Interface Compositions as Determinants of Resveratrol Stability in Nanoemulsion Delivery Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bovine and Caprine Caseins
2.3. Preparation of Caprine β-Casein
2.4. Fluorescence Spectroscopy
2.5. Circular Dichroism (CD) Spectroscopy
2.6. Resveratrol Binding to Bovine and Caprine Caseins in Solution
2.7. Preparation of Resveratrol-Loaded Nanoemulsions
2.8. Physical Characterization of Resveratrol-Loaded Nanoemulsions
2.9. Quantification of Resveratrol in Nanoemulsions
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Bovine and Caprine Caseins
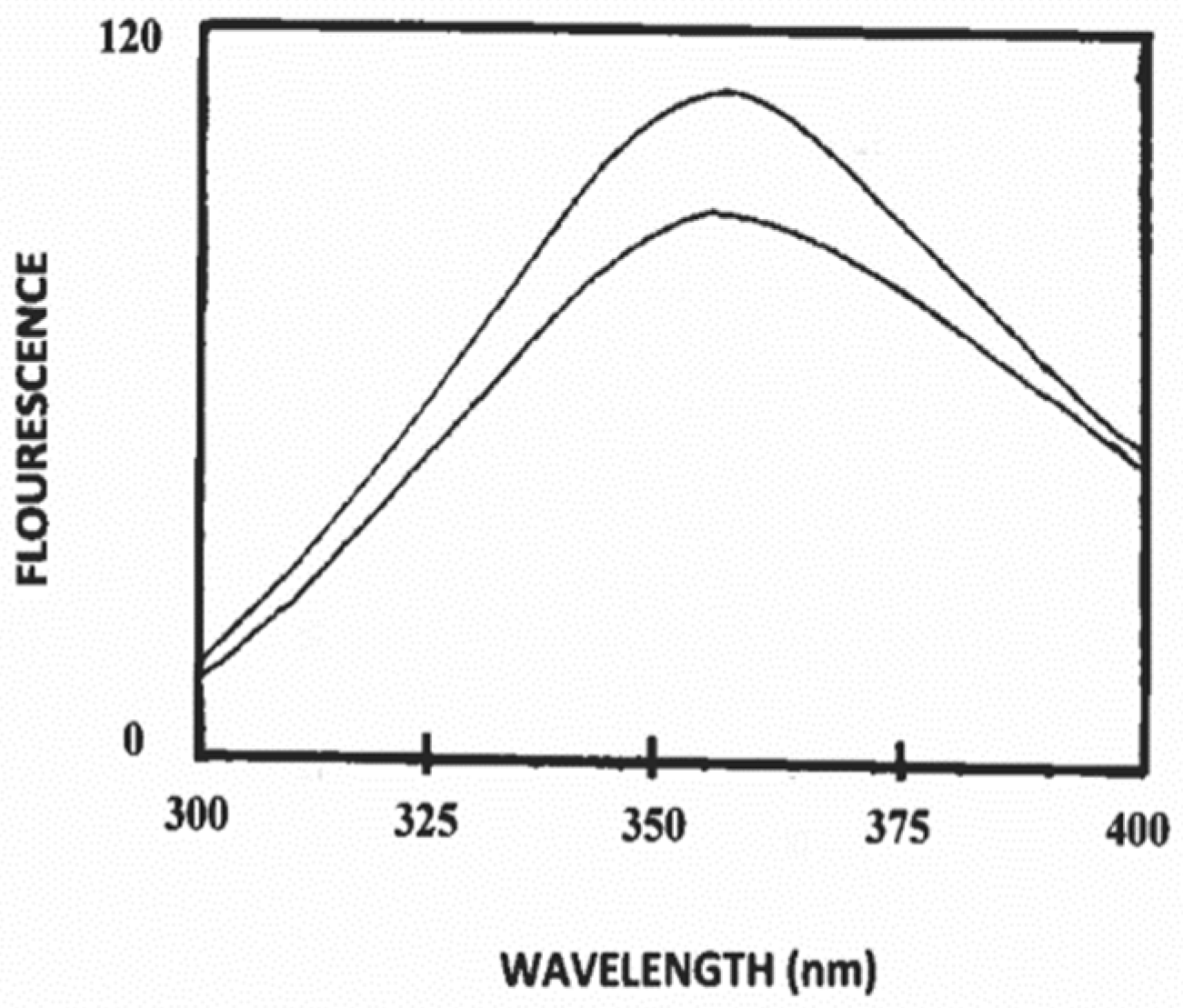
3.2. Binding of Polysorbate-20 to Caprine Casein in Solution
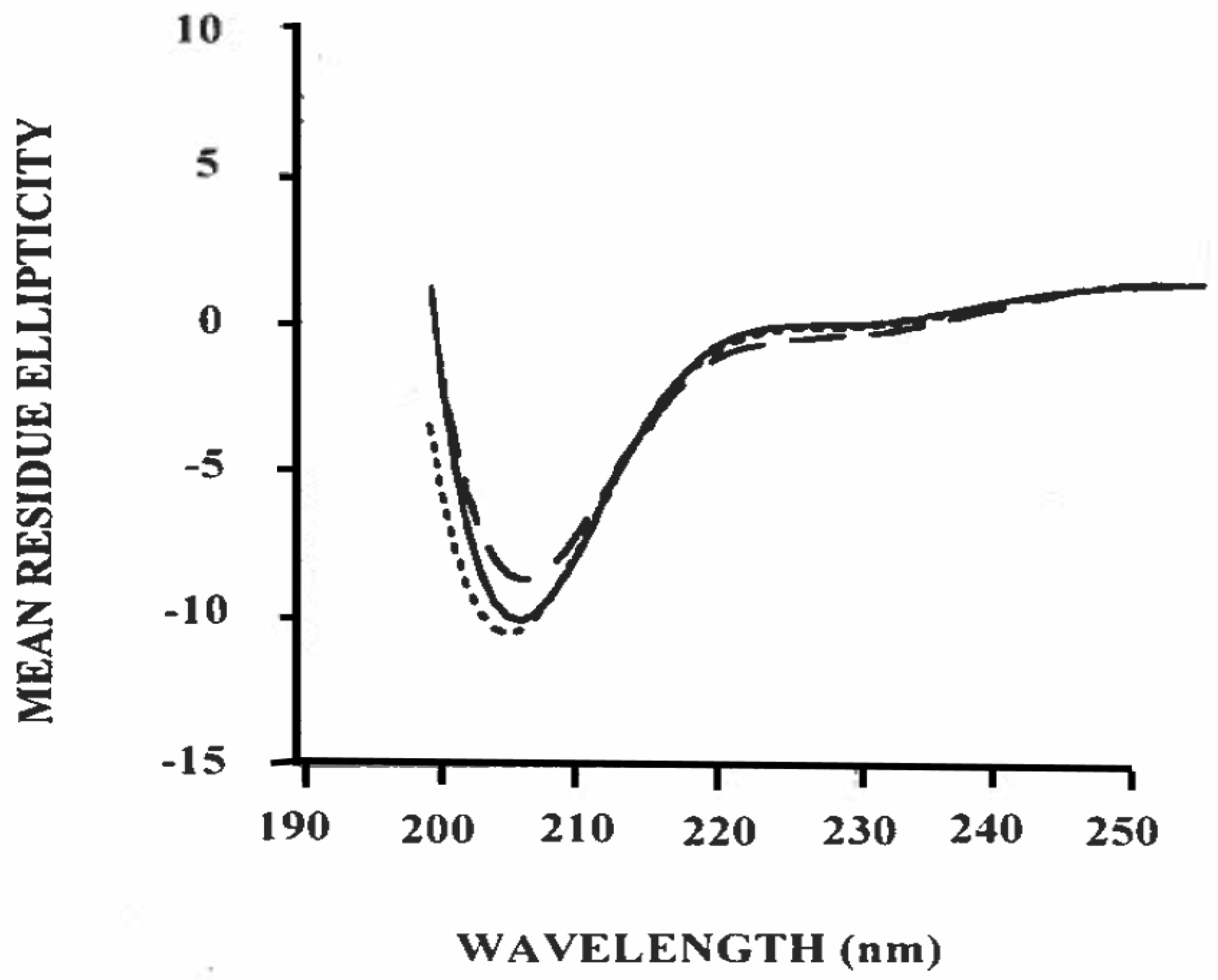
3.3. Estimation of Secondary Structure of β-Casein/Polysorbate-20 Complex in Solution
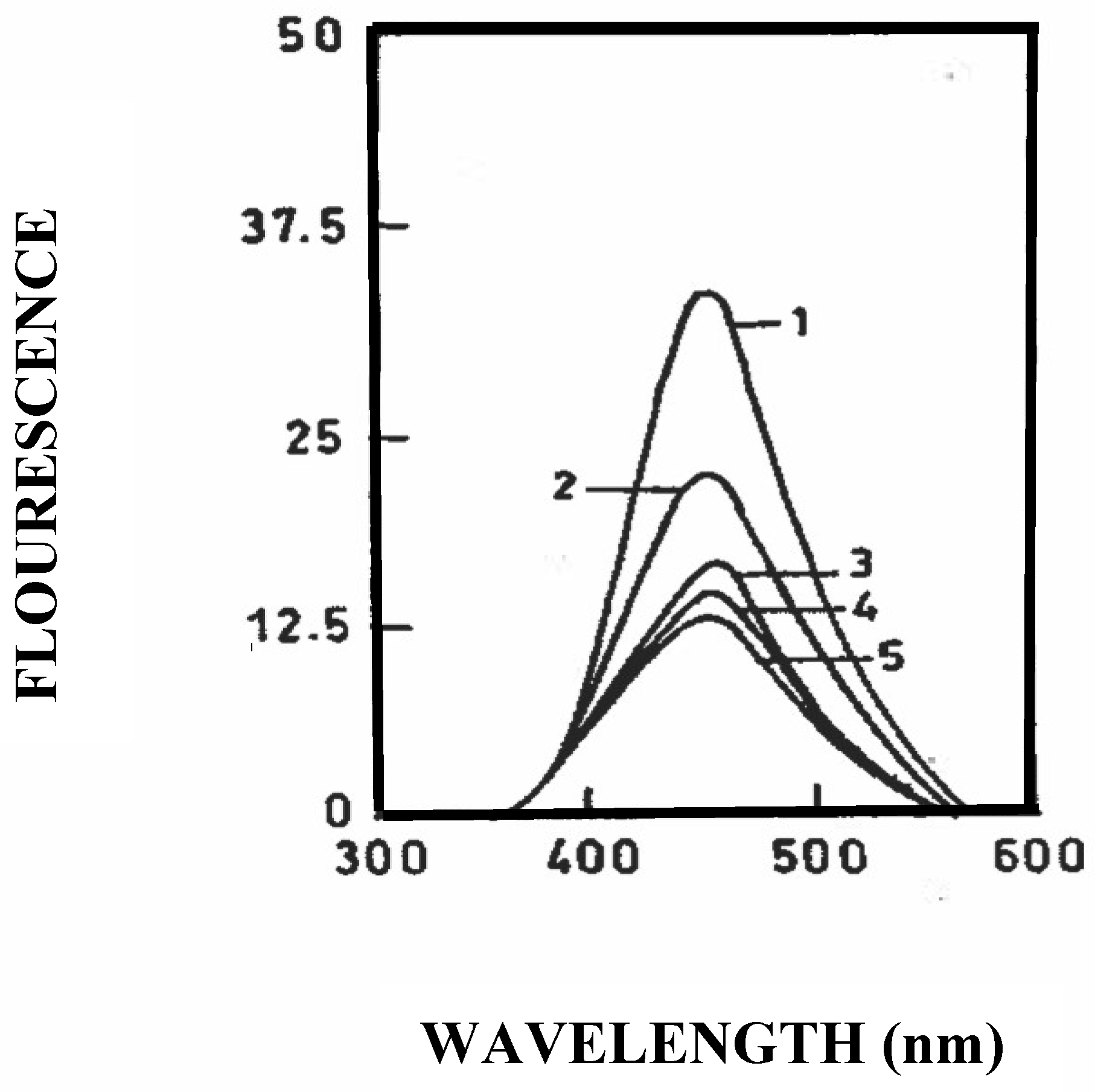
3.4. Binding of Resveratrol to Bovine and Caprine Caseins in Solution
3.5. Effects of Polysorbate-20 on the Physicochemical Properties of Resveratrol-Loaded Nanoemulsions Stabilized by Bovine and Caprine Caseins
3.6. Stability of Resveratrol
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Statement
References
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Sustained cerebrovascular and cognitive benefits of resveratrol in postmenopausal women. Nutrients 2020, 12, 828. [Google Scholar] [CrossRef]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release 2012, 158, 182–193. [Google Scholar] [CrossRef]
- Francioso, A.; Mastromarino, P.; Masci, A.; d’Erme, M.; Mosca, L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014, 10, 237–245. [Google Scholar] [CrossRef]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef]
- McClements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccessibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-grade nanoemulsions: Preparation, stability and application in encapsulation of bioactive compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Leaver, J.; Horne, D.S.; Law, A.J.R. Interactions of proteins and surfactants at oil-water interfaces: Influence of a variety of physical parameters on the behavior of milk proteins. Int. Dairy J. 1999, 9, 319–322. [Google Scholar] [CrossRef]
