Influence of the Technological Process on the Biochemical Composition of Fresh Roe and Bottarga from Liza ramada and Mugil cephalus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Processing Step
2.3. Chemicals
2.4. Moisture and Ash
2.5. Metals and Metalloids
2.6. Analysis of the Lipid Fraction
2.6.1. Total Lipid
2.6.2. Squalene and Cholesterol
2.6.3. Fatty Acid
2.7. Analysis of Protein Fraction
2.7.1. Total Protein
2.7.2. Amino Acid Analysis
2.8. Geosmine and 2-Methylisoborneol
2.9. Statistical Analysis
3. Results
3.1. Physicochemical Analysis
3.2. Metals and Metalloids
3.3. Proteins and Amino Acids
3.4. Lipid Fraction
3.5. Geosmine and 2-Methyl Isoborneol
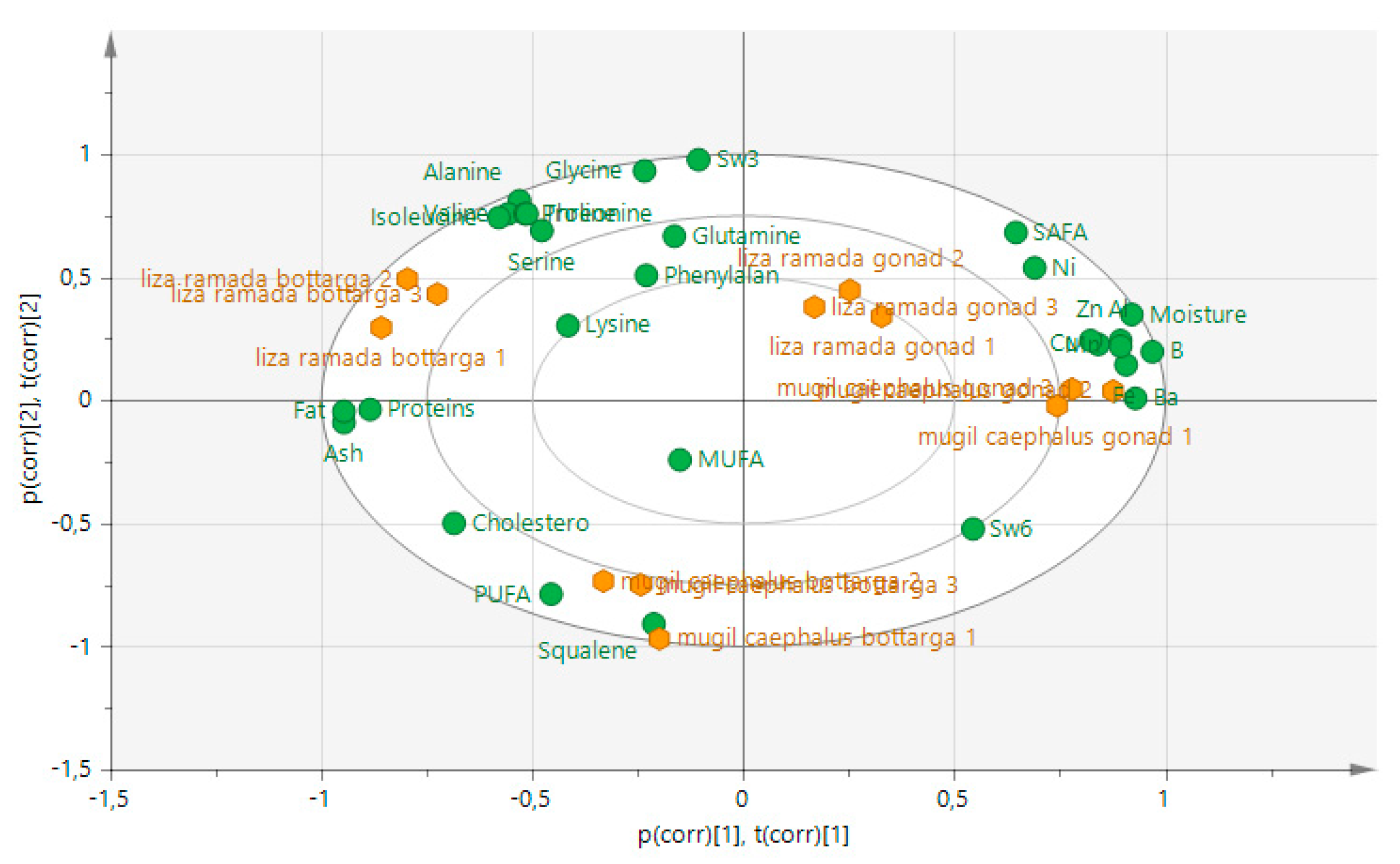
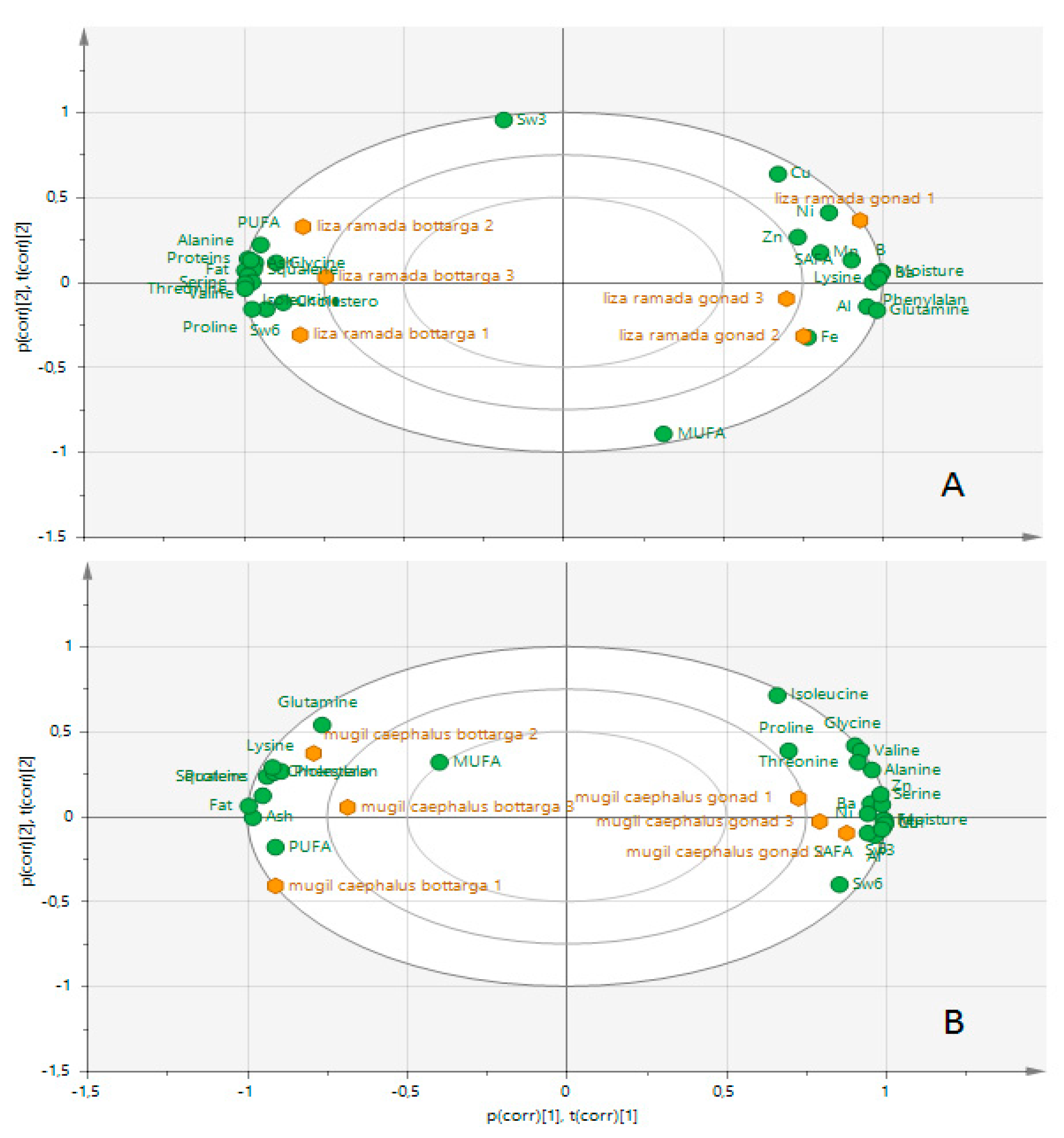
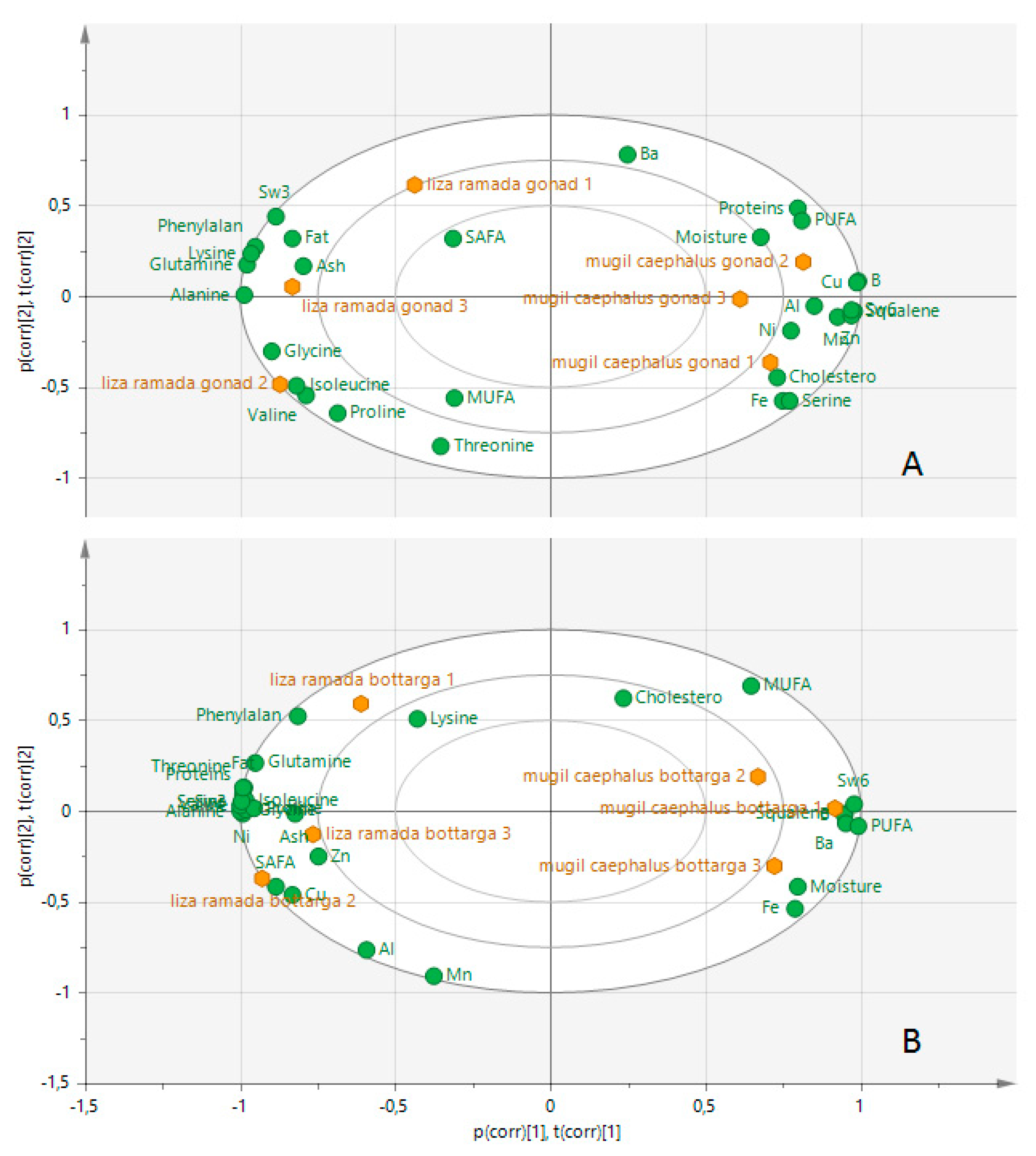
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crosetti, D.; Blaber, S.J.M. (Eds.) Biology, Ecology and Culture of Grey Mullets (Mugilidae); CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Saleh, M.A. Cultured