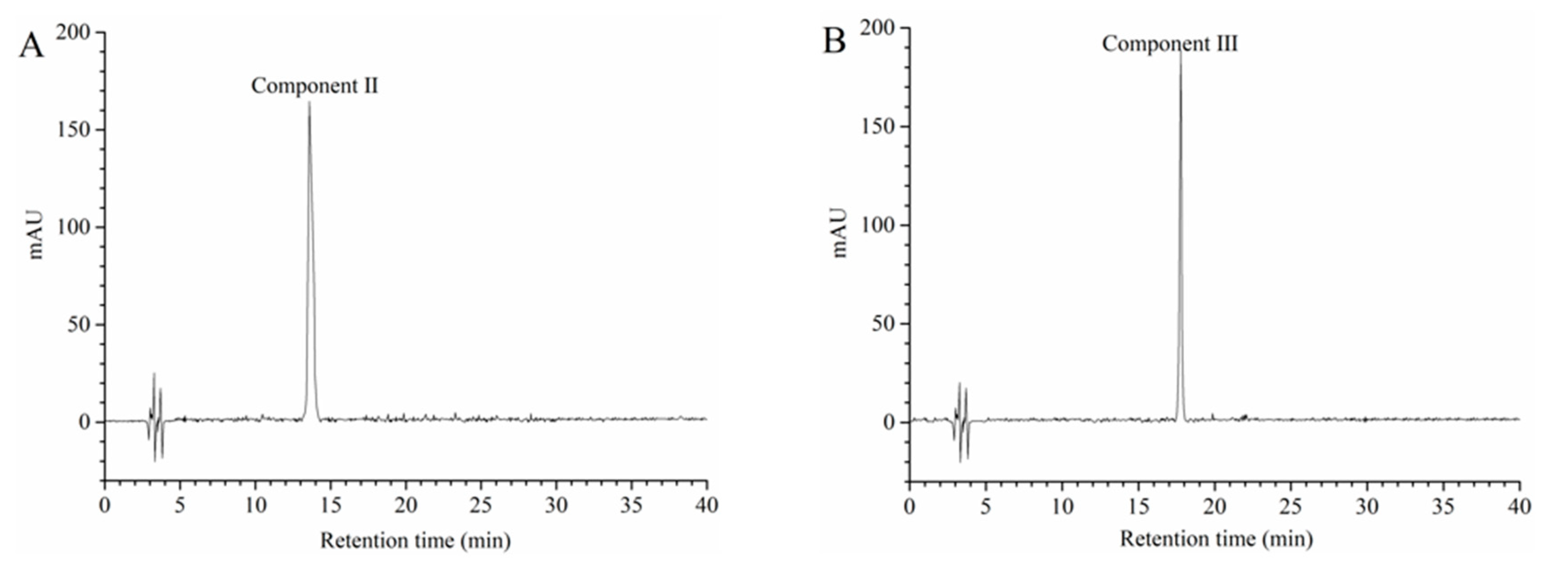
3.8. Identification of Separated Peaks
3.8.1. UV-Visible Spectrophotometer Analysis
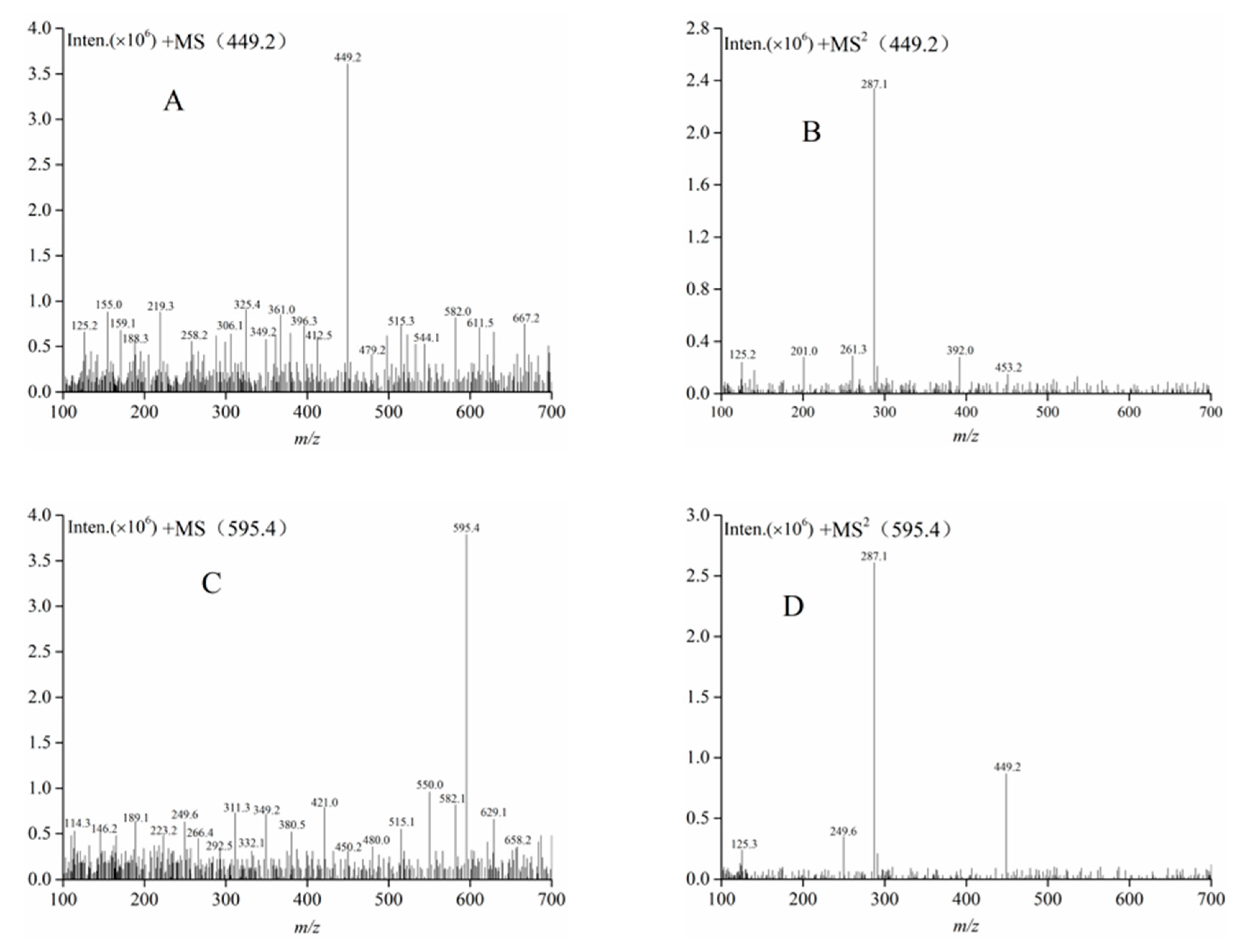
3.8.2. High-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry and Nuclear Magnetic Resonance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sette, P.; Franceschinis, L.; Schebor, C.; Salvatori, D. Fruit snacks from raspberries: Influence of drying parameters on colour degradation and bioactive potential. Int. J. Food Sci. Technol. 2017, 52, 313–328. [Google Scholar] [CrossRef]
- Chen, J.; Du, J.; Li, M.; Li, C. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT-Food Sci. Technol. 2020, 128, 109448. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Oancea, S.; Grosu, C.; Ketney, O.; Stoia, M. Conventional and ultrasound-assisted extraction of anthocyanins from blackberry and sweet cherry cultivars. Acta Chim. Slov. 2013, 60, 383–389. [Google Scholar] [PubMed]
- Vázquez-González, M.; Fernández-Prior, Á.; Oria, A.B.; Rodríguez-Juan, E.M.; Pérez-Rubio, A.G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Utilization of strawberry and raspberry waste for the extraction of bioactive compounds by deep eutectic solvents. LWT-Food Sci. Technol. 2020, 130, 109645. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Z.; Ma, S.; Rasool, M.A.; Wang, L.; Zhang, J. Optimization of ultrasonic-assisted extraction, refinement and characterization of water-soluble polysaccharide from Dictyosphaerium sp. and evaluation of antioxidant activity in vitro. J. Food Meas. Charact. 2020, 14, 963–977. [Google Scholar] [CrossRef]
- Jiménez, N.; Bassama, J.; Bohuon, P. Estimation of the kinetic parameters of anthocyanins degradation at different water activities during treatments at high temperature (100–140 °C) using an unsteady-state 3D model. J. Food Eng. 2020, 279, 109951. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; Lin, L. Effects of extraction methods on structural characteristics and bile acid-binding capacities of Moringa oleifera leaf polysaccharide fractions. Int. J. Food Sci. Technol. 2020, 55, 1539–1546. [Google Scholar] [CrossRef]