- Fuller, G.T.; Considine, T.; MacGibbon, A.; Golding, M.; Matia-Merino, L. Effect of Tween emulsifiers on the shear stability of partially crystalline oil-in-water emulsions stabilized by sodium caseinate. Food Biophys. 2018, 13, 80–90. [Google Scholar] [CrossRef]
- Cheong, J.N.; Mirhosseini, H.; Tan, C.P. Effect of polyoxyethylene sorbitan esters and sodium caseinate on physicochemical properties of palm-based functional lipid nanodispersions. Int. J. Food Sci. Nutr. 2010, 61, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Perugini, L.; Cinelli, G.; Cofelice, M.; Ceglie, A.; Lopez, F.; Cuomo, F. Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloid Surf. B. 2018, 168, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tual, A.; Bourles, E.; Barey, P.; Houdoux, A.; Desprairies, M.; Courthaudon, J.L. Effect of surfactant sucrose ester on physical properties of dairy whipped emulsions in relation to those of o/w interfacial layers. J. Colloid Interface Sci. 2006, 295, 495–503. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Kumosinski, T.F.; Farrell, H.M. Quantification of αs1-casein in goat milk from French-Alpine and Anglo-Nubian breeds using reversed-phase high performance liquid chromatography. J. Dairy Sci. 1991, 74, 3303–3307. [Google Scholar] [CrossRef]
- Basch, J.J.; Farrell, H.M.; Walsh, R.A.; Konstance, R.P.; Kumosinski, T.F. Development of a quantitative model for enzyme-catalyzed, time-dependent changes in protein composition of cheddar cheese during storage. J. Dairy Sci. 1989, 72, 591–603. [Google Scholar] [CrossRef]
- Mercier, J.C.; Maubois, J.L.; Poznańzky, S.; Ribadeau-Dumas, B. Preparative fractionation of cows’ and ewes’ milk caseins by chromatography on DEAE-cellulose in a medium containing urea and 2-mercaptoethanol. Bull. Soc. Chim. Biol. 1968, 50, 521–530. [Google Scholar]
- Moatsou, G.; Mollé, D.; Moschopoulou, E.; Valérie, G. Study of caprine β-casein using reversed-phase high-performance liquid chromatography and mass spectroscopy: Identification of a new genetic variant. Protein J. 2007, 26, 562–568. [Google Scholar] [CrossRef]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef]
