Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients?
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mealworm Powder and Oil Samples
2.2. Proximate Compositions and Amino Acid Compositions
2.3. Protein Solubility
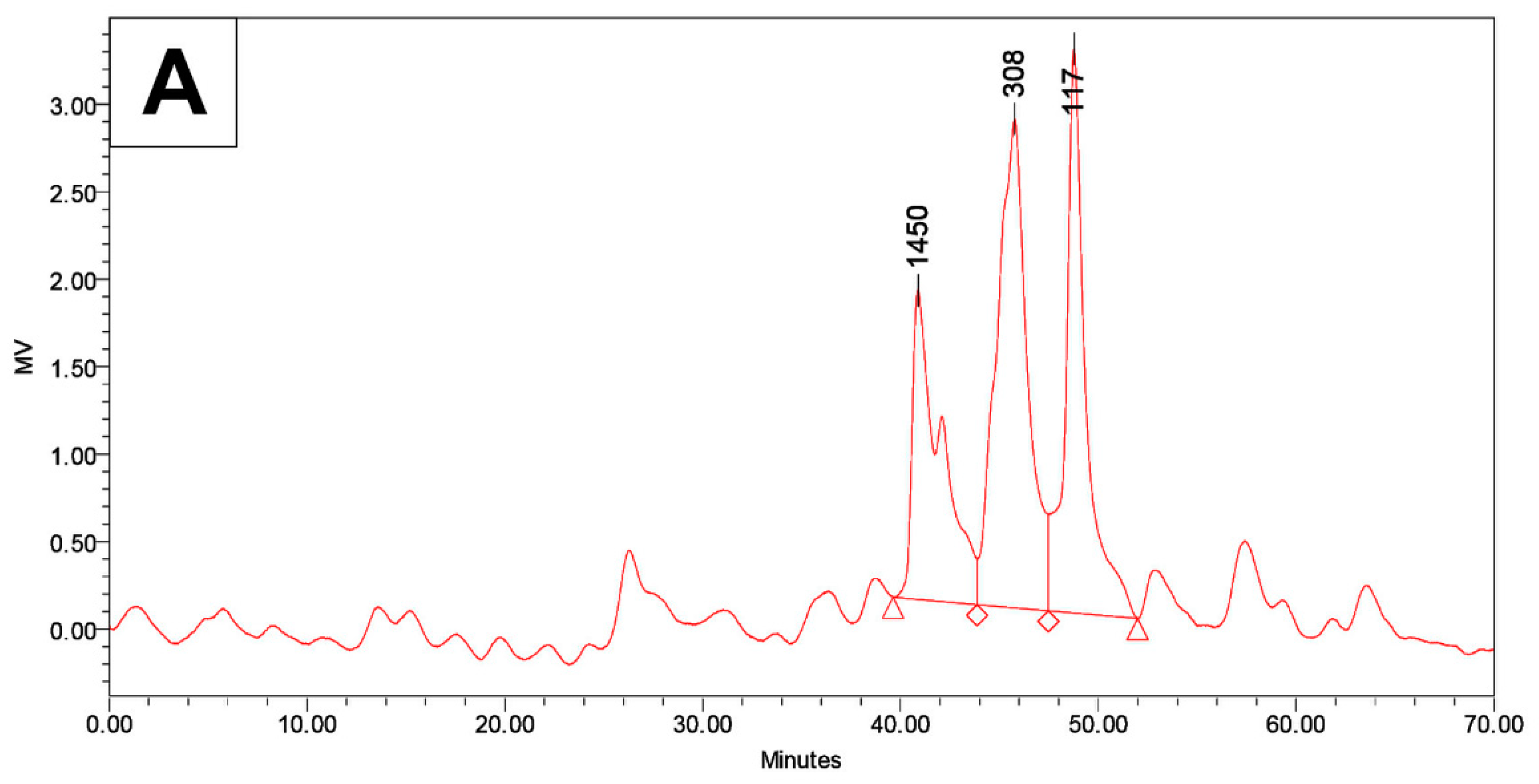
2.4. Gel Permeation Chromatograph Analysis
2.5. Fatty Acid Composition
2.6. Physicochemical Properties of Mealworm Oil
2.7. Minor Nutrients in Mealworm Oil
2.8. Bioactive Nutrients of Mealworm Powder and Antioxidant Capacity
2.9. NO Reduction in Lipopolysaccharide-Induced RAW 264.7 Cell Line
2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Compositions and Amino Acid Compositions of Mealworm
3.2. Protein Solubility and Soluble Particle Size Distributions of Mealworm Powders
3.3. Fatty Acid Composition of Mealworm Oil
3.4. Physicochemical Properties and Minor Nutrients of Mealworm Oil
3.5. Bioactive Nutrients, Antioxidant Capacity, and Anti-Inflammation Activity of Mealworms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Zhao, Z.R.; Liu, H. Feasibi lity of feeding yellow mealworm (Tenebrio molitor L.) in bioregenerative life support systems as a source of animal protein for humans. Acta Astronaut. 2013, 92, 103–109. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicaz, M.; Skibniewska, K.A.; Polak-Juszczak, L. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 4, 287–291. [Google Scholar]
- Han, S.R.; Lee, B.S.; Jung, K.J.; Yu, H.J.; Yun, E.Y.; Hwang, J.S.; Moon, K.S. Safety assessment of freeze-dried powdered Tenebrio molitor larvae (yellow mealworm) as novel food source: Evaluation of 90-day toxicity in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2016, 77, 206–212. [Google Scholar] [CrossRef]
- Ravazanaadii, N.; Kim, S.H.; Choi, W.H.; Hong, S.J.; Kim, N.J. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Indus. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Makkar, P.S.H.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Foster, D.K.; Bronlund, E.J.; Paterson, T.A.H.J. The contribution of milk fat towards the caking of dairy powders. Int. Dairy J. 2005, 15, 85–91. [Google Scholar] [CrossRef]
- Sharma, L.; Singh, C. Sesame protein based edible films: Development and characterization. Food Hydrocoll. 2016, 61, 139–147. [Google Scholar] [CrossRef]
- AOAC. Methods 932.06, 925.09, and 923.03. In Official Method of Analysis of AOAC International, 16th ed.; Association of Official Analytical Communities: Arlington, VA, USA, 1995. [Google Scholar]
- Son, Y.J.; Lee, J.C.; Hwang, I.K.; Nho, C.W.; Kim, S.H. Physicochemical properties of mealworm (Tenebrio molitor) powders manufactured by different industrial processes. LWT-Food Sci. Technol. 2019, 116, 108514. [Google Scholar] [CrossRef]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; van Buren, J.P.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A collaborative study to develop a standardized food protein solubility procedure. J. Food. Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Grubisic, Z.; Rempp, P.; Benoit, H. A universal calibration for gel permeation chromatography. Polym. Lett. 1967, 5, 753–759. [Google Scholar] [CrossRef]