- Hadidi, M.; Ibarz, A.; Pagan, J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. 2020, 309, 125786. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.P.D.F.; Sumere, B.R.; Mekaru, C.; Martinez, J.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of polyphenols and antioxidants from pomegranate peel using ultrasound: Influence of temperature, frequency and operation mode. Int. J. Food Sci. Technol. 2019, 54, 2792–2801. [Google Scholar] [CrossRef]
- Kurapati, V.B.; Kommineni, R.; Sundarrajan, S. Statistical analysis and mathematical modeling of dry sliding wear parameters of 2024 aluminium hybrid composites reinforced with fly ash and Sic particles. Trans. Indian. Inst. Met. 2018, 71, 1809–1825. [Google Scholar] [CrossRef]
- Rezazi, S.; Hanini, S.; Si-Moussa, C.; Abdelmalek, S. Modeling and optimization of the operating conditions of Marrubium vulgare L. essential oil extraction process: Kinetic parameters estimation through genetic algorithms. J. Essent. Oil Bear. Plants 2016, 19, 843–853. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, Y.; Wu, Y.; Jia, Q.; Gao, G.; Nie, F. Enzyme-assisted solvent extraction for extraction of blueberry anthocyanins and separation using resin adsorption combined with extraction technologies. Int. J. Food Sci. Technol. 2016, 51, 2567–2573. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef]
- Zou, H.; Ma, Y.; Xu, Z.; Liao, X.; Chen, A.; Yang, S. Isolation of strawberry anthocyanins using high-speed counter-current chromatography and the copigmentation with catechin or epicatechin by high pressure processing. Food Chem. 2018, 247, 81–88. [Google Scholar] [CrossRef]
- Chen, T.; Yang, X.; Wang, N.; Li, H.; Zhao, J.; Li, Y. Separation of six compounds including two n-butyrophenone isomers and two stibene isomers from Rheum tanguticum Maxim by recycling high speed counter-current chromatography and preparative high-performance liquid chromatography. IEEE J. Solid-State Circ. 2018, 41, 3660–3668. [Google Scholar] [CrossRef]