- Wiedemann, C.; Bellstedt, P.; Görlach, M. CAPITO-a web server-based analysis and plotting tool for circular dichroism data. Bioinformatics 2013, 29, 1750–1757. [Google Scholar] [CrossRef]
- Steiner, B.M.; Shukla, V.; McClements, D.J.; Li, Y.O.; Sancho-Madriz, M.; Davidov-Pardo, G. Encapsulation of lutein in nanoemulsions stabilized by resveratrol and Maillard conjugates. J. Food Sci. 2019, 84, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Markwell, M.A.; Haas, S.M.; Bieber, L.L.; Tolbert, N.E. A Modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Ambrosoli, R.; Di Stasio, L.; Mazzocco, P. Content of αs1-casein and coagulation properties in goat milk. J. Dairy Sci. 1988, 71, 24–28. [Google Scholar] [CrossRef]
- Grosclaude, F.; Mahé, M.F.; Brignon, G.; Di Stasio, L.; Jeunet, R. A Mendelian polymorphism underlying quantitative variations of goat αs1-casein. Genet. Sel. Evol. 1987, 19, 399–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaubert, A.; Martin, P. Reverse-phase HPLC analysis of goat caseins. Identification of αs1 and αS2 genetic variants. Lait 1992, 72, 235–247. [Google Scholar] [CrossRef]
- Kumosinski, T.F.; Brown, E.M.; Farrell, H.M. Three-dimensional molecular modeling of bovine caseins: An energy-minimized β-casein structure. J. Dairy Sci. 1993, 76, 931–945. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Attaie, R.; Farrell, H.M. Lipid oxidation in algae oil-in-water emulsions stabilized by bovine and caprine caseins. J. Agric. Food Chem. 2010, 58, 5131–5139. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Attaie, R.; Núñez de González, M.T.; Jung, Y.; Woldesenbet, S.; Marquez, S.A. Complexes of lutein with bovine and caprine caseins and their impact on lutein chemical stability in emulsion systems: Effect of arabinogalactan. J. Dairy Sci. 2018, 101, 18–27. [Google Scholar] [CrossRef]
- Lakowicz, J.R. On spectral relaxation in proteins. Photochem. Photobiol. 2000, 72, 421–437. [Google Scholar] [CrossRef]
- Kelkar, D.; Chanudhuri, A.; Haldar, S.; Chattopadhyay, A. Exploring tryptophan dynamics in acid-induced molten globule state of bovine α-lactalbumin: A wavelength-selective fluorescence approach. Eur. Biophys. J. 2010, 39, 1453–1463. [Google Scholar] [CrossRef]