Aquatic Species Information Programme; Mugil cephalus; FAO Fisheries and Aquaculture Department: Rome, Italy, 2006. [Google Scholar]
- Saleh, M. Capture-based aquaculture of mullets in Egypt. In Capture-Based Aquaculture, Technical Paper; A. Lovatelli, A., Holthus, P.F., Eds.; FAO Fisheries: Rome, Italy, 2008. [Google Scholar]
- Thomson, J.M. Mugelidae. In Check List of the Fishes of the Eastern Tropical Atlantic; Quero, J.C., Hureau, J.C., Post, C.A., Saldanha, L., Eds.; UNESCO: Paris, France, 1990. [Google Scholar]
- Bledsoe, G.E.; Bledsoe, C.D.; Rasco, B. Caviars and fish roe products. Crit. Rev. Food Sci. Nutr. 2003, 43, 317–356. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Atzeri, A.; Deiana, M.; Melis, M.P.; Ancani, A.; Loru, D.; Cabboi, B.; Dessì, M.A. Mullet bottarga as functional ingredient. Aliment. Funz. 2014, 6, 2–11. [Google Scholar]
- Brownlie, I. The Rule of Law in International Affairs: International Law at the Fiftieth Anniversary of the United Nations; Martinus Nijhoff Publishers: Leiden, Netherlands, 1998. [Google Scholar]
- Commission Implementing Regulation (EU). EU 2017/1925 of 12 October 2017, Amending Annex I to Counci Regulation (EEC) No 2658/87 on the Tariff and Statistical Nomenclature and on the Common Customs Tariff; Commission Implementing Regulation (EU): Brussels, Belgium, 2017. [Google Scholar]
- Food and Forestry Policies (MIPAAF). Update of the National List of Traditional Agri-Food Products Pursuant to Article 12, Paragraph 1, of the Law of 12 December 2016, n. 238; Ministry of Agricolture: Roma, Itay, 2018. [Google Scholar]
- Angioni, A.; Cau, A.; Addis, P. Gas chromatographic mass spectrometry determination of geosmin and 2-methylisoborneol off-Flavor in Mugil cephalus roe. Food Anal. Methods 2014, 8, 6. [Google Scholar] [CrossRef]
- FAO Fisheries and Aquaculture Department Rome. FAO Major Fishing Areas. Available online: http://www.fao.org/fishery/area/search/en (accessed on 4 April 2020).