- Weisz, A.; Ridge, C.D.; Perez-Gonzalez, M.; Ito, Y. Separation using high-speed counter-current chromatography and identification of 1,3-bis(4-phenylazophenyl)triazene, an impurity in the color additive D&C Red No. 17 (Sudan III). J. Chromatogr. A 2019, 1594, 120–128. [Google Scholar]
- Ali, M.C.; Chen, J.; Zhang, H.; Li, Z.; Zhao, L.; Qiu, H. Effective extraction of flavonoids from Lycium barbarum L. fruits by deep eutectic solvents-based ultrasound-assisted extraction. Talanta 2019, 203, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Oktaviyanti, N.D.; Mun’im, A. Application and optimization of ultrasound-assisted deep eutectic solvent for the extraction of new skin-lightening cosmetic materials from Ixora javanica flower. Heliyon 2019, 5, e02950. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, K.; Liang, Y.; Liu, G.; Sang, J.; Li, C. Extraction optimization and purification of anthocyanins from Lycium ruthenicum Murr. and evaluation of tyrosinase inhibitory activity of the anthocyanins. J. Food Sci. 2020, 85, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, H.; Xu, R.; Zhang, X.; Zhang, W.; Du, M.; Fan, L. Simultaneous optimization of the acidified water extraction for total anthocyanin content, total phenolic content, and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L.) using response surface methodology. Food Sci. Nutr. 2019, 7, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Z.; Jin, C.; Mu, Y.; Liu, C.; Chen, Z.; Lin, Z. Purification characteristics and parameters optimization of anthocyanin extracted from blueberry. Int. J. Agric. Biol. Eng. 2015, 8, 135–144. [Google Scholar]
- Xue, H.; Li, P.; Zhong, X.; Liu, C.; Li, Q. Separation and purification of anthocyanins from mulberry fruit by high-speed counter-current chromatography and their antioxidant activity. Food Sci. 2020, 41, 96–104. [Google Scholar]
- Qiu, G.; Wang, D.; Song, X.; Deng, Y.; Zhao, Y. Degradation kinetics and anti-oxidant capacity of anthocyanins in air-impingement jet dried purple potato slices. Food Res. Int. 2018, 105, 121–128. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.E.; Jang, H.W.; Lim, T.G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Xue, H.; Shen, L.; Wang, X.; Liu, C.; Liu, C.; Liu, H.; Zheng, X. Isolation and purification of anthocyanin from blueberry using macroporous resin combined Sephadex LH-20 techniques. Food Sci. Technol. Res. 2019, 25, 29–38. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches Extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.T.; Pauletto, R.; da Cavalheiro, S.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Kauer, M.; Belova-Magri, V.; Cairós, C.; Linka, G.; Mettin, R. High-speed imaging of ultrasound driven cavitation bubbles in blind and through holes. Ultrason. Sonochem. 2018, 48, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H.; Luo, Z. Optimization model for ultrasonic-assisted and scale-up extraction of anthocyanins from Pyrus communis ‘Starkrimson’ fruit peel. Food Chem. 2019, 297, 124993. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Perioli, L.; Blasi, F.; Bastianini, M.; Chiesi, C.; Cossignani, L. Optimisation of phenol extraction from wine using layered double hydroxides and technological evaluation of the bioactive-rich powder. Int. J. Food Sci. Technol. 2017, 52, 2582–2588. [Google Scholar] [CrossRef]
- Quan, W.; He, W.; Lu, M.; Yuan, B.; Zeng, M.; Gao, D.; He, Z. Anthocyanin composition and storage degradation kinetics of anthocyanins-based natural food colourant from purple-fleshed sweet potato. Int. J. Food Sci. Technol. 2019, 54, 2529–2539. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of heat- and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef]
- Wathon, M.H.; Beaumont, N.; Benohoud, M.; Blackburn, R.S.; Rayner, C.M. Extraction of anthocyanins from Aronia melanocarpa skin waste as a sustainable source of natural colorants. Color Technol. 2019, 135, 5–16. [Google Scholar] [CrossRef]
- Ferarsa, S.; Zhang, W.; Moulai-Mostefa, N.; Ding, L.; Jaffrin, M.Y.; Grimi, N. Recovery of anthocyanins and other phenolic compounds from purple eggplant peels and pulps using ultrasonic-assisted extraction. Food Bioprod. Process. 2018, 109, 19–28. [Google Scholar] [CrossRef]
