High Power Ultrasound Treatments of Red Young Wines: Effect on Anthocyanins and Phenolic Stability Indices
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Solvents
2.2. Wine Samples
2.3. Ultrasound Treatments
2.4. Analytical Methods
2.4.1. Anthocyanins Content
2.4.2. Color Intensity (C.I.)
2.4.3. Flavan-3-ols Content
2.4.4. Condensed Tannins
2.4.5. Polymerized Pigments Index (PPI)
2.4.6. HCl Index
2.4.7. Analysis of Anthocyanins by High Performance Liquid Chromatography (HPLC)
2.5. Statistical Analysis
3. Results and Discussion
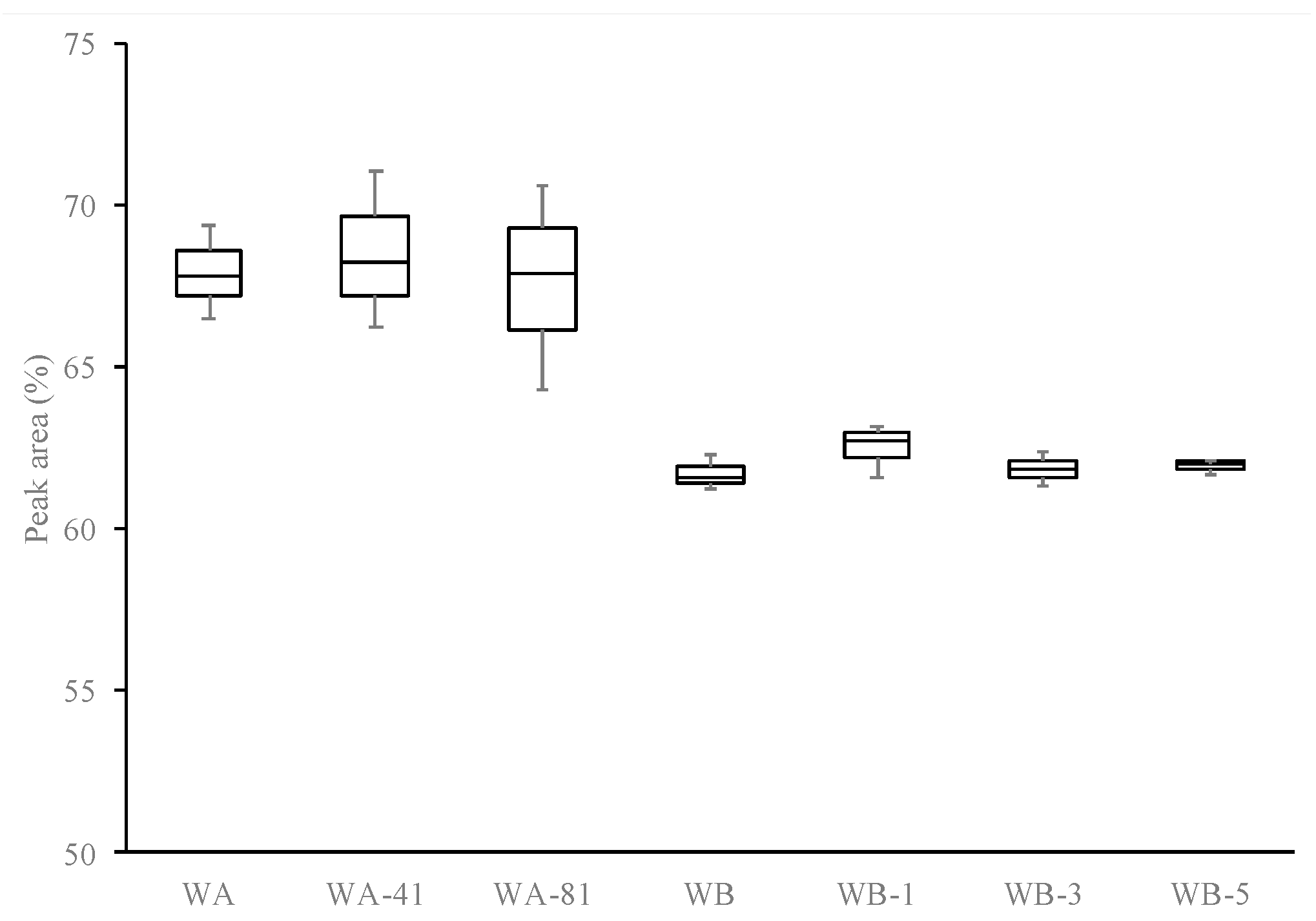
3.1. Effect of Ultrasound Amplitude
3.2. Effect of Ultrasound Time (tUS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heras-Roger, J.; Diaz-Romero, C.; Darias-Martin, J. What gives a wine its strong red color? Main correlations affecting copigmentation. J. Agric. Food Chem. 2016, 64, 6567–6574. [Google Scholar] [CrossRef] [PubMed]
- Bridle, P.; Timberlake, C.F. Anthocyanins as natural food colours–selected aspects. Food Chem. 1997, 58, 103–109. [Google Scholar] [CrossRef]
- Morrot, G.; Brochet, F.; Dubourdieu, D. The color of odors. Brain Lang. 2001, 79, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Kunsági-Máté, S.; Ortmann, E.; Kollár, L.; Nikfardjam, M.P. Entropy-driven complex formation of malvidin-3-O-glucoside with common polyphenols in ethanol-water binary solutions. Spectrochi. Acta A 2008, 70, 860–865. [Google Scholar] [CrossRef]
- Darias-Martín, J.; Carrillo, M.; Díaz, E.; Boulton, R.B. Enhancement of red wine colour by pre-fermentation addition of copigments. Food Chem. 2002, 73, 217–220. [Google Scholar] [CrossRef]
- Avizcuri, J.M.; Sáenz-Navajas, M.P.; Echávarri, J.F.; Ferreira, V.; Fernández-Zurbano, P. Evaluation of the impact of initial red wine compositionon changes in color and anthocyanin content during bottle storage. Food Chem. 2016, 213, 123–134. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol.Vitic. 2001, 52, 67–87. [Google Scholar]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- González-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the copigmentation between anthocyanins and flavan-3-ols and their influence in the colour expression of red wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.; González-Manzano, S.; Escribano-Bailón, M.T.; Heredia, F.J.; Santos-Buelga, C. Influence of different phenolic copigments on the color of Malvidin 3-glucoside. J. Agric. Food Chem. 2006, 54, 5422–5429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Wang, T.T. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017, 234, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Lukíc, K.; Brnčić, N.; Tomašević, M.; Valinger, D.; Denoya, G.I.; Barba, F.J.; Kovačević Ganić, K. Effects of high power ultrasound treatments on the phenolic, chromatic and aroma composition of young and aged red wine. Ultrason. Sonochem. 2019, 59, 104725. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C. The effects of gamma irradiation on rice wine maturation. Food Chem. 2003, 83, 323–327. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.M.; Saraiva, J.A.; Coimbra, M.A. Impact of high pressure treatments on the physicochemical properties of a sulphur dioxide-free white wine during bottle storage: Evidence for Maillard reaction acceleration. Innov. Food Sci. Emerg. Technol. 2013, 20, 51–58. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.W.; Górecki, A.; Blaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jeliński, T. A preliminary study about the influence of high hydrostatic pressure processing in parallel with oak chip maceration on the physicochemical and sensory properties of a young red wine. Food Chem. 2016, 194, 545–554. [Google Scholar] [CrossRef]
- Chemat, F.; Humma, Z.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Jiménez-Martínez, M.D.; Jurado, R.; Iniesta, J.A.; Terrades, S.; Andrés, A.; Gómez-Plaza, E. Application of high-power ultrasounds during red wine vinification. Int. J. Food Sci. Tech. 2017, 52, 1314–1323. [Google Scholar] [CrossRef]
- Cocito, C.; Gaetano, G.; Delfini, C. Rapid extraction of aroma compounds in must and wine by means of ultrasound. Food Chem. 1995, 52, 311–320. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Dipalmo, T.; Rizzello, C.G.; Corbo, F.; Crupi, P. Emerging technology to develop novel red winemaking practices: An overview. Innov. Food Sci. Emerg. Technol. 2016, 38, 41–56. [Google Scholar] [CrossRef]
- Jiranek, V.; Grbin, P.; Yap, A.; Barnes, M.; Bates, D. High power ultrasonics as a novel tool offering new opportunities for managing wine microbiology. Biotechnol. Lett. 2008, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extractions from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.M.; Loarce, L.; Alarcón, M.; Díaz-Maroto, M.C.; Alañón, M.E. Revalorization of winery by-products as source of natural preservatives obtained by means of green extraction techniques. Ind. Crop. Prod. 2018, 112, 617–625. [Google Scholar] [CrossRef]
- Tao, Y.; García, J.F.; Sun, D.W. Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Critic. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- García Martín, J.F.; Sun, D.W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Tech. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Ferrarretto, P.; Celotti, E. Preliminary study of the effects of ultrasounds on red wine polyphenols. CyTA J. Food 2016, 14, 529–535. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Shen, Y.; Fan, X.H.; García-Martín, J.F. Preliminary study of the effect of ultrasound on physicochemical properties of red wine. CyTA J. Food 2016, 14, 55–64. [Google Scholar] [CrossRef]