- Barra, A.; Garau, V.L.; Dessi, S.; Sarais, G.; Cereti, E.; Arlorio, M.; Coisson, J.D.; Cabras, P. Chemical characterization and DNA tracking of Sardinian botargo by Mugil cephalus from different geographical origins. J. Agric. Food Chem. 2008, 56, 10847–10852. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Piras, C.; Mereu, S.; Cesare Marincola, F.; Scano, P. 1H NMR Metabolite fingerprint and pattern recognition of Mullet (Mugil cephalus) Bottarga. J. Agric. Food Chem. 2011, 59, 9497–9505. [Google Scholar] [CrossRef]
- Scano, P.; Rosa, A.; Locci, E.; Dessì, M.A.; Lai, A. NMR study of the lipid profile of mullet raw roe and bottarga. Eur. J. Lipid Sci. Technol. 2009, 11, 505–512. [Google Scholar] [CrossRef]
- Scano, P.; Rosa, A.; Locci, E.; Manzo, G.; Dessì, M.A. Modifications of the 1H NMR metabolite profile of processed mullet (Mugil cephalus) roes under different storage conditions. Magn. Reson. Chem. 2012, 50, 436–442. [Google Scholar] [CrossRef]
- Çelik, U.; Altınelataman, C.; Dinçer, T.; Acarlı, D. Comparison of Fresh and Dried Flathead Grey Mullet (Mugil cephalus, Linnaeus 1758) Caviar by means of Proximate Composition and Quality Changes during Refrigerated Storage at 4 ± 2 °C. Turk. J. Fish. Aquat. Sci. 2012, 12, 1–5. [Google Scholar] [CrossRef]
- Caredda, M.; Addis, M.; Pes, M.; Fois, N.; Sanna, G.; Piredda, G.; Sanna, G. Physico-chemical, colorimetric, rheological parameters and chemometric discrimination of the origin of Mugil cephalus’ roes during the manufacturing process of bottarga. Food Res. Int. 2008, 108, 128–135. [Google Scholar] [CrossRef]
- Murenu, M.; Olita, A.; Sabatini, A.; Follesa, M.C.; Cau, A. Dystrophy effects on the Liza ramada (Pisces, Mugilidae) population in the Cabras lagoon (Central-Western Sardinia). Chem. Ecol. 2004, 20, 425–433. [Google Scholar] [CrossRef]
- Cataudella, S.; Crosetti, D.; Massa, F. Mediterranean coastal lagoons: Sustainable management and interactions among aquaculture, capture fisheries and the environments. Int. Stud. Rev. 2015, 95, 1–294. [Google Scholar]
- Padedda, B.M.; Pulina, S.; Magni, P.; Sechia, N.; Luglie, A. Phytoplankton dynamics in relation to environmental changes in a phytoplankton-dominated Mediterranean lagoon (Cabras Lagoon, Italy). Adv. Oceanogr. Limnol. 2012, 3, 147–169. [Google Scholar] [CrossRef]
- World Organization of Animal Health. Welfare Aspects of Stunning and Killing of Farmed Fish for Human Consumption. Available online: https://www.oie.int/en/standard-setting/aquatic-code/access-online/ (accessed on 20 September 2020).
- Corrias, F.; Atzei, A.; Addis, P.; Secci, M.; Russo, M.; Angioni, A. Integrated environmental evaluation of heavy metals and metalloids bioaccumulation in invertebrates and seaweeds from different marine coastal areas of Sardinia, Mediterranean Sea. Environ. Pollut. 2020, 266, 115048. [Google Scholar] [CrossRef] [PubMed]
- Beach, L.M. Determination of As, Sb and Se in Difficult Environmental Samples by Hydride Generation. Available online: https://www.agilent.com/cs/library/applications/aa105.pdf (accessed on 3 October 2020).