- Pariyarath, A.M.; Anal, A.K.; Sadiq, M.B. Optimization of ultrasonic assisted extraction (UAE) of anthocyanins from black glutinous rice and evaluation of their antioxidant properties. ETP Int. J. Food Eng. 2018, 4, 288–292. [Google Scholar] [CrossRef]
- Mazza, K.E.L.; Santiago, M.C.P.A.; do Nascimento, L.S.M.; Godoy, R.L.O.; Souza, E.F.; Brígida, A.I.S.; Tonon, R.V. Syrah grape skin valorisation using ultrasound-assisted extraction: Phenolic compounds recovery, antioxidant capacity and phenolic profile. Int. J. Food Sci. Technol. 2018, 54, 641–650. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Jambo, S.A.; Abdulla, R.; Marbawi, H.; Gansau, J.A. Response surface optimization of bioethanol production from third generation feedstock-Eucheuma cottonii. Renew. Energy. 2019, 132, 1–10. [Google Scholar] [CrossRef]
- Liu, P.; Li, W.; Hu, Z.; Qin, X.; Liu, G. Isolation, purification, identification, and stability of anthocyanins from Lycium ruthenicum Murr. LWT-Food Sci. Technol. 2020, 126, 109334. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Pacheco-Hernández, M.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Chen, F.; Sang, J.; Zhang, Y.; Sang, J. Development of a green two-dimensional HPLC-DAD/ESI-MS method for the determination of anthocyanins from Prunus cerasifera var. atropurpurea leaf and improvement of their stability in energy drinks. Int. J. Food Sci. Technol. 2018, 53, 1494–1502. [Google Scholar] [CrossRef]
- De Castro, V.C.; da Silva, P.H.A.; de Oliveira, E.B.; Desobry, S.; Humeau, C. Extraction, identification and enzymatic synthesis of acylated derivatives of anthocyanins from jaboticaba (Myrciaria cauliflora) fruits. Int. J. Food Sci. Technol. 2014, 49, 196–204. [Google Scholar] [CrossRef]
- Choi, S.J.; Choi, J.; Lee, C.U.; Yoon, S.H.; Bae, S.K.; Chin, Y.W. Rapid separation of cyanidin-3-glucoside and cyanidin-3-rutinoside from crude mulberry extract using high-performance countercurrent chromatography and establishment of a volumetric scale-up process. J. Sep. Sci. 2015, 38, 1828–1836. [Google Scholar] [CrossRef]
- Choi, S.J.; Choi, J.; Jeon, H.; Bae, S.K.; Ko, J.; Kim, J.; Yoon, K.D. Application of high-performance countercurrent chromatography for the isolation of steroidal saponins from Liriope plathyphylla. IEEE J. Solid-State Circ. 2015, 38, 18–24. [Google Scholar] [CrossRef] [PubMed]
