Mung Bean Protein Hydrolysates Protect Mouse Liver Cell Line Nctc-1469 Cell from Hydrogen Peroxide-Induced Cell Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Protein Hydrolysates and Ultrafiltered Fractions
2.3. Cell Culture
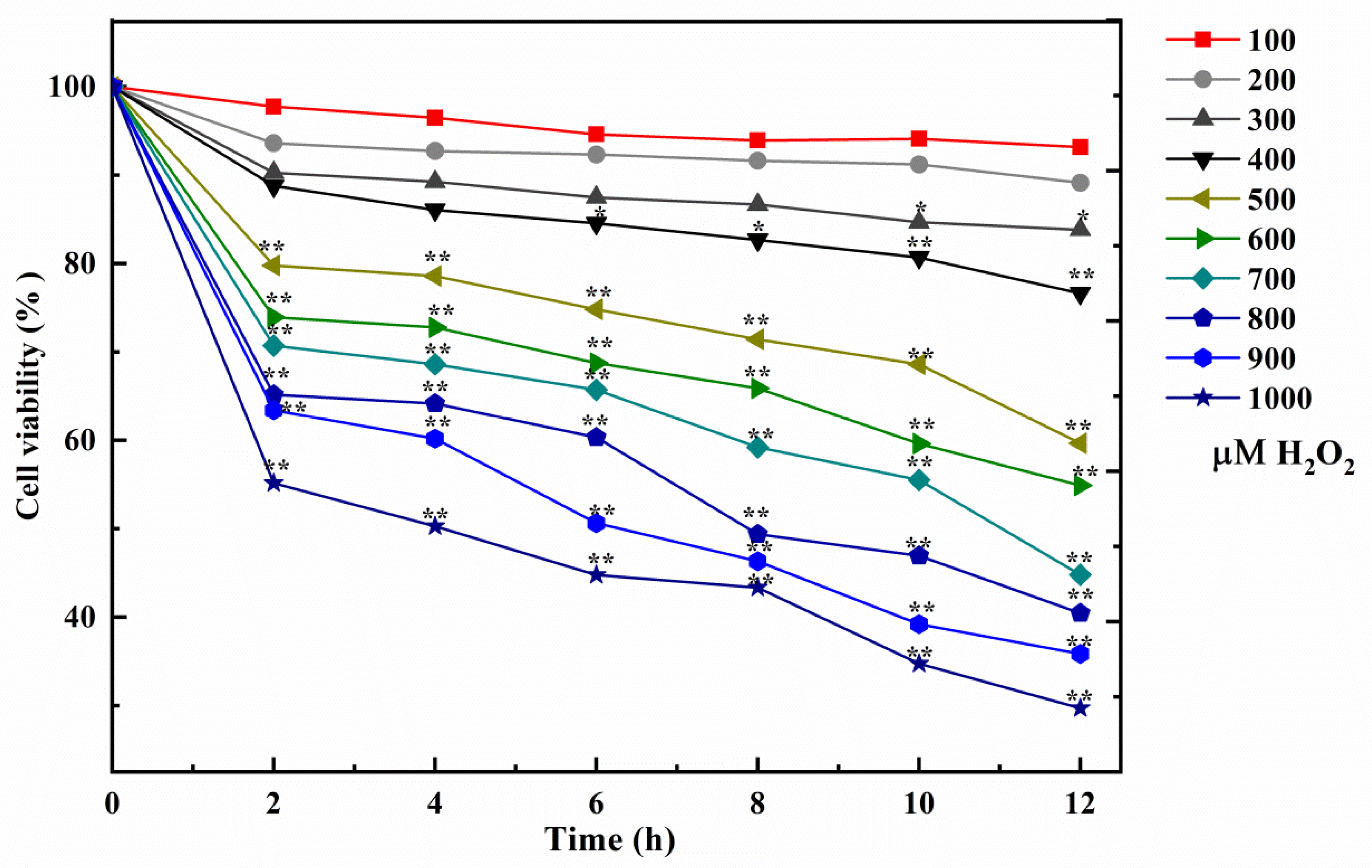
2.4. Influence of Cell Oxidative Damage Induced by H2O2
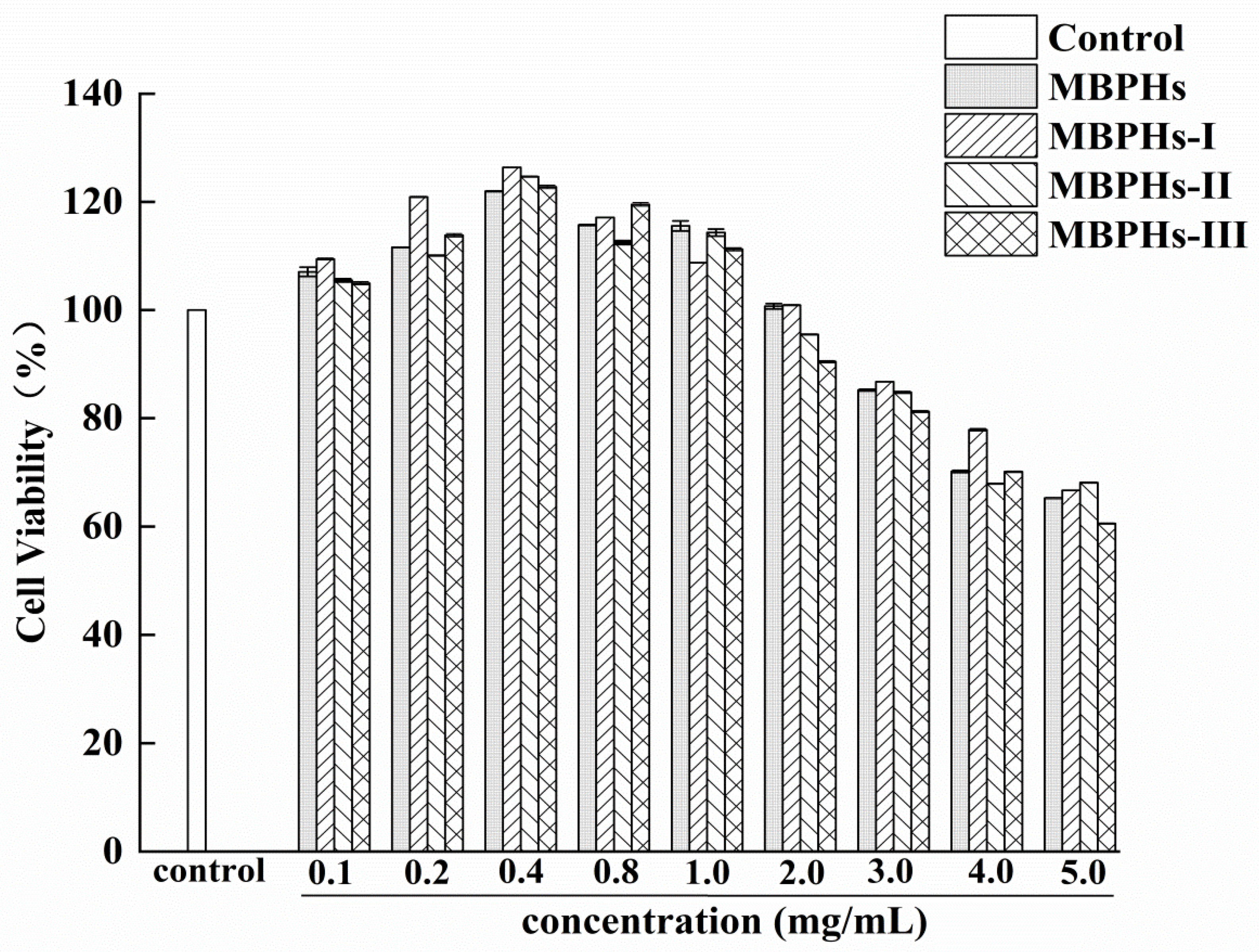
2.5. Assessment of Cytotoxicity of Protein Hydrolysates Using CCK-8 Assay
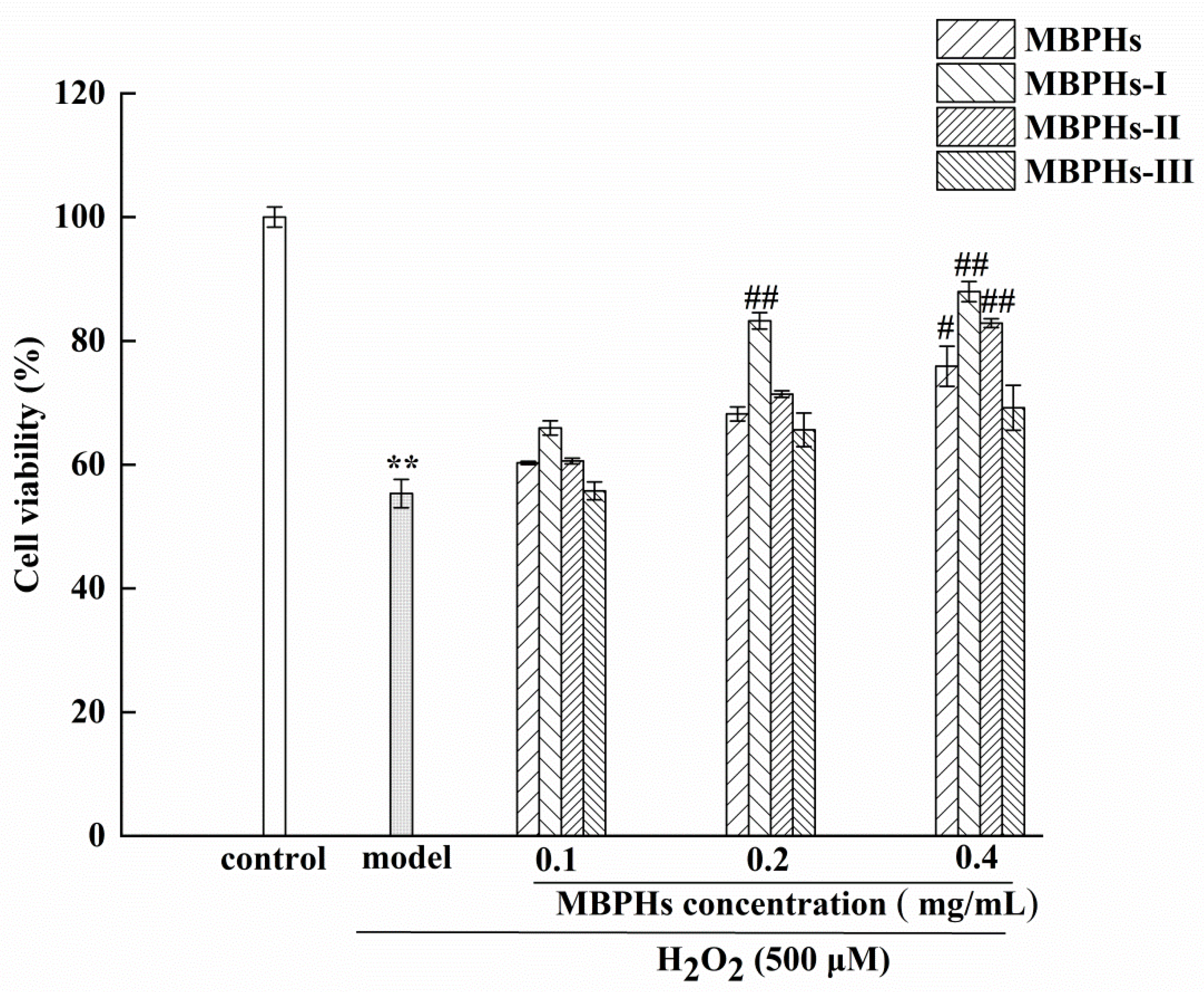
2.6. Protection Effect of MBPHs and Three Fractions on Oxidation-Induced Cell Damage
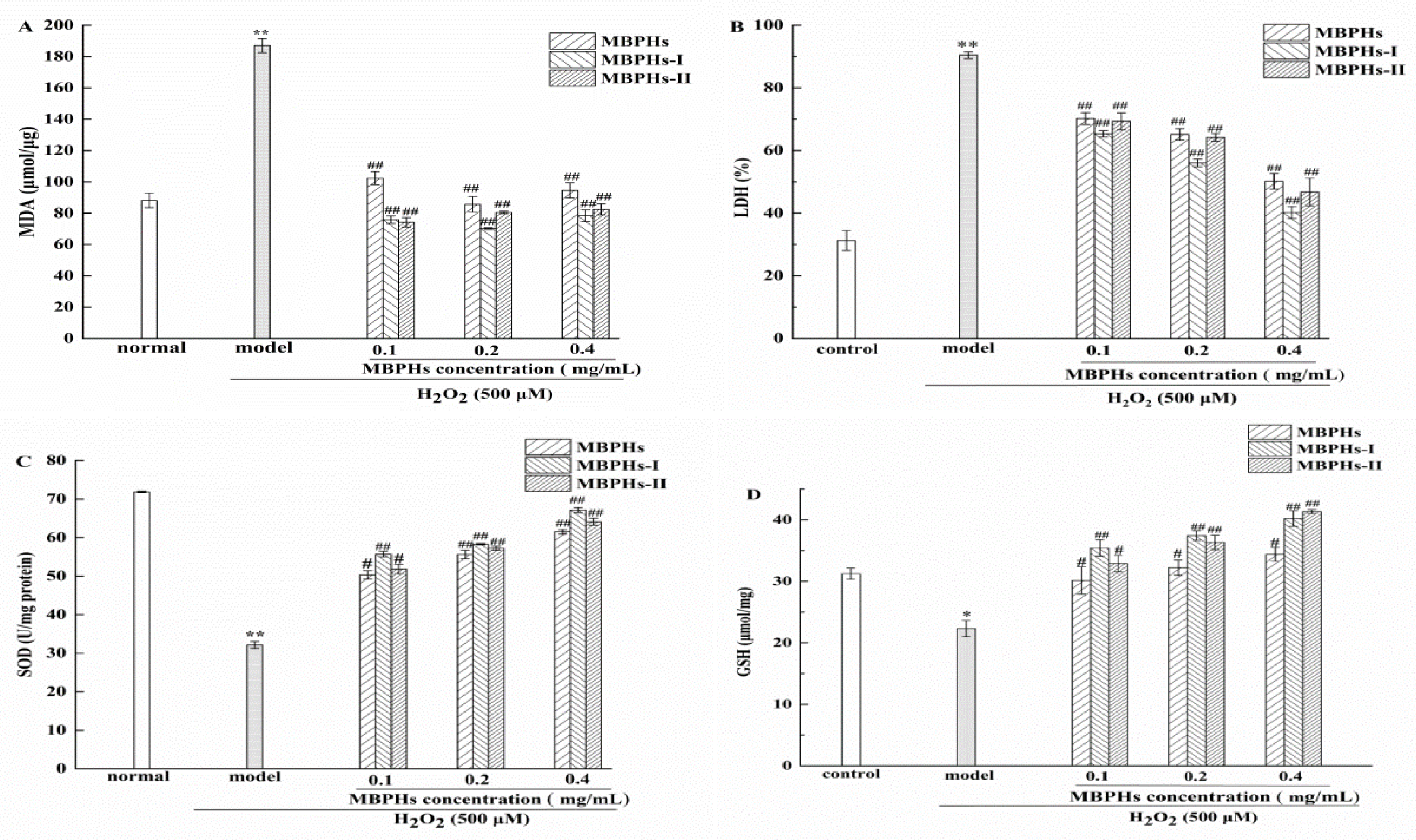
2.7. Determination of MDA, GSH, SOD, and LDH
2.8. Determination of Intracellular ROS
2.9. Amino Acid Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Cell Model Induced by H2O2
3.2. Cytotoxicity of Mbphs and the Ultrafiltration Fractions
3.3. Protective Effects of MBPHs and Ultrafiltration Fractions
3.4. Changes of MDA, GSH, SOD, and LDH on Injured NCTC-1469 Cells
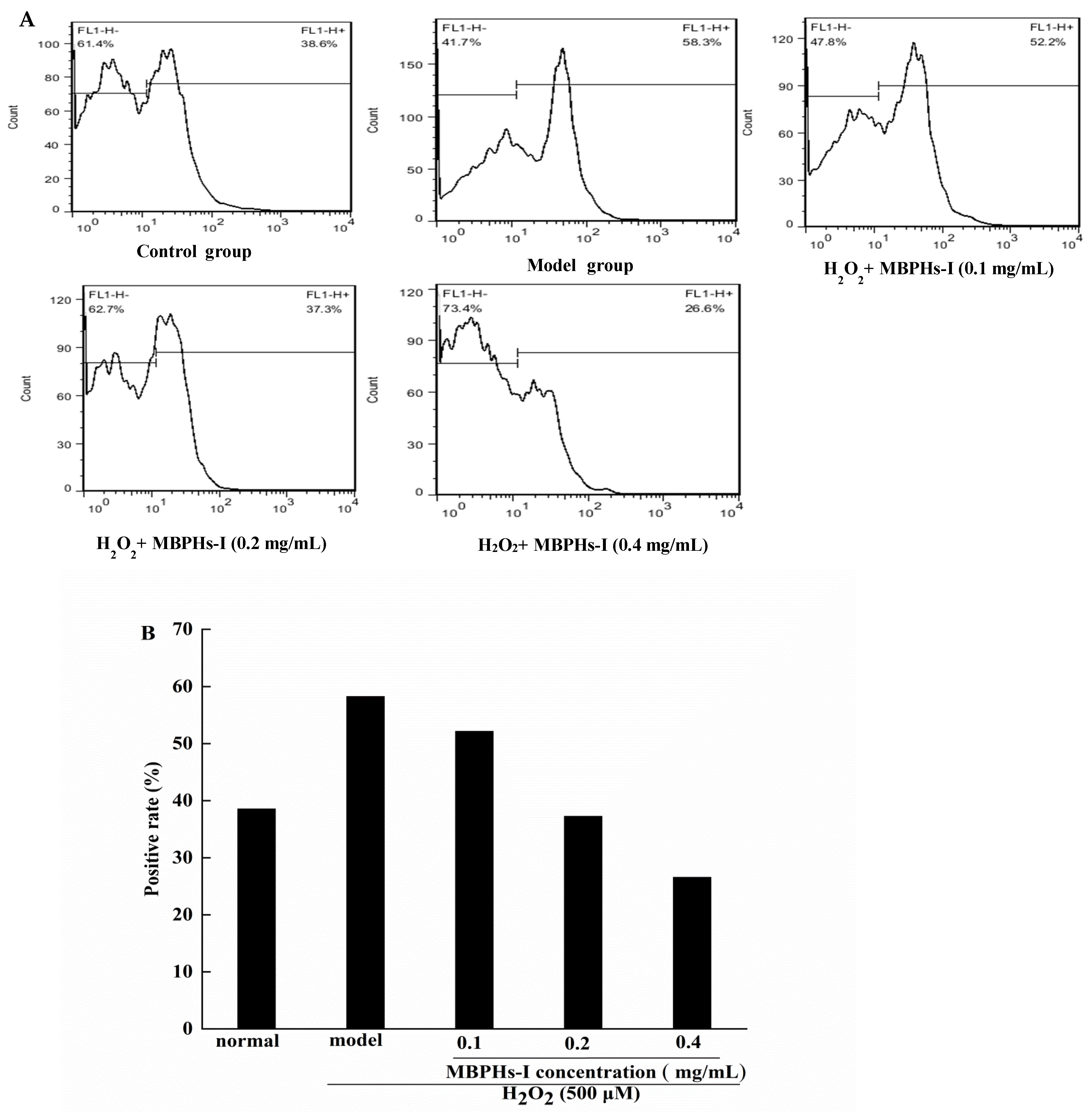
3.5. Effect of MBPHs-I on the Level of ROS on Cells
3.6. Analysis of Amino Acid Composition of MBPHs and MBPHs-I
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cao, J.X.; Zhou, C.Y.; Wang, Y.; Sun, Y.Y.; Pan, D.D. The effect of oxidation on the structure of G-actin and its binding ability with aroma compounds in carp grass skeletal muscle. Food Chem. 2018, 240, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Ding, L.; Yu, Z.P.; Zhang, T.; Ma, S.; Liu, J.B. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Kim, D.; Jeon, Y.J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Masoud, H.T.; Ahmad, A.; Mozhgan, S. Cytotoxic and antioxidant capacity of camel milk peptides: Effects of isolated peptide on superoxide dismutase and catalase gene expression. J. Food. Drug Anal. 2017, 25, 567–575. [Google Scholar]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Sharma, N. Free Radicals, Antioxidants and Disease. Biol. Med. 2014, 6, 1. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Yue, Y.; Wu, S.C.; Zhang, H.F.; Zhang, X.Y.; Niu, Y.H.; Cao, X.Q.; Huang, F.W.; Ding, H. Characterization and hepatoprotective effect of polysaccharides from Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou sarcocarp. Food Chem. Toxicol. 2014, 74, 76–84. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Pigeon pea enzymatic protein hydrolysates and ultrafiltration peptide fractions as potential sources of antioxidant peptides: An in vitro study. LWT Food Sci. Technol. 2018, 97, 269–278. [Google Scholar] [CrossRef]
- Cai, L.Y.; Wu, X.S.; Zhang, Y.H.; Li, X.X.; Ma, S.; Li, J.R. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Food. 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Liu, J.H.; Huang, Y.S.; Tian, Y.G.; Nie, S.P.; Xie, J.H.; Wang, Y.; Xie, M.Y. Purification and identification of novel antioxidative peptide released from Black-bone silky fowl (Gallus gallus domesticus Brisson). Eur. Food Res. Technol. 2013, 237, 253–263. [Google Scholar] [CrossRef]
