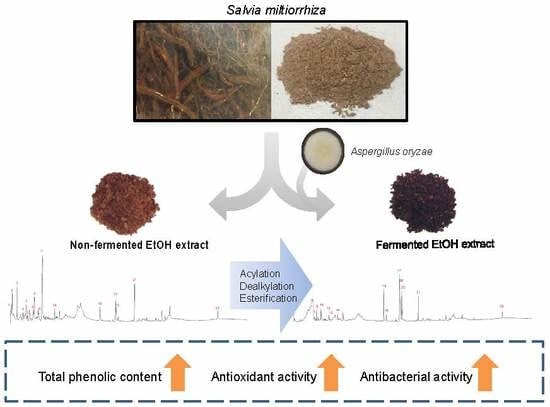
Enhancement of Antioxidant and Antibacterial Activities of Salvia miltiorrhiza Roots Fermented with Aspergillus oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganisms and Growth Conditions
2.3. Sample Preparation of S. miltiorrhiza Ethanol Extracts
2.4. Solid-State Fermentation
2.5. Determination of Total Carbohydrate Content
2.6. Determination of Phenolic and Flavonoid Contents
2.7. Antioxidant Assay
2.8. Antibacterial Activity Assay
2.9. Liquid Chromatography and Mass Spectrometry Analysis
2.10. Gas Chromatography and Mass Spectrometry Analysis
2.11. Statistical Analysis
3. Results
3.1. Extraction Yield and TCC of SME and SMBE
3.2. TPC and TFC of SME and SMBE
3.3. Antioxidant Activity of SME and SMBE
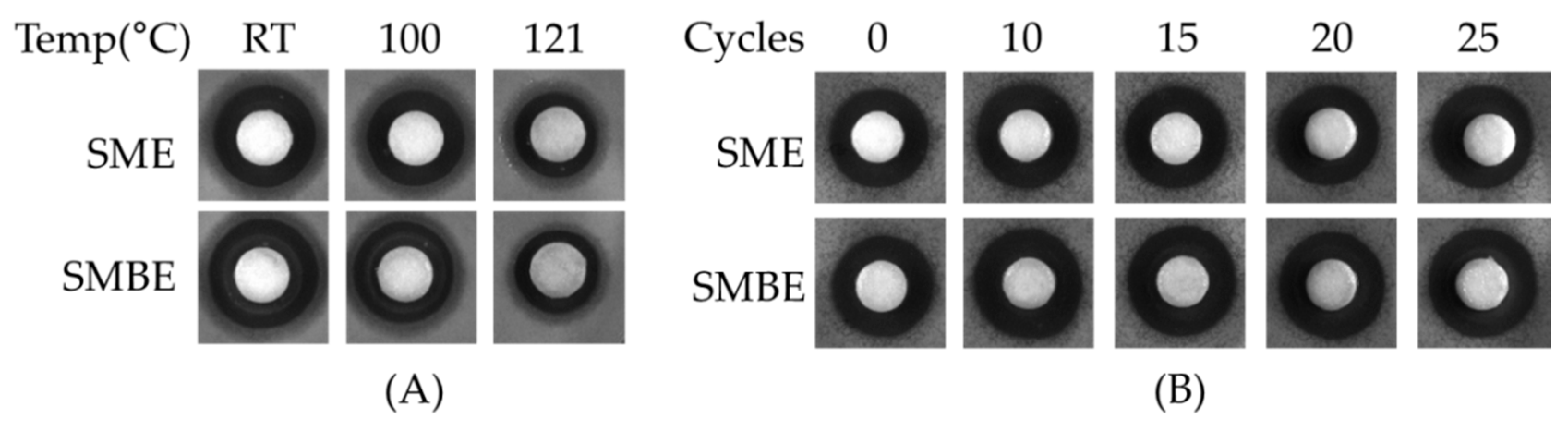
3.4. Antibacterial Activity and Stability of SME and SMBE
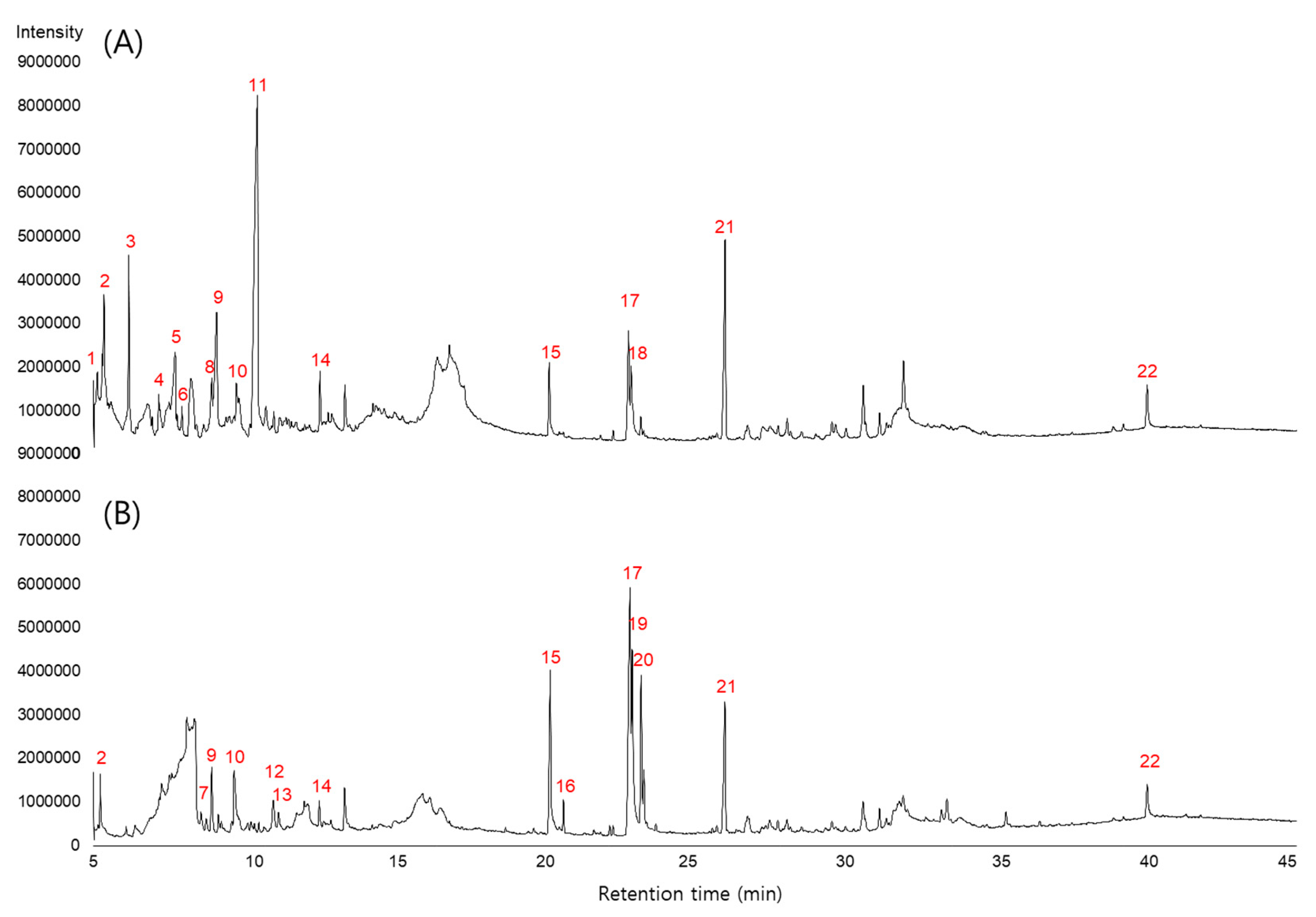
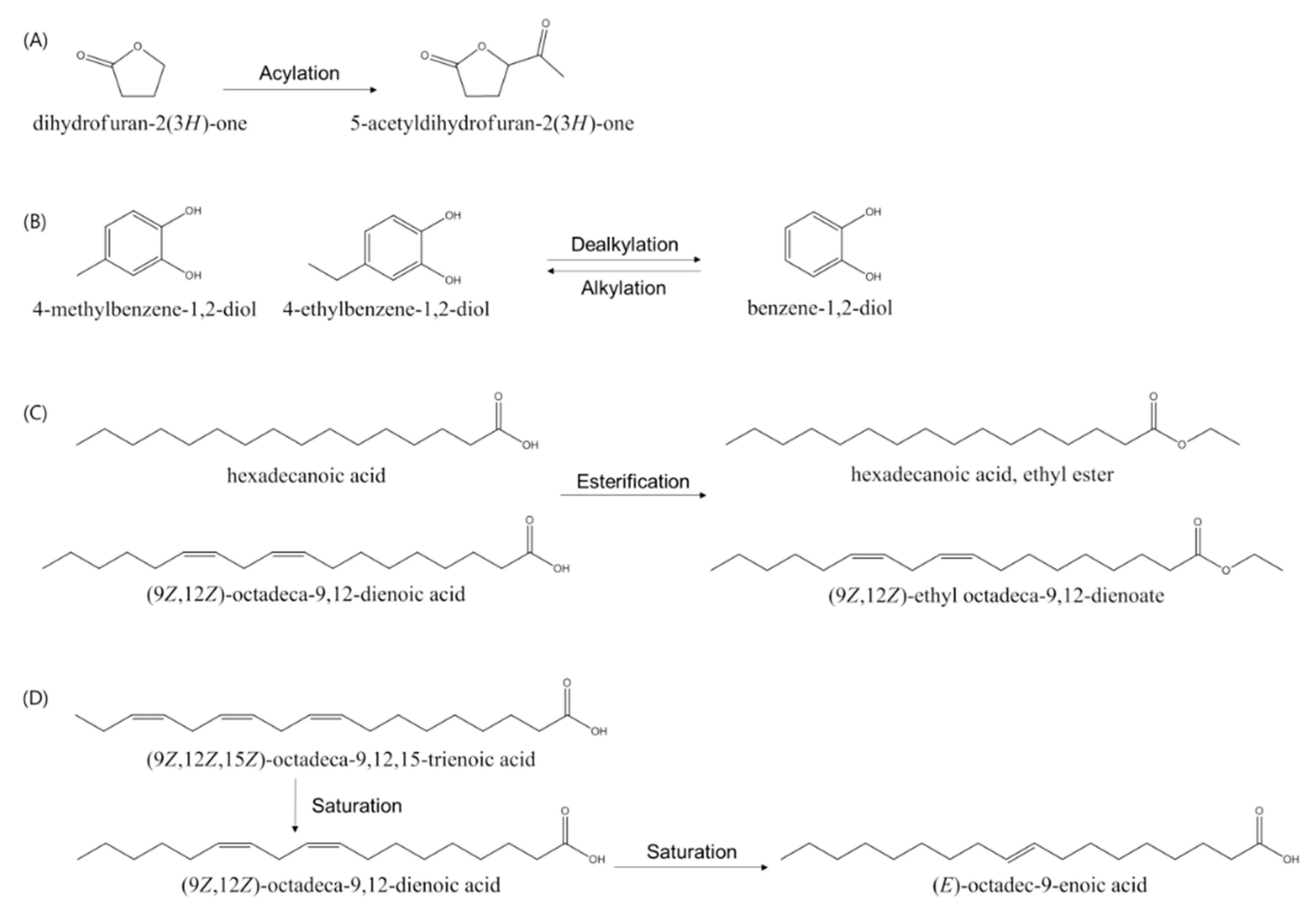
3.5. Identification and Analysis of Metabolites of SME and SMBE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, B.Q. Salvia miltiorrhiza: Chemical and pharmacological review of a medicinal plant. J. Med. Plants Res. 2010, 4, 2813–2820. [Google Scholar]
- Deng, J.; Zhang, D.; Yang, W. An in vitro experiment on the antimicrobial effects of ethanol extract from Salvia miltiorrhiza Bunge on several oral pathogenic microbes. Shanghai Kou Qiang Yi Xue 2006, 15, 210–212. [Google Scholar] [PubMed]
- Lee, H.S.; Kim, Y. Antifungal activity of Salvia miltiorrhiza against Candida albicans is associated with the alteration of membrane permeability and (1, 3)-β-d-glucan synthase activity. J. Microbiol. Biotechnol. 2016, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lou, J.; Mou, Y.; Li, P.; Wu, J.; Zhou, L. Diterpenoid tanshinones and phenolic acids from cultured hairy roots of Salvia miltiorrhiza Bunge and their antimicrobial activities. Molecules 2011, 16, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Mao, Z.; Shan, T.; Wang, Q.; Zhou, L. Chemical composition, antibacterial and antioxidant properties of the essential oils from the roots and cultures of Salvia miltiorrhiza. J. Essent. Oil Bear. Plants 2014, 17, 380–384. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Cen, Y.; Yang, D.; Qi, Z.; Hou, Z.; Han, S.; Cai, Z.; Liu, K. Bivariate correlation analysis of the chemometric profiles of Chinese wild Salvia miltiorrhiza based on UPLC-Qqq-MS and antioxidant activities. Molecules 2018, 23, 538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Song, Z.; Fang, X.; Pan, Y.; Guo, L.; Liu, T.; Wang, J. Effect of genotype and environment on Salvia miltiorrhiza roots using LC/MS-based metabolomics. Molecules 2016, 21, 414. [Google Scholar] [CrossRef]
- He, C.E.; Lu, L.L.; Jin, Y.; Wei, J.H.; Christie, P. Effects of nitrogen on root development and contents of bioactive compounds in Salvia miltiorrhiza Bunge. Crop Sci. 2013, 53, 2028–2039. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Chen, S.B.; Gao, G.Y.; Feng, Y.X.; Yang, S.L.; Xu, L.Z.; Du, L.J.; Lin, J.; Li, M. Content of inorganic elements of Salvia miltiorrhiza root in different area and physicochemical properties of its growing soil. Zhongguo Zhong Yao Za Zhi 2004, 29, 844–850. [Google Scholar]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Handayani, R.; Sulistyo, J.; Rahayu, R.D. Extraction of coconut oil (Cocos nucifera L.) through fermentation system. Biodiversitas 2009, 10, 151–157. [Google Scholar] [CrossRef]
- Lee, M.; Cho, J.Y.; Lee, Y.G.; Lee, H.J.; Lim, S.I.; Park, S.L.; Moon, J.H. Bioconversion of Capsaicin by Aspergillus oryzae. J. Agric. Food Chem. 2015, 63, 6102–6108. [Google Scholar] [CrossRef]
- Razak, D.L.A.; Rashid, N.Y.A.; Jamaluddin, A.; Sharifudin, S.A.; Kahar, A.A.; Long, K. Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar]
- Lee, S.; Seo, M.H.; Oh, D.K.; Lee, C.H. Targeted metabolomics for Aspergillus oryzae-mediated biotransformation of soybean isoflavones, showing variations in primary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Kim, M.J.; John, K.M.; Choi, J.N.; Lee, S.; Kim, A.J.; Kim, Y.M.; Lee, C.H. Changes in secondary metabolites of green tea during fermentation by Aspergillus oryzae and its effect on antioxidant potential. Food Res. Int. 2013, 53, 670–677. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Xiang, H.; Cao, F.; Ming, D.; Zheng, Y.; Dong, X.; Zhong, X.; Mu, D.; Li, B.; Zhong, L.; Cao, J.; et al. Aloe-emodin inhibits Staphylococcus aureus biofilms and extracellular protein production at the initial adhesion stage of biofilm development. Appl. Microbiol. Biotechnol. 2017, 101, 6671–6681. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, B.A.; Burt, T.M.; Tirey, D.A.; Williams, L.E.; Kuzmak, B.R.; Stanton, D.T.; Morand, K.L.; Nelson, S.L. The effect of freeze/thaw cycles on the stability of compounds in DMSO. J. Biomol. Screen. 2003, 8, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Su, S.; Xiang, X.; Sha, X.; Zhu, Z.; Wang, Y.; Guo, S.; Yan, H.; Qian, D.; Duan, J. Comparative analysis of the major chemical constituents in Salvia miltiorrhiza roots, stems, leaves and flowers during different growth periods by UPLC-TQ-MS/MS and HPLC-ELSD methods. Molecules 2017, 22, 771. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Sung, W.S.; Jung, H.J.; Lee, I.S.; Kim, H.S.; Lee, D.G. Antimicrobial effect of furaneol against human pathogenic bacteria and fungi. J. Microbiol. Biotechnol. 2006, 16, 349–354. [Google Scholar]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res. Int. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, H.S. 1, 2-benzendiol isolated from persimmon roots and its structural analogues show antimicrobial activities against food-borne bacteria. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 429–433. [Google Scholar] [CrossRef]
- Scopel, M.; Abraham, W.R.; Antunes, A.L.; Terezinha Henriques, A.; Macedo, J.; Jose, A. Mevalonolactone: An inhibitor of Staphylococcus epidermidis adherence and biofilm formation. Med. Chem. 2014, 10, 246–251. [Google Scholar] [CrossRef]
- Krishnan, K.R.; James, F.; Mohan, A. Isolation and characterization of n-hexadecanoic acid from Canthium parviflorum leaves. J. Chem. Pharm. Res. 2016, 8, 614–617. [Google Scholar]