- Evans, S.J.; Johnson, M.S.; Leah, R.T. Determination of Mercury in Fish Tissue, a Rapid, Automated Technique for Routine Analysis. Available online: https://www.agilent.com/cs/library/applications/AA060.pdf (accessed on 3 October 2020).
- Wenzl, T.; Haedrich, J.; Schaechtele, A.; Robouch, P.; Stroka, J. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food. EUR 28099; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Srigley, C.T.; Mossoba, M.M. Current Analytical Techniques for Food Lipids. In Food Safety: Innovative Analytical Tools for Safety Assessment; Spizzirri, U.G., Cirillo, G., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2017. [Google Scholar]
- Atherton, H.J.; Bailey, N.J.; Zhang, W.; Taylor, J.; Major, H.; Shockcor, J.; Clarke, K.; Griffin, J.L. A combined 1H NMR spectroscopy and mass spectrometry-based metabolomic study of the PPAR-null mutant mouse defines profound systemic changes in metabolism linked to the metabolic syndrome. Physiol. Genomics 2006, 27, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Pigott, G.M.; Tuker, B.W. Fish oils. In Encyclopaedia of Food Sciences and Nutrition, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 4, pp. 2491–2501. [Google Scholar]
- Chomérat, N.; Garnier, R.; Bertrand, C.; Cazaubon, A. Seasonal succession of cyanoprokaryotes in a hyperetrophic oligo-mesohaline lagoon from the South of France. Estuar. Coast. Shelf Sci. 2007, 72, 591–602. [Google Scholar] [CrossRef]
- Pulina, S.; Padedda, B.M. The dominance of cyanobacteria in Mediterranean hypereutrophic lagoons: A case study of Cabras Lagoon (Sardinia, Italy). Sci. Mar. 2011, 75, 111–120. [Google Scholar] [CrossRef]
- Scheffer, M.; Rinaldi, S.; Gragnani, A.; Mur, L.R.; Van Nes, E.H. On the dominance of filamentous cyanobacteria in shallow. Turbid Lakes. Environ. 2007, 78, 272–282. [Google Scholar]
- Sorokin, Y.I.; Sorokin, P.Y.; Gnes, A. Structure and functioning of antropogenically transformed Comacchio lagoon ecosystem (Ferrara, Italy). Mar. Ecol. Prog. Ser. 1996, 133, 57–71. [Google Scholar] [CrossRef]
- Hafez, N.E.; Awad, A.M.; Ibrahim, S.M.; Mohamed, H.R.; El-Lahamy, A.A. Effect of Salting Process on Fish Quality. Nutr. Food Process. 2019, 2. [Google Scholar] [CrossRef]
- Lu, J.Y.; Ma, Y.M.; Williams, C.; Chung, R.A. Fatty and amino acid composition of salted mullet roe. J. Food Sci. 1979, 44, 676–677. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Nomikos, T.; Chiou, A.; Fragopoulou, E.; Antonopoulou, S. Chemical composition of Greek Avgotaracho prepared from mullet (Mugil cephalus): Nutritional and health benefits. J. Agric. Food Chem. 2008, 56, 5916–5925. [Google Scholar] [CrossRef] [PubMed]
- Scano, P.; Rosa, A.; Cesare Marincola, F.; Locci, E.; Melis, M.P.; Dessì, M.A.; Lai, A. 13C NMR, GC and HPLC characterization of lipid components of the salted and dried mullet (Mugil cephalus) roe “bottarga”. Chem. Phys. Lipids 2008, 151, 69–76. [Google Scholar] [CrossRef]
- Gallagher, M.L.; McLeod, S.H.; Rulifson, R. Seasonal variations in fatty acids of striped bass Morone saxatilis. J. World Aquac. Soc. 1989, 20, 38–45. [Google Scholar] [CrossRef]