- OIV. Tretament of Crushed Grapes with Ultrasound to Promote the Extraction of Their Compounds Resolution OIV-OENO 616-2019; OIV: Paris, France, 2019. [Google Scholar]
- OIV. Compendium of International Methods of Analysis of Wines and Musts; OIV: Paris, France, 2019; ISBN 978-2-85038-003-7. [Google Scholar]
- Celotti, E.; Ferrarretto, P. Studies for the ultrasound application in winemaking for a low impact enology. In Proceedings of the 39th World Congress of Vine and Wine 2016, Bento Gonçalves, Brazil, 24–28 October 2016; pp. 104–106, ISBN 979-10-91799-62-1. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Le dosage des anthocyanes et des tannins dans le vin rouge. Bull. Soc. Chim. France 1965, 9, 2649–2652. [Google Scholar]
- Glories, Y. La couleur des vins rouges. 1re partie. Les équilibres des anthocyanes et des tannins. Conn. Vigne Vin 1984, 18, 195–217. [Google Scholar]
- Zironi, R.; Buiatti, S.; Celotti, E. Evaluation of a new colorimetric method for the determination of catechins in musts and wines. Wein Wissenschaft 1992, 47, 1–7. [Google Scholar]
- Bate-Smith, E.-C. Leucoanthocyanins. I. Detection and identification of anthocyanin formed from leucoanthocyanins in plant tissues. Biochem. J. 1954, 11, 1153–1156. [Google Scholar]
- Glories, Y. Recherches sur la Matiére Colorante des Vins Rouges. Ph.D. Thesis, Université de Bordeaux II, Bordeaux, France, 1978. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, J.; Xu, D.; Wang, S.; Yuan, Y.; Cao, Y. Investigation of (+)-catechin stability under ultrasonic treatment and its degradation kinetic modeling. J. Food Proc. Eng. 2018, 41, e12904. [Google Scholar] [CrossRef]
- Kidak, R.; Ince, N.H. Ultrasonic destruction of phenol and substituted phenols: A review of current research. Ultrason.Sonochem. 2006, 13, 195–199. [Google Scholar] [CrossRef]
- Saucier, C.; Lopes, P.; Mirabel, M.; Guerra, C.; Glories, G. Tannin-Anthocyanin interactions: Influence on wine color. In: Red wine color, 1st ed. Waterhouse, A.L.; Kennedy, J.A. Am. Chem. Soc. 2004, 886, 265–273. [Google Scholar]
- Morera, J.M.; Bartoli, E.; Combalía, F.; Borràs, E.; Castell, J.C.; Sorolla, S. Study of the application of ultrasound in a vegetable tannage. J. Am. Leather Chem. As. 2010, 105, 369–375. [Google Scholar]