- Jeon, Y.H.; Son, Y.J.; Kim, S.H.; Yun, E.Y.; Kang, H.J.; Hwang, I.K. Physicochemical properties and oxidative stabilities of mealworm (Tenebrio molitor) oils under different roasting conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Korean Food Standards Codex II; Ministry of Food and Drug Safety: Seoul, Korea, 2015; p. 48.
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Gómez-Rico, A.; Salvador, M.D.; Moriana, A.; Pérez, D.; Olmedilla, N.; Ribas, F.; Fregapane, G. Influence of different irrigation strategies in a traditional Cornicabra cv. olive orchard on virgin olive oil composition and quality. Food Chem. 2007, 100, 568–578. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Skiorska, E. Simple reversed-phase liquid chromatography method for determination of tocopherols in edible plant oils. J. Chromatogr. 1992, 1048, 1184–1188. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Belcher, R.; Nutten, A.J.; Sambrook, C.M. The determination of glucosamine. Analyst 1954, 79, 201–208. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, F.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline content in Burkina Fasan honey, as well as their radical scavenging acitivity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes-Extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.P.; List, G.R. Characterization of soybean oil extracted by supercritical carbon dioxide and hexane. J. Agric. Food Chem. 1982, 30, 192–193. [Google Scholar] [CrossRef]
- Brewer, M.S. Reducing the fat content in ground beef without sacrificing quality: A review. Meat. Sci. 2012, 91, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Lee, S.K.; Kim, H.K. Chemical compositions of the four lines of Korean native chickens. Korean J. Poult. Sci. 2016, 43, 119–128. [Google Scholar] [CrossRef]
- Franco, D.; González, L.; Bispo, E.; Rodríguez, P.; Garabal, J.I.; Moreno, T. Study of hydrolyzed protein composition, free amino acid, and taurine content in different muscles of galician blonde beef. J. Muscle Foods 2010, 21, 769–784. [Google Scholar] [CrossRef]
- FAO/WHO/UNU. Energy and Protein Requirements; World Health Organization Technical Report Series 724; FAO/WHO/UNU: Geneva, Switzerland, 1985. [Google Scholar]
- Jones, L.D.; Cooper, R.W.; Harding, R.S. Composition of mealworm Tenebrio molitor larvae. J. Zoo Anim. Med. 1972, 3, 34–41. [Google Scholar] [CrossRef]
- Enser, M.; Hallett, K.; Hewitt, B.; Fursey, G.A.J.; Wood, J.D. Fatty acid content and composition of English beef, lamb and pork at retail. Meat. Sci. 1996, 42, 443–456. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid. Sci. Technol. 2006, 58, 1051–1061. [Google Scholar] [CrossRef]