- Musa, A.M.; Ibrahim, M.A.; Aliyu, A.B.; Abdullahi, M.S.; Tajuddeen, N.; Ibrahim, H.; Oyewale, A.O. Chemical composition and antimicrobial activity of hexane leaf extract of Anisopus mannii (Asclepiadaceae). J. Intercult. Ethnopharmacol. 2015, 4, 129. [Google Scholar] [CrossRef]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch. Oral Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Yoo, J.S.; Lee, T.G.; Cho, H.Y.; Kim, Y.H.; Kim, W.G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.I.; Hwang, Y.H.; Sugamoto, K.; Matsui, T. Antimicrobial activity of heartwood components of sugi (Cryptomeria japonica) against several fungi and bacteria. J. Wood Sci. 2006, 52, 552–556. [Google Scholar] [CrossRef]
- Deyab, M.A.; Abou-Dobara, M.I. Antibacterial activity of some marine algal extracts against most nosocomial bacterial infections. Egypt. J. Exp. Biol. (Bot.) 2013, 9, 281–286. [Google Scholar]
- Kim, M.H.; Kim, S.I.; Seo, D.W.; Ryu, J.C.; Choi, H.Y. Antioxidant activity of Salvia miltiorrhiza Bunge, a novel foodstuff. Mol. Cell Toxicol. 2010, 6, 65–72. [Google Scholar] [CrossRef]
- Wen, Y.L.; Yan, L.P.; Chen, C.S. Effects of fermentation treatment on antioxidant and antimicrobial activities of four common Chinese herbal medicinal residues by Aspergillus oryzae. J. Food Drug Anal. 2013, 21, 219–226. [Google Scholar] [CrossRef]
- Xing, Y.; Cai, L.; Yin, T.P.; Chen, Y.; Yu, J.; Wang, Y.R.; Ding, Z.T. Improving the antioxidant activity and enriching salvianolic acids by the fermentation of Salvia miltiorrhizae with Geomyces luteus. J. Zhejiang Univ. Sci. B 2016, 17, 391–398. [Google Scholar] [CrossRef]
- Dos Santos Oliveira, M.; Feddern, V.; Kupski, L.; Cipolatti, E.P.; Badiale-Furlong, E.; de Souza-Soares, L.A. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 2011, 102, 8335–8338. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Xin, X.; Tang, Y.; Zhao, G.; Xiao, X. Efficient acylation of gastrodin by Aspergillus oryzae whole-cells in non-aqueous media. RSC Adv. 2019, 9, 16701–16712. [Google Scholar] [CrossRef]