- Fischer, C.H.; Hart, E.J.; Henglein, A.J. Hydrogen/deuterium isotope exchange in the hydrogen deuteride-water system under the influence of ultrasound. Phys. Chem. 1986, 90, 3059–3060. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Rad. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Ince, N.H.; Tezcanli, G.; Belen, R.K.; Apikyan, P.G. Ultrasound as a catalyzer of aqueous reaction systems: The state of the art and environmental applications. Appl. Catal. B 2001, 29, 167–176. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The physical and chemical effect of ultrasound. In Ultrasound Technologies for Food and Bioprocessing, 1st ed.; Feng, H., Barbosa-Cánovas, G.V., Weiss, J., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2010; pp. 7–11. [Google Scholar]
- Zhang, Q.A.; Shen, Y.; Fan, X.; García Martín, J.F.; Wang, X.; Song, Y. Free radical generation induced by ultrasound in red wine and model wine: And EPR spin trapping study. Ultrason. Sonochem. 2015, 27, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TRAC Trend Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Cao, X.; Cai, C.; Wang, Y.; Zheng, X. Effects of ultrasound processing on physicochemical parameters, antioxidants, and color quality of bayberry juice. J. Food Qual. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006, 57, 289–297. [Google Scholar]
- Gambuti, A.; Rinaldi, A.; Ugliano, M.; Moio, L. Evolution of phenolic comporunds and astringency during aging of red wine: Effect of oxygen exposure before and after bottling. J. Agric. Food Chem. 2013, 61, 1618–1627. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Sharp, E.; de Villiers, A.; du Toit, W. The Impact of Different Tannin to Anthocyanin Ratios and of Oxygen on the Phenolic Polymerisation over Time in a Wine-like Solution. S. Af. J. Enol. Viticult. 2019, 40, 278–289. [Google Scholar] [CrossRef]
| Wine | Alcohol (%) | pH | Total Acidity (g L−1) | Free SO2 (mg L−1) | Total SO2 (mg L−1) |
|---|---|---|---|---|---|
| WA | 11.5 | 3.72 | 5.20 | 20 | 60 |
| WB | 11.5 | 3.23 | 8.35 | 29 | 55 |
| Wine | Sample Code | Amp (%) | tUS (min) |
|---|---|---|---|
| A | WA * | 0 | 3 |
| WA-41 | 41 | 3 | |
| WA-81 | 81 | 3 | |
| B | WB # | 81 | 0 |
| WB-1′ | 81 | 1 | |
| WB-3′ | 81 | 3 | |
| WB-5′ | 81 | 5 |
| Sample | Amplitude | tstor. | Anthocyanins | Flavan-3-ols | Tannins | P.P.I. | HCl Index | C.I. |
|---|---|---|---|---|---|---|---|---|
| (%) | (day) | (mg/L) | (mg/L) | (g/L) | (-) | (-) | (-) | |
| WA | 0 | 0 | 185.99 ± 1.58 a * | 201.07 ± 7.13 c | 1.52 ± 0.16 ab | 45.61 ± 1.09 c | 8.82 ± 0.73 c | 2.63 ± 0.11 a |
| 0 | 15 | 169.27 ± 3.43 b | 227.94 ± 3.92 b | 1.55 ± 0.09 ab | 45.65 ± 0.60 c | 18.22 ± 1.01 b | 2.83 ± 0.11 a | |