- Wirth, M.; Kirschbaum, F.; Gessner, J.; Williot, P.; Patriche, N.; Billard, R. Fatty acid composition in sturgeon caviar from different species: Comparing wild and farmed origins. Int. Rev. Hydrobiol. 2002, 87, 629–636. [Google Scholar] [CrossRef]
- Porarinsdottir, K.A. The Influence of Salting Procedures on the Characteristics of Heavy Salted Cod. Ph.D. Thesis, Lund University, Lund, Sweden, 2010. [Google Scholar]
- Amir, R.M.; Randhawa, M.A.; Sajid, M.W.; Nadeem, M.; Ahmad, A.; Watto, F.M. Evaluation of various soaking agents as a novel tool for heavy metal residues mitigation from spinach. Food Sci. Technol. 2019, 39, 76–180. [Google Scholar] [CrossRef]
- Oluk, C.A.; Karaca, O.B. Functional food ingredients and nutraceuticals, milk proteins as nutraceuticals nanoscience and food industry. In Nutraceuticals, Nanotechnology in the Agri-Food Industry; Grumezescu, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 4. [Google Scholar]
- Shirai, N.; Higuchi, T.; Suzuki, H. Analysis of lipid classes and the fatty acid composition of the salted fish roe food products, Ikura, Tarako, Tobiko, and Kazunoko. Food Chem. 2006, 94, 61–67. [Google Scholar] [CrossRef]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstate, M.; Das, A. Anti-inflammatory ω-3 endocannabinoid epoxides. PNAS Early Ed. 2017, 114, E6034–E6043. [Google Scholar] [CrossRef]
- WHO. Protein and Amino Acid Requirements in Human Nutrition. In WHO Technical Report Series 935. Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2002. [Google Scholar]
| Species | Sample | Humidity (%) g/100 g FW | Ash g/100 g FW | Total Protein g/100 g FW | Total Lipid g/100 g FW | Squalene mg/100 g | Cholesterol mg/100 g |
|---|---|---|---|---|---|---|---|
| L. ramada | fresh | 47.6 a | 5.9 a | 24.5 a | 2.7 a | 11.3 a | 474.7 a |
| bottarga | 13.6 b | 8.6 b | 24.7 a | 11.3 b | 24.6 b | 621.0 b | |
| M. cephalus | fresh | 50.7 a | 4.7 a | 26.4 a | 2.5 a | 49.2 c | 648.9 b |
| bottarga | 24.9 c | 7.2 b | 21.3 a | 7.5 c | 68.2 d | 722.0 c |
| LOQ | ||||
|---|---|---|---|---|
| Metal | λ | (µg/kg) | Linear Regression Equation | r2 |
| Al | 396.15 | 0.05 | y = 670.89x − 45.48 | 0.9978 |
| As | 193.69 | 0.05 | y = 79.38x − 10.14 | 0.9991 |
| B | 249.67 | 0.025 | y = 5151.33x + 789.8 | 0.9995 |
| Ba | 455.40 | 0.05 | y = 8035x − 289.9 | 0.9993 |
| Be | 313.10 | 0.001 | y = 259,128x − 6848 | 0.9985 |
| Cd | 214.43 | 0.005 | y = 4804x − 3.55 | 0.9995 |
| Co | 238.89 | 0.01 | y = 6622.7x + 58.6 | 0.9997 |
| Cr | 205.56 | 0.025 | y = 9908.7x + 97.7 | 0.9994 |
| Cu | 324.75 | 0.01 | y = 17,117.2x − 95.93 | 0.9996 |
| Fe | 238.20 | 0.10 | y = 3059.9x + 80.8 | 0.9992 |
| Hg | 194.16 | 0.10 | y = 392.2x + 11 | 0.9994 |
| Mn | 257.61 | 0.005 | y = 70,780.6x − 8029 | 0.9990 |
| Mo | 204.59 | 0.05 | y = 604.4x − 5.5 | 0.9988 |
| Ni | 231.60 | 0.025 | y = 1642.2x − 22.3 | 0.9986 |
| Pb | 220.35 | 0.10 | y = 297.3x − 19.4 | 0.9967 |
| Sb | 206.83 | 0.10 | y = 49.7x − 5.8 | 0.9981 |
| Sn | 189.92 | 0.10 | y = 74.3x − 4.9 | 0.9986 |
| Sr | 407.71 | 0.01 | y = 829,945.3x + 17943 | 0.9984 |
| Te | 214.28 | 0.50 | y = 106.6x − 10.1 | 0.9972 |
| Ti | 336.12 | 0.10 | y = 14,275x + 521.8 | 0.9976 |