- Wu, R.B.; Wu, C.L.; Liu, D.; Yang, X.H.; Huang, J.F.; Zhang, J.; Liao, B.Q.; He, H.L. Antioxidant and anti-freezing peptides from salmon collagen hydrolysate prepared by bacterial extracellular protease. Food Chem. 2018, 248, 346–352. [Google Scholar] [CrossRef]
- Wang, H.M.; Pan, J.L.; Chen, C.Y.; Chiu, C.C.; Yang, M.H.; Chang, H.W.; Chang, J.S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B.J. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Yongsawatdigul, J. Immunomodulatory activity of protein hydrolysates derived from Virgibacillus halodenitrificans SK1-3-7 proteinase. Food Chem. 2017, 224, 320–328. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ma, L.; Otte, J. Optimization of hydrolysis conditions for production of angiotensin-converting enzyme inhibitory peptides from Basa fish skin using response surface methodology. J. Aquat. Food Prod. Technol. 2016, 25, 684–693. [Google Scholar] [CrossRef]
- Ranamukhaarachchi, S.; Meissner, L.; Moresoli, C. Production of antioxidant soy protein hydrolysates by sequential ultrafiltration and nanofiltration. J. Membr. Sci. 2013, 429, 81–87. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Dong, S.T.; Sun, B.; Liu, J.B. Optimisation of antioxidant peptide preparation from corn gluten meal. J. Sci. Food Agric. 2013, 93, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.C.; Bamdad, F.; Ganzle, M.; Chen, L.Y. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.X.; Zhou, H.M.; Qian, H.F. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O. Physicochemical and flavor characteristics of flavoring agent from mungbean protein hydrolyzed by bromelain. J. Agric. Food Chem. 2011, 59, 8475–8483. [Google Scholar] [CrossRef]
- Lapsongphon, N.; Yongsawatdigul, J. Production and purification of antioxidant peptides from a mungbean meal hydrolysate by Virgibacillus sp. SK37 proteinase. Food Chem. 2013, 141, 992–999. [Google Scholar] [CrossRef]
- Li, G.H.; Wan, J.Z.; Le, G.W.; Shi, Y.H. Novel angiotensin I-converting enzyme inhibitory peptides isolated from Alcalase hydrolysate of mung bean protein. J. Pept. Sci. 2006, 12, 509–514. [Google Scholar] [CrossRef]
- Xie, J.H.; Du, M.X.; Shen, M.Y.; Wu, T.; Lin, L.H. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Soucek, J.; Soucek, J.; Pouckova, P.; Matougek, J.; Slavik, T.; Matousek, J. Mung bean sprout (Phaseolus aureus) nuclease and its biological and antitumor effects. Neoplasma 2005, 53, 402–409. [Google Scholar]
- Du, M.X.; Xie, J.H.; Gong, B.; Xin, X.; Tang, W.; Li, X.; Li, C.; Xie, M.Y. Extraction, physicochemical characteristics and functional properties of mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Tang, W.; Shen, M.Y.; Xie, J.H.; Liu, D.; Du, M.X.; Lin, L.H.; Gao, H.; Hamaker, B.R.; Xie, M.Y. Physicochemical characterization, antioxidant activity of polysaccharides from Mesona chinensis Benth and their protective effect on injured NCTC-1469 cells induced by H2O2. Carbohydr. Polym. 2017, 175, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence. Lancet 1994, 344, 721. [Google Scholar] [CrossRef]