- Kratz, F.; Elsadek, B. Clinical impact of serum proteins on drug delivery. J. Control. Release 2012, 161, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Lucey, J.A.; Otter, D.; Horne, D.S. A 100-Year Review: Progress on the chemistry of milk and its components. J. Dairy Sci. 2017, 100, 9916–9932. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oseliero Filho, P.L.; Oliveira, C.L.P. α-Lactalbumin and sodium dodecyl sulfate aggregates: Denaturation, complex formation and time stability. Food Hydrocoll. 2017, 62, 10–20. [Google Scholar] [CrossRef]
- Perticaroli, S.; Nickels, J.D.; Ehlers, G.; Mamontov, E.; Sokolov, A.P. Dynamics and rigidity in an intrinsically disordered protein, β-casein. J. Phys. Chem. B 2014, 118, 7317–7326. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Zhang, X.; Chen, D.; Li, N.; Hemar, Y.; Yu, B.; Tang, S.; Sun, Y. How much can we trust polysorbates as food protein stabilizers—The case of bovine casein. Food Hydrocoll. 2019, 96, 81–92. [Google Scholar] [CrossRef]
- Sun, Y.; Filho, P.L.O.; Bozelli, J.C.; Carvalho, J.; Schreier, S.; Oliveira, C.L.P. Unfolding and folding pathway of lysozyme induced by sodium dodecyl sulfate. Soft Matter 2015, 11, 7769–7777. [Google Scholar] [CrossRef]
- Gorji, E.G.; Rocchi, E.; Schleining, G.; Bender-Bojalil, D.; Furtmüller, P.G.; Piazza, L.; Iturri, J.J.; Toca-Herrera, J.L. Characterization of resveratrol-milk protein interaction. J. Food Eng. 2015, 167, 217–225. [Google Scholar] [CrossRef]
- Cheema, M.; Hristov, A.N.; Harte, F.M. The binding of orally dosed hydrophobic active pharmaceutical ingredients to casein micelles in milk. J. Dairy Sci. 2017, 100, 8670–8679. [Google Scholar] [CrossRef]
- Głąb, T.K.; Boratyński, J. Potential of casein as a carrier for biologically active agents. Top. Curr. Chem. 2017, 375, 71. [Google Scholar] [CrossRef]
- Kamigaki, T.; Ito, Y.; Nishino, Y.; Miyazawa, A. Microstructural observation of casein micelles in milk by cryo-electron microscopy of vitreous sections (CEMOVIS). Microscopy 2018, 67, 164–170. [Google Scholar] [CrossRef]