- Bramley, P.M.; Elmadfa, I.; Kafatos, A.; Kelly, F.J.; Manios, Y.; Roxborough, H.E.; Schuch, W.; Sheehy, P.J.A.; Wagner, K.H. Review: Vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Maraschiello, C.; Sárraga, C.; Regueiro, J.A.G. Glutathione peroxidase activity, TBARS, and α-tocopherol in meat from chickens fed different diets. J. Agric. Food Chem. 1999, 47, 867–872. [Google Scholar] [CrossRef]
- Surai, P.F.; Sparks, N.H.C. Tissue-specific fatty acid and α-tocopherol profiles in male chickens depending on dietary tuna oil and vitamin E provision. Poult. Sci. 2000, 70, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Okogeri, O.; Tasioula-Margari, M. Changes occurring in phenolic compounds and α-tocopherol of virgin olive oil during storage. J. Agric. Food Chem. 2002, 50, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R. The effect of operational parameters of the rancimat methods on the determination of the oxidative stability measures and shelf-life prediction of soybean oil. J. Am. Oil Chem. Soc. 2007, 84, 205–209. [Google Scholar] [CrossRef]
- Lourenço, R.; Camilo, M.E. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar]
- Largo, R.; Alvarez-Soria, M.A.; Díez-Ortego, I.; Calvo, E.; Sánchez-Pernaute, O.; Egido, J.; Herrero-Beaumont, G. Glucosamine inhibits IL-1β-induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthr. Cartil. 2003, 11, 290–298. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Crozier, A.; Aruoma, O. Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J. Sci. Food Agric. 2004, 84, 1553–1561. [Google Scholar] [CrossRef]
- Je, J.Y.; Park, P.J.; Kim, S.K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]



| WF-M † | DF-M ‡ | |
|---|---|---|
| Protein (%) | 52.2 ± 0.6 | 70.8 ± 5.8 |
| Essential amino acids (g/100 g protein) | ||
| Histidine | 3.1 ± 0.0 | 2.9 ± 0.2 |
| Lysine | 5.1 ± 0.1 | 5.1 ± 0.4 |
| Methionine | 0.5 ± 0.0 | 1.2 ± 0.1 |
| Phenylalanine | 3.9 ± 0.0 | 3.9 ± 0.2 |
| Threonine | 4.8 ± 0.0 | 4.3 ± 0.2 |
| Isoleucine | 4.5 ± 0.0 | 4.5 ± 0.2 |
| Leucine | 7.5 ± 0.0 | 7.5 ± 0.4 |
| Valine | 6.4 ± 0.0 | 6.4 ± 0.4 |
| Sub total | 35.8 ± 0.0 | 35.6 ± 0.3 |
| Non-essential amino acids (g/100 g protein) | ||
| Alanine | 8.1 ± 0.0 | 8.1 ± 0.6 |
| Aspartic acid | 8.4 ± 0.0 | 8.4 ± 0.4 |
| Arginine | 5.7 ± 0.0 | 5.7 ± 0.3 |
| Cysteine | N.D. | N.D. |
| Glutamic acid | 13.0 ± 0.0 | 13.2 ± 0.5 |
| Glycine | 5.3 ± 0.0 | 5.3 ± 0.4 |
| proline | 5.2 ± 0.0 | 5.4 ± 0.4 |
| Serine | 4.8 ± 0.0 | 4.8 ± 0.3 |
| Tyrosine | 7.3 ± 0.0 | 7.6 ± 0.5 |
| Sub total | 57.7 ± 0.0 | 58.5 ± 0.4 |
| Total (E + NE) § | 93.5 ± 0.0 | 94.1 ± 0.4 |
| E/NE | 62.0 | 60.9 |
| BCAA contents (%) ¶ | 19.4 ± 0.0 | 18.3 ± 0.4 |
| FAO/WHO Ref. (1985) | WF-M † | DF-M ‡ | |
|---|---|---|---|
| Histidine | 20 | 30.7 | 28.9 |
| Lysine | 55 | 51.1 | 50.8 |