- Xue, Z.H.; Wen, H.C.; Zhai, L.J.; Yu, Y.Q.; Li, Y.N.; Yu, W.C.; Cheng, A.Q.; Wang, C.; Kou, X.H. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Ma, L.; Wang, X.M. Correlation between Protein Hydrolysates and Color during Fermentation of Mucor-Type Douchi. Int. J. Food Prop. 2015, 18, 2800–2812. [Google Scholar] [CrossRef]
- Huang, L.X.; Huang, M.; Shen, M.Y.; Wen, P.W.; Wu, T.; Hong, Y.Z.; Yu, Q.; Chen, Y.; Xie, J.H. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef]
- Xie, J.H.; Xie, M.Y.; Nie, S.P.; Shen, M.Y.; Wang, Y.X.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Mehdi, M.Z.; Azar, Z.M.; Srivastava, A.K. Role of receptor and nonreceptor Protein Tyrosine Kinases in H2O2-induced PKB and ERK1/2 Signaling. Cell Biochem. Biophys. 2007, 47, 1–10. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, J.H.; Yang, Y.J.; Zhang, F.; Wang, S.N.; Wu, T.; Shen, M.Y.; Xie, M.Y. Sulfated Cyclocarya paliurus polysaccharides markedly attenuates inflammation and oxidative damage in lipopolysaccharide-treated macrophage cells and mice. Sci. Rep. 2017, 7, 40402. [Google Scholar] [CrossRef]
- Park, J.E.; Yang, J.H.; Yoon, S.J.; Lee, J.H.; Yang, E.S.; Park, J.W. Lipid peroxidation-mediated cytotoxicity and DNA damage in U937 cells. Biochimie 2002, 84, 1198–1204. [Google Scholar] [CrossRef]