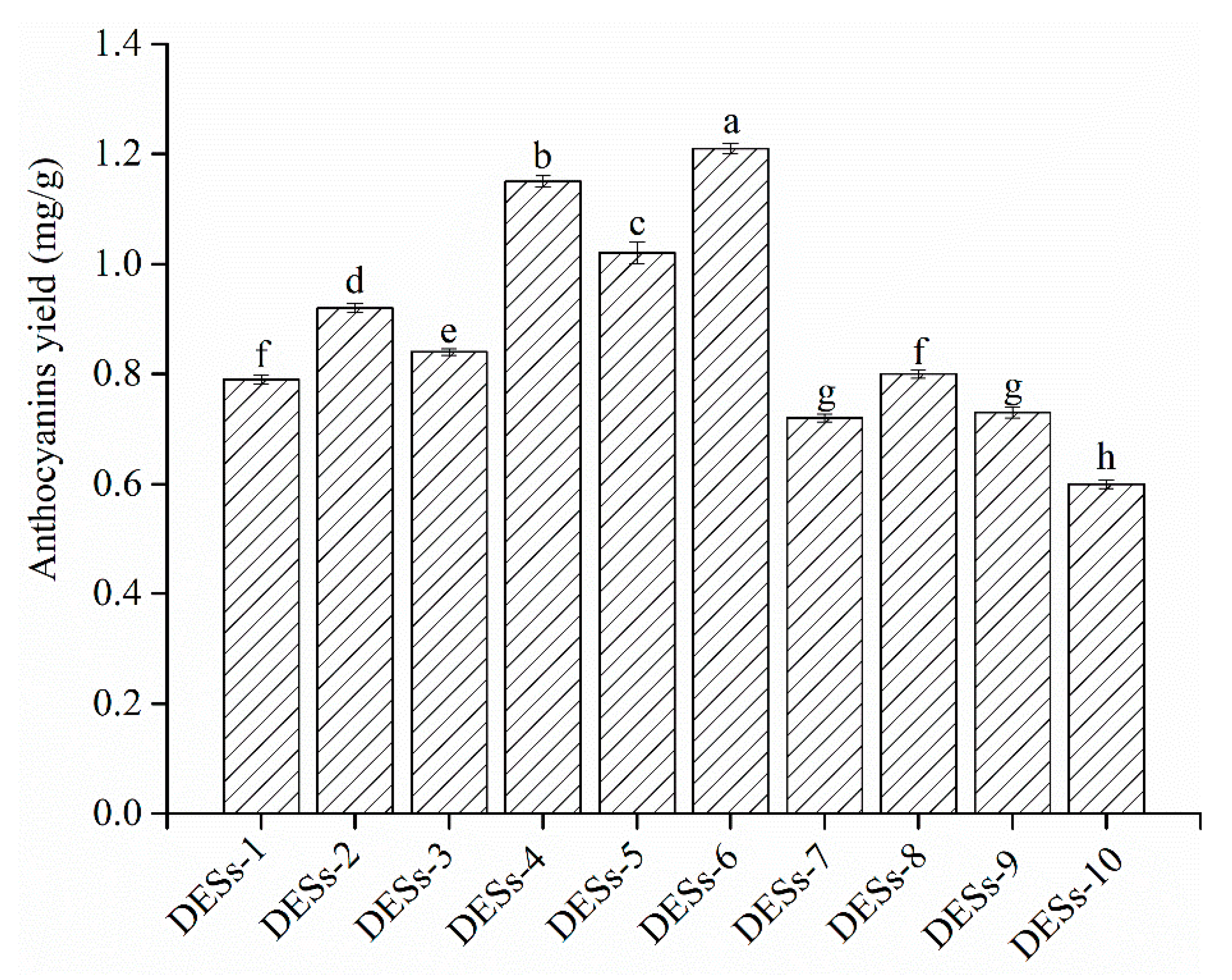

| NO. | HBA | HBD | Mole Ratio | DESs:Water (v/v) |
|---|---|---|---|---|
| DESs-1 | choline chloride | glycerol | 1:2 | 80:20 |
| DESs-2 | choline chloride | glycerol | 1:3 | 80:20 |
| DESs-3 | choline chloride | 1,3-butanediol | 1:2 | 80:20 |
| DESs-4 | choline chloride | 1,3-butanediol | 1:3 | 80:20 |
| DESs-5 | choline chloride | 1,4-butanediol | 1:2 | 80:20 |
| DESs-6 | choline chloride | 1,4-butanediol | 1:3 | 80:20 |
| DESs-7 | choline chloride | glycol | 1:2 | 80:20 |
| DESs-8 | choline chloride | glycol | 1:3 | 80:20 |
| DESs-9 | choline chloride | glucose | 1:1 | 80:20 |
| DESs-10 | choline chloride | glucose | 1:2 | 80:20 |
| Levels | Independent Variables | |||
|---|---|---|---|---|
| Water Content (X1)/% | Ultrasonic Power (X2)/W | Extraction Temperature (X3)/°C | Extraction Time (X4)/min | |
| −1 | 20 | 200 | 45 | 20 |
| 0 | 30 | 300 | 50 | 30 |
| 1 | 40 | 400 | 55 | 40 |
| No. | Variable | Anthocyanins Yield | |||
|---|---|---|---|---|---|
| X1 (%) | X2 (W) | X3 (°C) | X4 (min) | ||
| 1 | −1 | −1 | 0 | 0 | 1.189 |
| 2 | 1 | −1 | 0 | 0 | 1.148 |
| 3 | −1 | 1 | 0 | 0 | 1.124 |
| 4 | 1 | 1 | 0 | 0 | 1.121 |
| 5 | 0 | 0 | −1 | −1 | 1.107 |
| 6 | 0 | 0 | 1 | −1 | 1.189 |
| 7 | 0 | 0 | −1 | 1 | 1.134 |
| 8 | 0 | 0 | 1 | 1 | 1.204 |
| 9 | −1 | 0 | 0 | −1 | 1.239 |
| 10 | 1 | 0 | 0 | −1 | 1.056 |
| 11 | −1 | 0 | 0 | 1 | 1.164 |
| 12 | 1 | 0 | 0 | 1 | 1.205 |
| 13 | 0 | −1 | −1 | 0 | 1.208 |
| 14 | 0 | 1 | −1 | 0 | 1.123 |
| 15 | 0 | −1 | 1 | 0 | 1.189 |
| 16 | 0 | 1 | 1 | 0 | 1.254 |
| 17 | −1 | 0 | −1 | 0 | 1.088 |
| 18 | 1 | 0 | −1 | 0 | 1.009 |
| 19 | −1 | 0 | 1 | 0 | 1.167 |
| 20 | 1 | 0 | 1 | 0 | 1.098 |
| 21 | 0 | −1 | 0 | −1 | 1.172 |
| 22 | 0 | 1 | 0 | −1 | 1.229 |
| 23 | 0 | −1 | 0 | 1 | 1.278 |
| 24 | 0 | 1 | 0 | 1 | 1.248 |
| 25 | 0 | 0 | 0 | 0 | 1.310 |
| 26 | 0 | 0 | 0 | 0 | 1.380 |
| 27 | 0 | 0 | 0 | 0 | 1.299 |
| 28 | 0 | 0 | 0 | 0 | 1.302 |
| 29 | 0 | 0 | 0 | 0 | 1.301 |
| Source of Variance | SQ | df | MS | F-value | p-value |