- Khmelnitsky, Y.L.; Budde, C.; Arnold, J.M.; Usyatinsky, A.; Clark, D.S.; Dordick, J.S. Synthesis of water-soluble paclitaxel derivatives by enzymatic acylation. J. Am. Chem. Soc. 1997, 119, 11554–11555. [Google Scholar] [CrossRef]
- Guo, Q.Q.; Zeng, J.H.; Lu, Y.; Shu, X.G. Effects of solubility, thermal stability and antioxidant properties of acylating dihydromyricetin. In Advanced Materials Research; Trans Tech Publications: Zurich, Switzerland, 2013; pp. 101–105. [Google Scholar]
- Li, R.; Narita, R.; Ouda, R.; Kimura, C.; Nishimura, H.; Yatagai, M.; Fujita, T.; Watanabe, T. Structure-dependent antiviral activity of catechol derivatives in pyroligneous acid against the encephalomycarditis virus. RSC Adv. 2018, 8, 35888–35896. [Google Scholar] [CrossRef]
- Ralston, A.W.; Hoerr, C.W. The solubilities of the normal saturated fatty acids. J. Org. Chem. 1942, 7, 546–555. [Google Scholar] [CrossRef]
- Sedgwick, R.S.; Hoerr, C.W.; Harwood, H.J. Solubilities of saturated fatty acid esters. J. Org. Chem. 1952, 17, 327–337. [Google Scholar] [CrossRef]
| Sample | Extraction Yield (g) | Phenolic Content (mg GAE/g) | Flavonoid Content (mg QE/g) | |||
|---|---|---|---|---|---|---|
| SME | SMBE | SME | SMBE | SME | SMBE | |
| n-Hexane | 0.97 | 0.41 | 33.6 ± 1.3 (32.6) 1 | 30.1 ± 2.6 *** (12.3) | 21.6 ± 0.2 (21.0) | 32.3 ± 0.0 *** (13.2 ***) |
| Chloroform | 0.59 | 0.22 | 41.2 ± 2.5 (24.3) | 103.2 ± 3.9 *** (22.7) | 28.3 ± 0.5 (16.7) | 40.6 ± 0.6 *** (8.9 ***) |
| EtOAc | 0.62 | 0.62 | 543.5 ± 19.0 (337.0) | 623.0 ± 12.6 ** (386.3 **) | 13.3 ± 0.4 (8.2) | 24.3 ± 0.9 *** (15.1 ***) |
| n-BuOH | 1.25 | 1.67 | 426.8 ± 17.3 (533.5) | 342.3 ± 2.7 *** (571.6 *) | 7.9 ± 0.5 (9.9) | 5.2 ± 0.9 * (8.7) |
| Water | 13.79 | 4.21 | 42.2 ± 2.8 (581.9) | 186.0 ± 2.5 *** (783.1 ***) | 1.3 ± 0.2 (17.9) | 5.3 ± 0.3 *** (22.3) |
| Sample | DPPH (μg QE/g) | FRAP (mg FeSO4/g) | ||
|---|---|---|---|---|
| SME | SMBE | SME | SMBE | |
| n-Hexane | 24.5 ± 0.3 (20.9) 1 | 17.6 ± 0.5 *** (7.2 ***) | 0.7 ± 0.1 (0.7) | 0.6 ± 0.1 * (0.2 **) |