- Courthaudon, J.L.; Girardet, J.M.; Campagne, S.; Rouhier, L.M.; Campagna, S.; Linden, G.; Lorient, D. Surface active and emulsifying properties of casein micelles compared to those of sodium caseinate. Int. Dairy J. 1999, 9, 411–412. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Ivanov, I.B.; Campbell, B. Coalescence stability of emulsions containing globular milk proteins. Adv. Colloid Interface Sci. 2006, 123–126, 259–293. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Bai, L.; Chung, C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Ritzoulis, C.; Povey, M.J. Stability of emulsions containing both sodium caseinate and Tween 20. J. Colloid Interface Sci. 1999, 212, 466–473. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Kennedy, D.; Wightman, E.; Khan, J.; Grothe, T.; Jackson, P. The acute and chronic cognitive and cerebral blood-flow effects of Nepalese pepper (Zanthoxylum Armatum DC.) extract-A randomized, double-blind, placebo-controlled study in healthy humans. Nutrients 2019, 11, 3022. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Saavedra-Leos, M.Z.; Cervantes-González, E.; Aguirre-Bañuelos, P.; Silva-Cázarez, M.B.; Álvarez-Salas, C. Spray drying of blueberry juice-maltodextrin mixtures: Evaluation of processing conditions on content of resveratrol. Antioxidants 2019, 8, 437. [Google Scholar] [CrossRef]
- Ho, R.J.Y.; Gibaldi, M. Clinical pharmacology, toxicology, and their therapeutics. In Biotechnology and Biopharmaceutical. Transforming Proteins and Genes into Drugs; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Kim, M.Y.; Ha, H.K.; Ayu, I.L.; Han, K.-S.; Lee, W.-J.; Lee, M.-R. Manufacture and physicochemical properties of chitosan oligosaccharide/A2 β-casein nano-delivery system entrapped with resveratrol. Food Sci. Anim. Resour. 2019, 39, 831–843. [Google Scholar] [CrossRef]


| Casein Fraction % | |||||
|---|---|---|---|---|---|
| Sample | αs2 | αs1 | αs1 | β | κ |
| Caprine casein high in αs1-casein-I | 9.2 | 4.0 | 21.1 | 51.6 | 13.8 |
| Caprine casein high in αs1-casein-II | 5.3 | 25.6 | 60.6 | 9.6 | |
| Bovine casein | 12.1 | 39.5 | 37.2 | 11.2 | |
| β-Casein/Polysorbate-20 Samples | ||||
|---|---|---|---|---|