- Xiao, X.H.; Liu, J.T.; Hu, J.W.; Zhu, X.P.; Yang, H.; Wang, C.Y.; Zhang, Y.H. Protective effects of protopine on hydrogen peroxide-induced oxidative injury of PC12 cells via Ca2+ antagonism and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 591, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Liu, Q.; Xiao, B.; Zhou, J.; Zhang, J.G.; Li, Y. The vascular protective properties of kinsenoside isolated from Anoectochilus roxburghii under high glucose condition. Fitoterapia 2013, 86, 163–170. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xia, C.L.; Pan, D.D.; Cao, J.X.; Sun, Y.Y. Proteomic responses to oxidative damage in meat from ducks exposed to heat stress. Food Chem. 2019, 295, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, F.; Li, Q.; Chen, H.; Zhang, W.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Structure characterization of one polysaccharide from Lepidium meyenii Walp., and its antioxidant activity and protective effect against H2O2-induced injury RAW264.7 cells. Int. J. Biol. Macromol. 2018, 118, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Ahbab, M.A.; Kolankaya, D.; Barlas, N. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 2010, 48, 2865–2871. [Google Scholar] [CrossRef]
- Pinho, R.A.; Andrades, M.E.; Oliveira, M.R.; Pirola, A.C.; Zago, M.S.; Silveira, P.C.L.; Dal-Pizzol, F.; Moreira, J.C.F. Imbalance in SOD/CAT activities in rat skeletal muscles submitted to treadmill training exercise. Cell Biol. Int. 2006, 30, 848–853. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Tong, X.H.; Li, Y.; Wang, H.; Wang, Z.J.; Qi, B.K.; Sui, X.N.; Jiang, L.Z. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef]