| Chloroform | 43.1 ± 0.2 (25.4) | 98.5 ± 4.1 (21.7) | 0.8 ± 0.1 (0.5) | 1.8 ± 0.0 * (0.4 *) |
| EtOAc | 750.9 ± 8.3 (465.6) | 699.2 ± 19.9 ** (433.5 **) | 14.9 ± 0.7 (9.2) | 13.4 ± 0.8 ** (8.3 **) |
| n-BuOH | 392.7 ± 5.4 (490.9) | 347.3 ± 25.7 ** (580.0 **) | 8.2 ± 0.5 (10.3) | 6.4 ± 0.1 ** (10.7) |
| Water | 39.7 ± 1.3 (547.5) | 160.1 ± 16.3 (674.0 *) | 0.9 ± 0.1 (12.4) | 3.1 ± 0.1 *** (13.1) |
| Bacteria | SME (μg/mL) | SMBE (μg/mL) | |
|---|---|---|---|
| Gram (+) | Bacillus cereus | 128 | 64 |
| Staphylococcus aureus | 256 | 256 | |
| Listeria monocytogenes | 2048 | 1024 | |
| Streptococcus iniae | 512 | 256 | |
| Streptococcus parauberis | 512 | 256 | |
| Gram (-) | Pseudomonas aeruginosa | ND 1 | ND |
| Klebsiella pneumoniae | ND | ND | |
| Escherichia coli | ND | ND | |
| Vibrio fluvialis | ND | ND | |
| Vibrio mimicus | ND | ND | |
| Solvent Fraction | SME (μg/mL) | SMBE (μg/mL) |
|---|---|---|
| EtOH | 128 | 64 |
| n-Hexane | <2 | 8 |
| Chloroform | 4 | 16 |
| EtOAc | 1024 | 256 |
| n-BuOH | ND 1 | 4096 |
| Water | ND | ND |
| Peak No. | RT (min) | Area | Name of Compound | Activity 2 | Ref | |
|---|---|---|---|---|---|---|
| SME | SMBE | |||||
| 1 | 5.1 | 2,021,039 | ND 1 | dihydrofuran-2(3H)-one | ||
| 2 | 5.3 | 6,839,225 | 2,364,683 | 2-hydroxycyclopent-2-enone | ||
| 3 | 6.2 | 11,684,632 | ND | 2,4-dihydroxy-2,5-dimethylfuran-3(2H)-one | ||
| 4 | 7.2 | 2,621,991 | ND | 3-nitrobut-1-ene | ||
| 5 | 7.7 | 10,327,938 | ND | 4-hydroxy-2,5-dimethylfuran-3(2H)-one | 2 | [25] |
| 6 | 7.9 | 1,108,052 | ND | 5-hydroxy-6-methyl-2H-pyran-4(3H)-one | ||
| 7 | 8.6 | ND | 1,085,995 | 5-acetyldihydrofuran-2(3H)-one | ||
| 8 | 8.9 | 5,064,272 | ND | 3-acetyl-3-hydroxydihydrofuran-2(3H)-one | ||
| 9 | 9.1 | 15,999,437 | 4,860,418 | 3,5-dihydroxy-6-methyl-2H-pyran-4(3H)-one | 2 | [26] |
| 10 | 9.7 | 3,175,165 | 6,158,327 | benzene-1,2-diol | 1,2 | [27] |
| 11 | 10.4 | 63,567,813 | ND | 5-(hydroxymethyl)furan-2-carbaldehyde | ||
| 12 | 10.9 | ND | 3,084,106 | 4-hydroxy-4-methyltetrahydro-2H-pyran-2-one | 2 | [28] |
| 13 | 11.1 | ND | 1,184,908 | 4-methylbenzene-1,2-diol | 1,2 | [27] |