|---|---|---|---|---|---|
| Model | 0.19 | 14 | 0.014 | 14.84 | <0.0001 ** |
| X1 | 9.926 × 10−0.003 | 1 | 9.926 × 10−0.003 | 10.13 | 0.0066 ** |
| X2 | 6.021 × 10−0.004 | 1 | 6.021 × 10−0.004 | 0.66 | 0.4314 |
| X3 | 0.016 | 1 | 0.016 | 16.95 | 0.0010 ** |
| X4 | 4.840 × 10−0.003 | 1 | 4.840 × 10−0.003 | 5.28 | 0.0376 * |
| X12 | 0.097 | 1 | 0.097 | 105.92 | <0.0001 ** |
| X22 | 9.375 × 10−0.003 | 1 | 9.375 × 10−0.003 | 10.22 | 0.0065 ** |
| X32 | 0.068 | 1 | 0.068 | 73.77 | <0.0001 ** |
| X42 | 0.013 | 1 | 0.013 | 14.49 | 0.0019 ** |
| X1X2 | 3.610 × 10−0.004 | 1 | 3.610 × 10−0.004 | 0.39 | 0.5405 |
| X1X3 | 2.500 × 10−0.005 | 1 | 2.500 × 10−0.005 | 0.027 | 0.8712 |
| X1X4 | 0.013 | 1 | 0.013 | 13.67 | 0.0024 ** |
| X2X3 | 5.625 × 10−0.003 | 1 | 5.625 × 10−0.003 | 6.13 | 0.0267 * |
| X2X4 | 1.892 × 10−0.003 | 1 | 1.892 × 10−0.003 | 2.06 | 0.1729 |
| X3X4 | 3.600 × 10−0.005 | 1 | 3.600 × 10−0.005 | 0.039 | 0.8458 |
| Residual | 0.013 | 14 | 9.173 × 10−0.004 | ||
| Lack of fit | 7.994 × 10−0.003 | 10 | 7.994 × 10−0.004 | 0.66 | 0.7302 |
| Pure error | 4.849 × 10−0.003 | 4 | 1.212 × 10−0.003 | ||
| Cor total | 0.20 | 28 | |||
| R2 = 0.9369 | R2adj = 0.8737 | C.V. = 0.7364 |
| Peaks | Retention Time(min) | Molecular ion (m/z) | Molecular Fragments (m/z) | Lost Fragments (m/z) | Compound |
|---|---|---|---|---|---|
| Component II | 13.58 | 449.2 | 287.1 | 162 | Cyanidin-3-glucoside |
| Component III | 21.69 | 595.4 | 449.2, 287.1 | 146, 162 | Cyanidin-3-rutinoside |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods 2020, 9, 1409. https://doi.org/10.3390/foods9101409
Xue H, Tan J, Li Q, Tang J, Cai X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods. 2020; 9(10):1409. https://doi.org/10.3390/foods9101409
Chicago/Turabian StyleXue, Hongkun, Jiaqi Tan, Qian Li, Jintian Tang, and Xu Cai. 2020. "Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm" Foods 9, no. 10: 1409. https://doi.org/10.3390/foods9101409
APA StyleXue, H., Tan, J., Li, Q., Tang, J., & Cai, X. (2020). Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods, 9(10), 1409. https://doi.org/10.3390/foods9101409