| V | 292.40 | 0.005 | y = 13,753.2x + 231.2 | 0.9996 |
| Zn | 213.85 | 0.10 | y = 4785.9x − 316 | 0.9986 |
| Liza ramada | Mugil cephalus | |||||
|---|---|---|---|---|---|---|
| Metal | Fresh | Bottarga | Decrease | Fresh | Bottarga | Decrease |
| μg/kg {\displaystyle \sigma} | % | μg/kg {\displaystyle \sigma} | % {\displaystyle \sigma} | |||
| Al | 88.2 a | 26.2 b | 70.3 | 121.4 a | 27.9 b | 77.0 |
| As | <LOQ | <LOQ | <LOQ | <LOQ | ||
| B | 47.1 a | 14.9 b | 68.4 | 67.5 a | 19.3 b | 71.4 |
| Ba | 5.4 a | 1.8 b | 66.7 | 4.8 a | 1.8 b | 62.5 |
| Be | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Cd | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Co | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Cr | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Cu | 17.3 c | 10.9 c | 37.0 | 43.6 a | 28.3 b | 35.1 |
| Fe | 206.9 b | 98.2 | 52.5 | 358.4 a | 159.3 b | 55.6 |
| Hg | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Mn | 11.4 a | 4.6 c | 59.6 | 18.4 a | 7.2 b | 60.9 |
| Mo | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Ni | 4.1 b | 2.6 c | 36.6 | 7.2 a | 4.6 b | 36.1 |
| Pb | <LOQ | 8.4 b | <LOQ | 14.1 a | ||
| Sb | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Sn | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Sr | 5.3 c | 1.9 b | 64.2 | 7.4 a | 2.9 b | 60.8 |
| Te | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Ti | <LOQ | <LOQ | <LOQ | <LOQ | ||
| V | <LOQ | <LOQ | <LOQ | <LOQ | ||
| Zn | 729.2 b | 441.0 c | 39.5 | 1143.5 a | 635.9 b | 44.4 |
| Aminoacid | Liza ramada | Mugil cephalus | ||
|---|---|---|---|---|
| Fresh | Bottarga | Fresh | Bottarga | |
| Alanine | 8.8 a* | 13.1 b | 7.3 a | 11.4 b |
| Glycine | 7.2 a | 8.4 a | 6.6 a | 12.8 b |
| Valine e | 6.3 a | 11.2 b | 5.6 a | 9.4 b |
| Isoleucine e | 5.1 a | 8.3 b | 4.3 a | 6.6 a |
| Proline | 5.2 a | 7.8 a | 4.7 a | 5.1 a |
| Serine | 7.4 a | 16.3 b | 9.3 a | 14.7 b |
| Threonine e | 6.4 a | 11.7 b | 6.3 a | 10.2 b |
| Glutamine | 25.6 a | 13.6 b | 20.1 a | 12.3 b |
| Phenylalanine e | 9.1 a | 5.2 b | 7.5 a | 6.3 a |
| Lysine e | 6.6 a | 4.0 b | 6.2 a | 7.1 a |
| Tyrosine e | 0.2 a | 1.2 b | ||
| TAA | 87.70 ± 3.31 | 99.80 ± 0.02 | 47.48 ± 0.54 | 38.78 ± 3.64 |
| EAA | 33.47 ± 2.35 | 40.46 ± 1.01 | 17.80 ± 0.11 | 17.11 ± 2.30 |
| EAA/TAA% | 38.17 ± 0.97 | 40.54 ± 0.99 | 37.24 ± 0.66 | 44.14 ± 3.00 |
| Fatty Acid | Liza ramada | Mugil cephalus | ||
|---|---|---|---|---|
| Fresh | Bottarga | Fresh | Bottarga | |
| anteiso C14 | 0.03 a* | 0.04 a | 0.03 a | 0.03 a |
| C14:0 | 3.55 b | 1.99 a | 2.73 b | 1.60 a |
| anteiso C15:0 | 0.18 b | 0.08 a | 0.14 b | 0.09 a |
| C15:0 + isomer | 2.04 c | 1.37 a | 1.64 b | 1.50 b |
| anteiso C16 | 1.28 b | 1.93 c | 1.83 c | 0.04 a |
| C16:0 | 12.60 b | 12.84 b | 9.60 a | 9.16 a |
| C17:0 | 0.42 b | 0.74 c | 0.25 a | 0.57 b |
| C18:0 | 1.76 b | 1.70 b | 1.01 a | 1.46 b |
| anteiso C19 | 0.05 | 0.04 a | 0.06 a | 0.05 |
| C20:0 | 0.09 a | 0.14 b | ||
| C14:1 + isomer | 0.11 a | 0.03 a | 0.38 b | 0.08 a |
| C15:1 + isomer | 0.72 b | 0.33 a | 0.77 b | 0.51 a |
| C16:1 + isomer | 30.45 b | 27.18 a | 30.09 b | 24.85 a |
| C16:2 | 0.32 a | 0.42 a | 0.40 a | |