- Feng, L.; Peng, F.; Wang, X.J.; Li, M.; Lei, H.J.; Xu, H.D. Identification and characterization of antioxidative peptodes derived derived from simulated in vitro gastrointestinal digestion of walnut meal protein. Food Res. Int. 2019, 116, 518–526. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, B.; He, J.G.; Qian, P. Structure-activity relationship study of antioxidative peptides by QSAR modeling: The amino acid next to C-terminus affects the activity. J. Pept. Sci. 2011, 17, 454–462. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Amin, I. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Li, B. Characterization of structure-antioxidant activity relationship of peptides in free radical systems using QSAR models: Key sequence positions and their amino acid properties. J. Theor. Biol. 2013, 318, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, C.F.; Fashakin, J.B.; Fagbemi, T.N.; Aluko, R.E. Effect of Peptide Size on Antioxidant Properties of African Yam Bean Seed (Sphenostylis stenocarpa) Protein Hydrolysate Fractions. Int. J. Mol. Sci. 2011, 12, 6685–6702. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid | MBPHs (g/100 g) | MBPHS-I (g/100 g) |
|---|---|---|
| Aspartic (Asp) | 9.580 | 11.292 |
| Threonine (Thr) | 3.022 | 3.319 |
| Serine (Ser) | 6.618 | 6.618 |
| Glx a | 20.251 | 22.231 |