| 14 | 12.5 | 3,903,321 | 1,754,609 | 4-ethylbenzene-1,2-diol | 1 | |
| 15 | 20.1 | 5,873,955 | 15,800,575 | hexadecanoic acid | 1,2 | [29] |
| 16 | 20.6 | ND | 1,634,484 | hexadecanoic acid, ethyl ester | 1,2 | [30] |
| 17 | 22.7 | 10,155,786 | 29,314,886 | (9Z,12Z)-octadeca-9,12-dienoic acid | 2 | [31] |
| 18 | 22.8 | 8,594,663 | ND | (9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid | 2 | [32] |
| 19 | 22.9 | ND | 16,887,502 | octadec-9-enoic acid | 2 | [31] |
| 20 | 23.2 | ND | 10,363,933 | (9Z,12Z)-ethyl octadeca-9,12-dienoate | 2 | [31] |
| 21 | 26.0 | 18,898,107 | 12,189,786 | ferruginol | 1,2 | [33] |
| 22 | 40.0 | 4,118,012 | 3,229,685 | γ-sitosterol | 1,2 | [34] |
| Peak No. | R.T. (min) | Ion Mode | Area | Name of the Compound | Activity 2 | Ref | |
|---|---|---|---|---|---|---|---|
| SME | SMBE | ||||||
| 1 | 6.0 | ES- | 1,434,150 | ND 1 | rosmarinic acid | 1,2 | [4] |
| 2 | 6.3 | ES- | 6,285,563 | ND | salvianolic acid B | 1,2 | [35] |
| 3 | 9.3 | ES+ | 10,401,970 | 1,136,992 | dihydrotanshinone I | 1,2 | [4] |
| 4 | 9.8 | ES+ | 57,574,444 | 71,574,320 | cryptotanshinone | 1,2 | [4] |
| 5 | 10.3 | ES+ | 65,527,940 | 95,715,976 | tanshinone IIA | 1,2 | [4] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, K.; Cha, J. Enhancement of Antioxidant and Antibacterial Activities of Salvia miltiorrhiza Roots Fermented with Aspergillus oryzae. Foods 2020, 9, 34. https://doi.org/10.3390/foods9010034
Moon K, Cha J. Enhancement of Antioxidant and Antibacterial Activities of Salvia miltiorrhiza Roots Fermented with Aspergillus oryzae. Foods. 2020; 9(1):34. https://doi.org/10.3390/foods9010034
Chicago/Turabian StyleMoon, Keumok, and Jaeho Cha. 2020. "Enhancement of Antioxidant and Antibacterial Activities of Salvia miltiorrhiza Roots Fermented with Aspergillus oryzae" Foods 9, no. 1: 34. https://doi.org/10.3390/foods9010034
APA StyleMoon, K., & Cha, J. (2020). Enhancement of Antioxidant and Antibacterial Activities of Salvia miltiorrhiza Roots Fermented with Aspergillus oryzae. Foods, 9(1), 34. https://doi.org/10.3390/foods9010034