| Methionine + Cysteine | 35 | 5.1 | 11.5 |
| Phenylalanine + Tyrosine | 60 | 111.6 | 114.5 |
| Threonine | 40 | 47.9 | 43.3 |
| Isoleucine | 40 | 44.6 | 44.5 |
| Leucine | 70 | 75.4 | 74.5 |
| Valine | 50 | 63.9 | 64.0 |
| Limiting amino acids | Met | Met | |
| Amino Acids Score (AAS) | 14.6 | 32.9 | |
| Fatty Acids | Composition (g/100 g Oil) |
|---|---|
| C4:0 | N.D. |
| C6:0 | N.D. |
| C8:0 | N.D. |
| C10:0 | N.D. |
| C11:0 | N.D. |
| C12:0 | 0.3 ± 0.0 |
| C13:0 | 0.1 ± 0.0 |
| C14:0 | 4.0 ± 0.2 |
| C14:1cis | N.D. |
| C16:0 | 15.8 ± 0.1 |
| C16:1cis | 1.8 ± 0.1 |
| C17:0 | 0.1 ± 0.0 |
| C18:0 | 2.3 ± 0.2 |
| C18:1cis | 44.5 ± 1.1 |
| C18:2 (n-6) | 19.5 ± 0.8 |
| C18:3 (n-3) | 0.4 ± 0.1 |
| C20:0 | 0.1 ± 0.0 |
| C20:1cis | 0.1 ± 0.0 |
| C20:2 (n-6) | 0.1 ± 0.0 |
| Total | 88.6 ± 0.6 |
| SFA † | 22.6 ± 0.1 |
| MUFA ‡ | 46.2 ± 1.6 |
| PUFA § | 19.8 ± 0.9 |
| USFA ¶ | 66.0 ± 0.7 |
| n-6:n-3 ratio | 47.0 ± 4.6 |
| P:S ratio | 0.9 ± 0.0 |
| Mealworm Oil | |
|---|---|
| Extraction Yield (%) | 29.5 ± 1.0 |
| Specific gravity (15 °C) | 0.8528 ± 0.0032 |
| Viscosity (cP) | 324.2 ± 6.3 |
| Color | |
| L (lightness) | 38.9 ± 0.3 |
| a (redness) | −1.9 ± 0.1 |
| b (yellowness) | 7.5 ± 0.6 |
| Peroxide value (meq/1000 g oil) | 3.5 ± 0.2 |
| Acid value (mg KOH/g oil) | 2.6 ± 0.0 |
| TBARS (mg MDA/1000 g oil) | 1.8 ± 0.1 |
| The contents of tocopherols (mg/1000 g oil) | |
| α-Tocopherol | 6.3 ± 0.1 |
| β-Tocopherol | 8.5 ± 0.6 |
| γ-Tocopherol | 123.5 ± 2.7 |
| δ-Tocopherol | 6.1 ± 0.3 |
| Total tocopherol | 144.3 ± 3.0 |
| Total polyphenol (mg GAE †/1000 g oil) | 18.0 ± 1.3 |
| Squalene (mg/1000 g oil) | 21.1 ± 5.0 |
| Induction time (h) | 36.0 ± 0.7 |
| Predicted shelf-life (day) ‡ | 305 |
| DF-M † | |
|---|---|
| GABA (mg/100 g dry basis) | 3.5 ± 0.1 |
| Taurine (mg/100 g dry basis) | 17.8 ± 0.3 |
| Glucosamine (g/100 g dry basis) | 7.0 ± 0.7 |
| Polyphenol (mg GAE ‡/100 g dry basis) | 8.2 ± 0.2 |
| Flavonoid (mg CE §/100 g dry basis) | 2.0 ± 0.2 |
| DPPH (mg TE ¶/g dry basis) | 21.5 ± 0.5 |
| ABTS (mg TE ¶/g dry basis) | 12.3 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.-J.; Choi, S.Y.; Hwang, I.-K.; Nho, C.W.; Kim, S.H. Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients? Foods 2020, 9, 40. https://doi.org/10.3390/foods9010040
Son Y-J, Choi SY, Hwang I-K, Nho CW, Kim SH. Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients? Foods. 2020; 9(1):40. https://doi.org/10.3390/foods9010040
Chicago/Turabian StyleSon, Yang-Ju, Soo Young Choi, In-Kyeong Hwang, Chu Won Nho, and Soo Hee Kim. 2020. "Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients?" Foods 9, no. 1: 40. https://doi.org/10.3390/foods9010040
APA StyleSon, Y.-J., Choi, S. Y., Hwang, I.-K., Nho, C. W., & Kim, S. H. (2020). Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients? Foods, 9(1), 40. https://doi.org/10.3390/foods9010040