| Alanine (Ala) | 3.964 | 4.615 |
| Cysteine (Cys) | 10.307 | 10.059 |
| Valine (Val) | 4.807 | 5.499 |
| Methionine (Met) | 1.107 | 1.650 |
| Isoleucine (Ile) | 3.816 | 4.928 |
| Leucine (Leu) | 7.317 | 9.151 |
| Tyrosine (Tyr) | 3.072 | 4.653 |
| Phenylalanine (Phe) | 5.831 | 6.913 |
| Histidine (His) | 3.287 | 3.044 |
| Lysine (Lys) | 6.558 | 6.913 |
| Arginine (Arg) | 7.251 | 7.325 |
| Proline (Pro) | 4.047 | 3.790 |
| HAA b | 32.904 | 38.317 |
| AAA c | 8.903 | 10.566 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Ye, H.; Du, M.; Yu, Q.; Chen, Y.; Shen, M. Mung Bean Protein Hydrolysates Protect Mouse Liver Cell Line Nctc-1469 Cell from Hydrogen Peroxide-Induced Cell Injury. Foods 2020, 9, 14. https://doi.org/10.3390/foods9010014
Xie J, Ye H, Du M, Yu Q, Chen Y, Shen M. Mung Bean Protein Hydrolysates Protect Mouse Liver Cell Line Nctc-1469 Cell from Hydrogen Peroxide-Induced Cell Injury. Foods. 2020; 9(1):14. https://doi.org/10.3390/foods9010014
Chicago/Turabian StyleXie, Jianhua, Hedan Ye, Mengxia Du, Qiang Yu, Yi Chen, and Mingyue Shen. 2020. "Mung Bean Protein Hydrolysates Protect Mouse Liver Cell Line Nctc-1469 Cell from Hydrogen Peroxide-Induced Cell Injury" Foods 9, no. 1: 14. https://doi.org/10.3390/foods9010014
APA StyleXie, J., Ye, H., Du, M., Yu, Q., Chen, Y., & Shen, M. (2020). Mung Bean Protein Hydrolysates Protect Mouse Liver Cell Line Nctc-1469 Cell from Hydrogen Peroxide-Induced Cell Injury. Foods, 9(1), 14. https://doi.org/10.3390/foods9010014