| 0 | 30 | 143.86 ± 3.32 c | 283.02 ± 3.22 a | 1.42 ± 0.04 b | 53.75 ± 0.55 ab | 33.73 ± 0.90 a | 2.70 ± 0.76 a | |
| WA-41 | 41 | 0 | 188.88 ± 3.52 a | 182.66 ± 11.96 c | 1.54 ± 0.52 ab | 45.31 ± 0.61 c | 8.62 ± 1.28 c | 3.07 ± 0.48 a |
| 41 | 15 | 168.23 ± 3.76 b | 224.78 ± 7.22 b | 1.47 ± 0.43 ab | 48.63 ± 2.96 bc | 18.32 ± 6.47 b | 3.10 ± 0.24 a | |
| 41 | 30 | 144.70 ± 4.72 c | 279.08 ± 8.99 a | 1.23 ± 0.40 ab | 54.52 ± 0.87 a | 36.30 ± 1.98 a | 2.94 ± 0.07 a | |
| WA-81 | 81 | 0 | 194.10 ± 2.50 a | 158.03 ± 6.00 d | 1.67 ± 0.08 a | 46.46 ± 3.87 c | 7.81 ± 2.48 c | 2.89 ± 0.18 a |
| 81 | 15 | 166.54 ± 5.80 b | 234.48 ± 9.30 b | 1.17 ± 0.14 b | 48.69 ± 2.90 bc | 20.89 ± 2.94 b | 3.14 ± 0.05 a | |
| 81 | 30 | 137.11 ± 9.78 c | 285.40 ± 5.38 a | 1.01 ± 0.06 c | 53.05 ± 1.28 ab | 33.38 ± 2.53 a | 2.97 ± 0.04 a |
| Compound | Wine A | Wine B | |||||
|---|---|---|---|---|---|---|---|
| WA | WA-41 | WA-81 | WB | WB-1 | WB-3 | WB-5 | |
| Delphinidin-3-monoglucoside | 1.60 ± 0.16 c * | 3.15 ± 0.26 b | 3.21 ± 0.19 b | 3.97 ± 0.36 a * | 3.94 ± 0.30 a | 3.88 ± 0.24 a | 4.15 ± 0.15 a |
| Cyanidin-3-monoglucoside | 0.46 ± 0.08 b | 0.68 ± 0.15 b | 0.69 ± 0.11 b | 0.98 ± 0.20 a | 0.93 ± 0.18 a | 0.95 ± 0.20 a | 0.99 ± 0.02 a |
| Petunidin-3-monoglucoside | 5.03 ± 0.23 a | 5.00 ± 0.21 a | 4.96 ± 0.24 a | 5.51 ± 0.29 a | 5.10 ± 0.78 a | 5.52 ± 0.14 a | 5.30 ± 0.33 a |
| Peonidin-3-monoglucoside | 7.65 ± 0.39 b | 9.88 ± 1.25 a | 9.55 ± 0.55 a | 10.39 ± 0.02 a | 10.75 ± 0.12 a | 10.30 ± 0.65 a | 10.94 ± 0.28 a |
| Vitisin A | 1.14 ± 0.10 b | 1.32 ± 0.11 b | 1.33 ± 0.11 b | 2.17 ± 0.07 a | 2.13 ± 0.16 a | 2.27 ± 0.10 a | 2.19 ± 0.08 a |
| Petunidin-3-monoglucoside acetylated | 0.55 ± 0.09 b | 0.66 ± 0.11 b | 0.55 ± 0.06 b | 0.73 ± 0.03 a | 0.71 ± 0.05 a | 0.74 ± 0.07 a | 0.69 ± 0.02 a |
| Peonidin-3-monoglucoside acetylated | 1.35 ± 0.17 a | 1.34 ± 0.38 a | 1.91 ± 0.44 a | 2.12 ± 0.36 a | 1.64 ± 0.68 a | 2.00 ± 0.16 a | 1.41 ± 0.59 a |
| Malvidin-3-monoglucoside acetylated | 7.37 ± 0.33 a | 7.99 ± 1.40 a | 7.01 ± 0.37 a | 6.39 ± 0.59 ab | 6.15 ± 0.58 ab | 6.41 ± 0.20 b | 6.12 ± 0.12 b |
| Delphinidin-3-monoglucoside p-coumarylated | 2.82 ± 0.16 a | 2.48 ± 0.20 a | 2.57 ± 0.40 a | 2.21 ± 0.10 a | 2.25 ± 0.07 a | 2.14 ± 0.09 a | 2.23 ± 0.05 a |
| Malvidin-3-monoglucoside p-coumarylated | 4.13 ± 0.26 a | 4.26 ± 0.26 a | 4.39 ± 0.51 a | 3.24 ± 0.12 a | 3.32 ± 0.05 a | 3.37 ± 0.03 a | 3.43 ± 0.13 a |
| Malvidin-3-monoglucoside vinylphenol | n.d. # | n.d. | n.d. | 0.15 ± 0.02 a | 0.16 ± 0.03 a | 0.19 ± 0.01 a | 0.18 ± 0.01 a |
| Malvidin-3-monoglucoside vinylphenol acetylated | n.d. | n.d. | n.d. | 0.41 ± 0.01 a | 0.37 ± 0.04 a | 0.39 ± 0.02 a | 0.39 ± 0.03 a |