| C16:1 n9 7 methyl | 0.15 a | 0.22 b | 0.12 a | 0.13 a |
| C17:1 | 1.68 a | 2.81 b | 1.57 a | 3.00 b |
| C18:1c | 16.32 b | 12.74 a | 16.06 b | 13.01 a |
| C18:1 Δ11 | 5.30 a | 10.33 b | 5.29 a | 11.42 b |
| C20:1 | 0.13 a | 0.13 a | 0.56 b | |
| C20:1n7 | 0.13 a | 0.32 b | 0.22 b | 0.10 a |
| C20:1n4 | 0.23 b | 0.17 a | 0.26 b | |
| C17:2 | 1.36 b | 0.80 a | 1.18 b | 2.68 c |
| C18:2 | 0.55 a | 2.65 c | 1.75 b | |
| C18:2n6t | 0.17 a | 0.42 b | 1.44 c | 1.76 c |
| C18:2 n6c | 1.17 c | 0.75 b | 0.20 a | 1.34 c |
| C18:2 | 0.66 b | 0.52 b | 1.27 c | 0.17 a |
| C18:3 | 0.22 a | 0.32 a | 0.70 b | 0.26 a |
| γ C18:3n6 | 0.34 a | 0.47 ab | 0.20 a | 0.53 b |
| αC18:3 | 1.16 c | 0.81 bc | 0.43 a | 0.55 b |
| unknow | 1.98 b | 1.74 a | 2.03 b | |
| C18:4n3 | 1.20 b | 0.60 a | 3.94 c | 4.28 c |
| CLA C18:2 | 1.21 b | 0.69 a | 1.11 b | 1.61 b |
| CLA C18:2 | 0.80 a | 2.59 c | 1.71 b | 2.57 c |
| C20:2 + isomer | 0.17 a | 0.23 b | 1.56 b | 0.15 a |
| C20:3 | 0.21 a | 2.36 b | 0.14 a | 0.25 a |
| C20:4 | 0.44 a | 1.06 b | 0.45 a | 1.35 b |
| C20:3n3 (11-14-17) | 0.07 a | 0.10 a | 0.08 a | 0.13 a |
| C20:4n3 | 0.74 a | 2.77 b | 0.90 a | 0.95 a |
| C20:5 EPA | 3.00 b | 1.41 a | 3.71 b | 2.19 ab |
| C20:3n9 | 0.11 a | 0.07 a | ||
| C21:4 | 0.36 b | 0.17 a | 0.42 b | 0.12 a |
| C21:5n3 | 0.36 b | 0.15 a | 0.23 a | 0.31 ab |
| C22:5 | 1.83 a | 2.12 b | 1.92 ab | |
| C22:6 DHA | 4.37 b | 2.00 a | 3.50 b | |
| Liza ramada | Mugil cephalus | |||
|---|---|---|---|---|
| Fresh | Bottarga | Fresh | Bottarga | |
| Σsaturated | 21.98 b | 20.84 b | 17.34 ab | 14.58 a |
| Σmonounsaturated | 55.55 a | 54.44 a | 54.80 a | 56.07 a |
| Σpolyunsaturated | 22.47 a | 24.72 ab | 26.21 bc | 29.36 c |
| PUFA/SAFA | 1.02 a | 1.59 | 1.19 a | 2.03 |
| Σω3 (%) | 14.01 c | 14.24 c | 9.95 b | 6.44 a |
| Σω6 (%) | 2.17 a | 3.28 a | 8.56 c | 6.07 b |
| ω3/ω6 | 6.44 b | 4.41 b | 1.17 a | 1.12 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrias, F.; Atzei, A.; Giglioli, A.; Pasquini, V.; Cau, A.; Addis, P.; Sarais, G.; Angioni, A. Influence of the Technological Process on the Biochemical Composition of Fresh Roe and Bottarga from Liza ramada and Mugil cephalus. Foods 2020, 9, 1408. https://doi.org/10.3390/foods9101408
Corrias F, Atzei A, Giglioli A, Pasquini V, Cau A, Addis P, Sarais G, Angioni A. Influence of the Technological Process on the Biochemical Composition of Fresh Roe and Bottarga from Liza ramada and Mugil cephalus. Foods. 2020; 9(10):1408. https://doi.org/10.3390/foods9101408
Chicago/Turabian StyleCorrias, Francesco, Alessandro Atzei, Angelica Giglioli, Viviana Pasquini, Alessandro Cau, Piero Addis, Giorga Sarais, and Alberto Angioni. 2020. "Influence of the Technological Process on the Biochemical Composition of Fresh Roe and Bottarga from Liza ramada and Mugil cephalus" Foods 9, no. 10: 1408. https://doi.org/10.3390/foods9101408
APA StyleCorrias, F., Atzei, A., Giglioli, A., Pasquini, V., Cau, A., Addis, P., Sarais, G., & Angioni, A. (2020). Influence of the Technological Process on the Biochemical Composition of Fresh Roe and Bottarga from Liza ramada and Mugil cephalus. Foods, 9(10), 1408. https://doi.org/10.3390/foods9101408