| Conformational Element * | Bovine β-Casein | Bovine β-Casein/PS-20 1 | Caprine β-Casein | Caprine β-Casein/PS-20 |
| α-helix (%) | 13.5 | 16.8 | 14.2 | 17.8 |
| β-strand (%) | 17.9 | 21.0 | 16.8 | 22.5 |
| Irregular (%) | 69.1 | 62.7 | 70.3 | 61.6 |
| Emulsifier | Particle Size (nm) 1 | Zeta Potential (mV) 1 |
|---|---|---|
| Bovine casein | 205.84 ± 1.57 a | −38.23 ± 0.29 c |
| Bovine casein + PS-20 2 | 202.89 ± 1.66 a | −37.39 ± 1.56 b,c |
| Caprine casein (αs1-I) | 200.80 ± 0.45 a | −34.95 ± 0.18 a |
| Caprine casein (αs1-I) + PS-20 | 192.17 ± 4.61 b | −33.74 ± 0.95 a |
| Caprine casein (αs1-II) | 200.16 ± 2.54 a | −35.10 ± 0.69 b |
| Caprine casein (αs1-II) + PS-20 | 191.09 ± 2.27 b | −33.89 ± 1.19 a |
| Persistence of Resveratrol (%) 2 | |
|---|---|
| Emulsifier 1 | |
| Bovine casein | 76.25 ± 2.67 c |
| Bovine casein + PS-20 2 | 78.83 ± 2.61 c |
| Caprine casein (αs1-I) | 83.58 ± 1.90 b |
| Caprine casein (αs1-I) + PS-20 | 88.33 ± 1.84 a |
| Caprine casein (αs1-II) | 83.50 ± 1.64 b |
| Caprine casein (αs1-II) + PS-20 | 89.08 ± 2.14 a |
| Storage time (hours) | |
| 6 | 92.11 ± 0.99 a |
| 12 | 86.72 ± 1.11 b |
| 24 | 78.06 ± 1.49 c |
| 48 | 76.17 ± 1.71 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Gutierrez, A.; Attaie, R.; Núñez de González, M.T.; Jung, Y.; Marquez, S.A. Interface Compositions as Determinants of Resveratrol Stability in Nanoemulsion Delivery Systems. Foods 2020, 9, 1394. https://doi.org/10.3390/foods9101394
Mora-Gutierrez A, Attaie R, Núñez de González MT, Jung Y, Marquez SA. Interface Compositions as Determinants of Resveratrol Stability in Nanoemulsion Delivery Systems. Foods. 2020; 9(10):1394. https://doi.org/10.3390/foods9101394
Chicago/Turabian StyleMora-Gutierrez, Adela, Rahmat Attaie, Maryuri T. Núñez de González, Yoonsung Jung, and Sixto A. Marquez. 2020. "Interface Compositions as Determinants of Resveratrol Stability in Nanoemulsion Delivery Systems" Foods 9, no. 10: 1394. https://doi.org/10.3390/foods9101394
APA StyleMora-Gutierrez, A., Attaie, R., Núñez de González, M. T., Jung, Y., & Marquez, S. A. (2020). Interface Compositions as Determinants of Resveratrol Stability in Nanoemulsion Delivery Systems. Foods, 9(10), 1394. https://doi.org/10.3390/foods9101394