| Sample | Time US | tstor. | Anthocyanins | Flavan-3-ols | Tannins | P.P.I. | HCl Index | C.I. |
|---|---|---|---|---|---|---|---|---|
| (min) | (day) | (mg/L) | (mg/L) | (g/L) | (-) | (-) | (-) | |
| WB | 0 | 0 | 280.99 ± 9.71 a * | 298.50 ± 0.72 bc | 1.26 ± 0.11 ab | 57.87 ± 4.61 b | 14.22 ± 0.54 b | 4.52 ± 0.06 c |
| 15 | 132.18 ± 11.87 c | 246.10 ± 0.86 e | 1.09 ± 0.07 b | 68.95 ± 3.72 ab | 28.51 ± 2.40 a | 5.55 ± 0.04 a | ||
| 30 | 182.12 ± 3.52 b | 262.23 ± 7.43 e | 1.08 ± 0.25 ab | 60.57 ± 2.96 ab | 30.98 ± 1.45 a | 5.38 ± 0.17 ab | ||
| WB-1 | 1 | 0 | 293.27 ± 12.56 a | 300.70 ± 6.22 b | 1.34 ± 0.04 a | 61.37 ± 1.04 ab | 13.13 ± 2.48 b | 4.54 ± 0.01 c |
| 15 | 133.67 ± 15.74 c | 251.03 ± 10.38 e | 1.09 ± 0.12 b | 71.89 ± 1.97 a | 27.66 ± 1.39 a | 5.42 ± 0.14 ab | ||
| 30 | 183.02 ± 4.70 b | 268.38 ± 2.78 de | 1.10 ± 0.24 ab | 60.45 ± 2.49 ab | 24.27 ± 2.39 a | 5.50 ± 0.06 ab | ||
| WB-3 | 3 | 0 | 283.68 ± 5.14 a | 299.18 ± 9.85 bc | 1.26 ± 0.14 ab | 67.55 ± 12.73 ab | 13.68 ± 2.43 b | 4.65 ± 0.01 c |
| 15 | 150.85 ± 10.23 c | 312.95 ± 26.55 ab | 1.05 ± 0.23 b | 68.11 ± 0.75 ab | 24.74 ± 3.45 a | 5.47 ± 0.03 ab | ||
| 30 | 187.25 ± 4.61 b | 271.50 ± 3.64 cde | 1.51 ± 0.18 a | 59.12 ± 0.97 b | 25.16 ± 2.44 a | 5.33 ± 0.25 ab | ||
| WB-5 | 5 | 0 | 273.44 ± 1.15 a | 292.75 ± 0.70 bcd | 1.19 ± 0.17 ab | 64.53 ± 1.87 ab | 12.59 ± 1.08 b | 4.68 ± 0.11 c |
| 15 | 150.33 ± 8.22 c | 331.35 ± 4.70 a | 0.98 ± 0.17 b | 63.14 ± 0.82 ab | 26.20 ± 3.38 a | 5.32 ± 0.12 ab | ||
| 30 | 190.28 ± 4.11 b | 269.73 ± 7.46 de | 1.32 ± 0.11 ab | 56.87 ± 1.73 b | 29.84 ± 4.38 a | 5.18 ± 0.11 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celotti, E.; Stante, S.; Ferraretto, P.; Román, T.; Nicolini, G.; Natolino, A. High Power Ultrasound Treatments of Red Young Wines: Effect on Anthocyanins and Phenolic Stability Indices. Foods 2020, 9, 1344. https://doi.org/10.3390/foods9101344
Celotti E, Stante S, Ferraretto P, Román T, Nicolini G, Natolino A. High Power Ultrasound Treatments of Red Young Wines: Effect on Anthocyanins and Phenolic Stability Indices. Foods. 2020; 9(10):1344. https://doi.org/10.3390/foods9101344
Chicago/Turabian StyleCelotti, Emilio, Stefano Stante, Paola Ferraretto, Tomás Román, Giorgio Nicolini, and Andrea Natolino. 2020. "High Power Ultrasound Treatments of Red Young Wines: Effect on Anthocyanins and Phenolic Stability Indices" Foods 9, no. 10: 1344. https://doi.org/10.3390/foods9101344
APA StyleCelotti, E., Stante, S., Ferraretto, P., Román, T., Nicolini, G., & Natolino, A. (2020). High Power Ultrasound Treatments of Red Young Wines: Effect on Anthocyanins and Phenolic Stability Indices. Foods, 9(10), 1344. https://doi.org/10.3390/foods9101344





