Effect of Moist Cooking Blanching on Colour, Phenolic Metabolites and Glucosinolate Content in Chinese Cabbage (Brassica rapa L. subsp. chinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Moist Cooking Treatment
- (i)
- blanching at 95 °C in water bath [thermostatically regulated water bath (PolyScience, Niles, IL, USA)] for 5 min in water, or in 5%, 10% or 20% lemon juice solutions;
- (ii)
- a microwave treatment (Defy) (household) working at 2450 MHz–900 W for 5 min in water, or in 5%, 10% or 20% lemon juice solutions;
- (iii)
- steaming in stainless steel steamer pot for 5 min in water or in 5%, 10% or 20% lemon juice solutions at 100 °C.
2.3. Chemicals
2.4. Colour Measurement
2.5. Chlorophyll
2.6. Total Phenol and Predominant Metabolic Profile
2.6.1. Total Phenol Content
2.6.2. Predominant Metabolic Profile
2.7. Total Antioxidant Capacities Were Determined Using the Following Assays
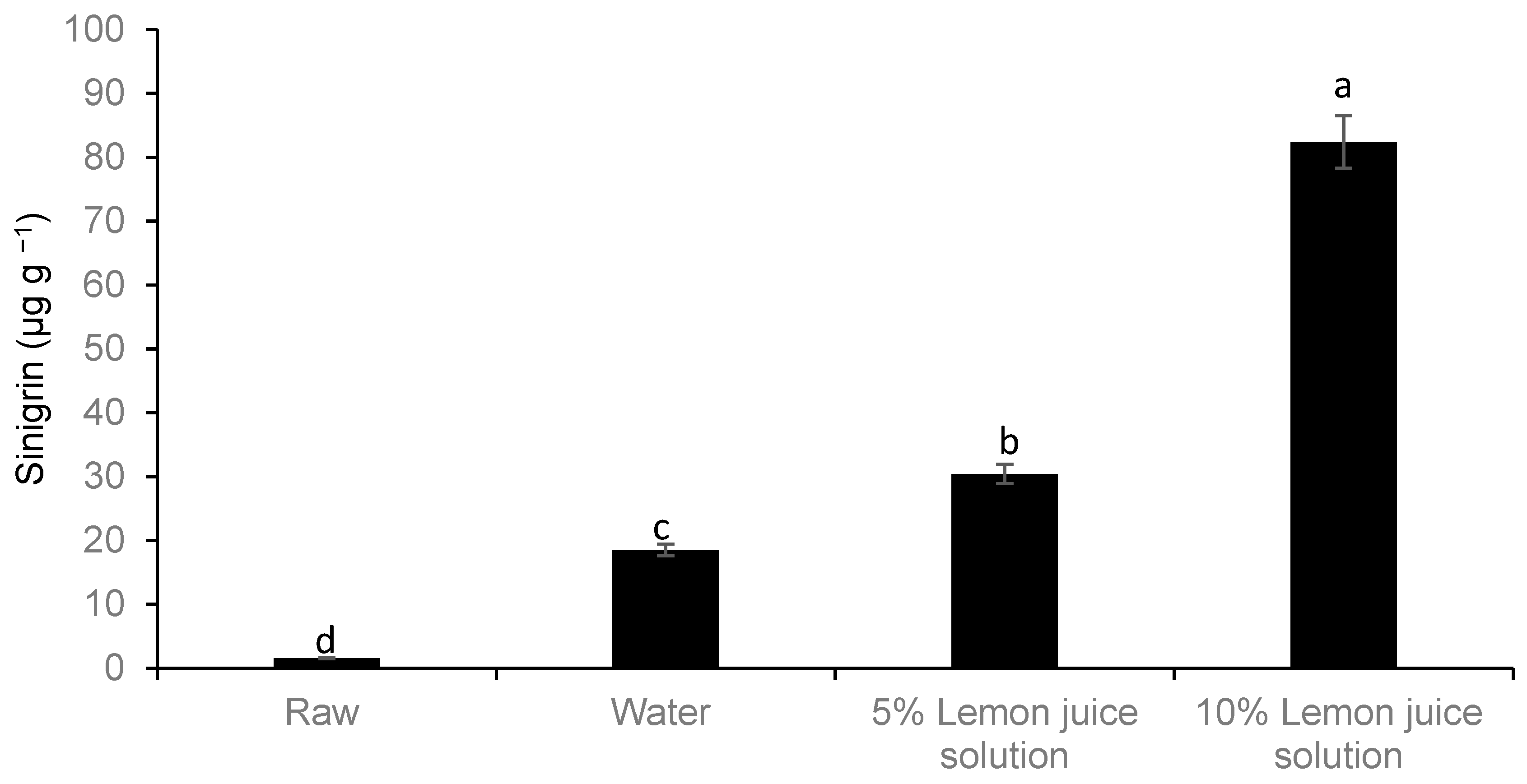
2.8. Glucosinolate
2.9. Statistical Analysis
3. Results
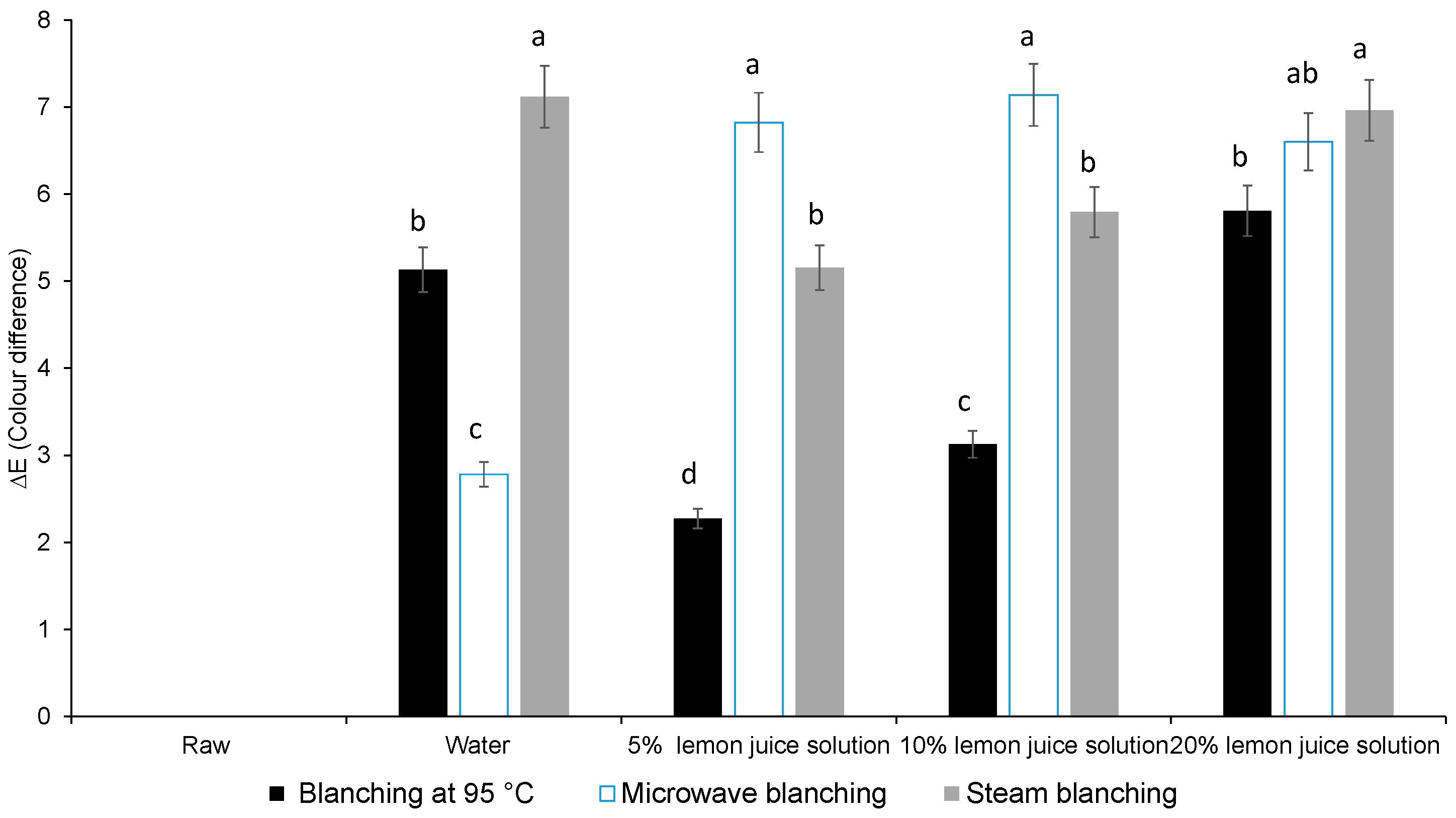
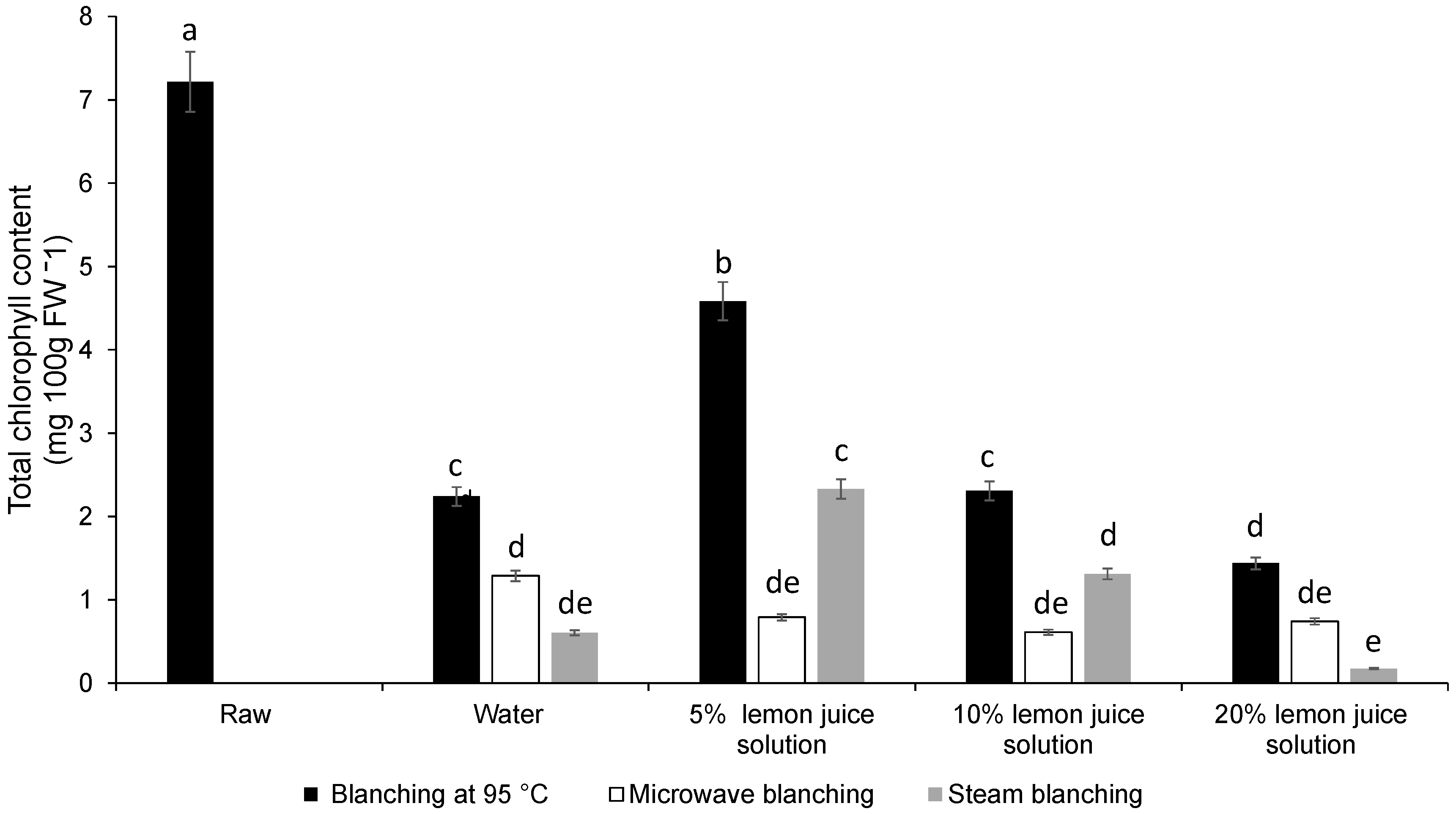
3.1. Colour Difference, Chlorophyll Content
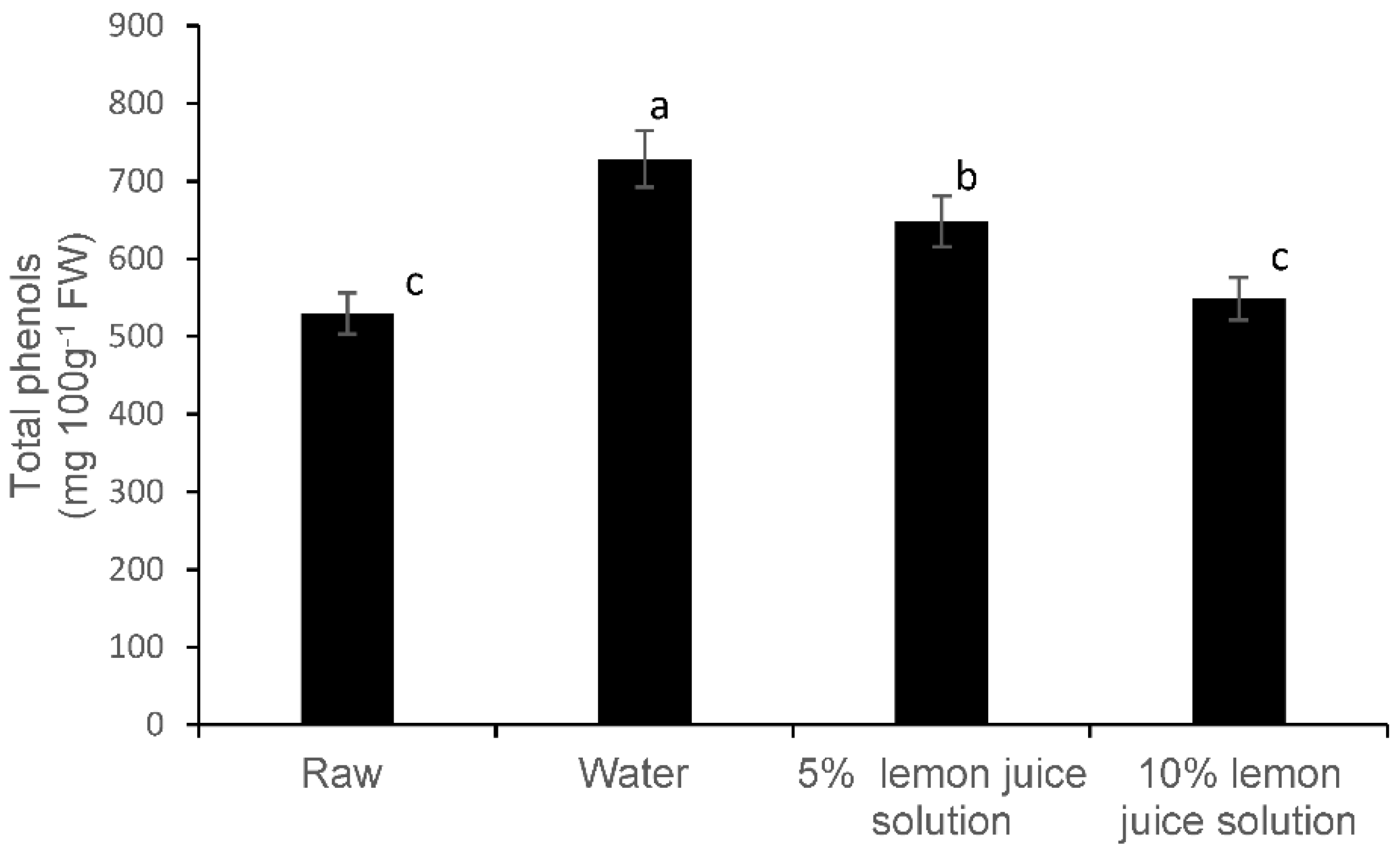
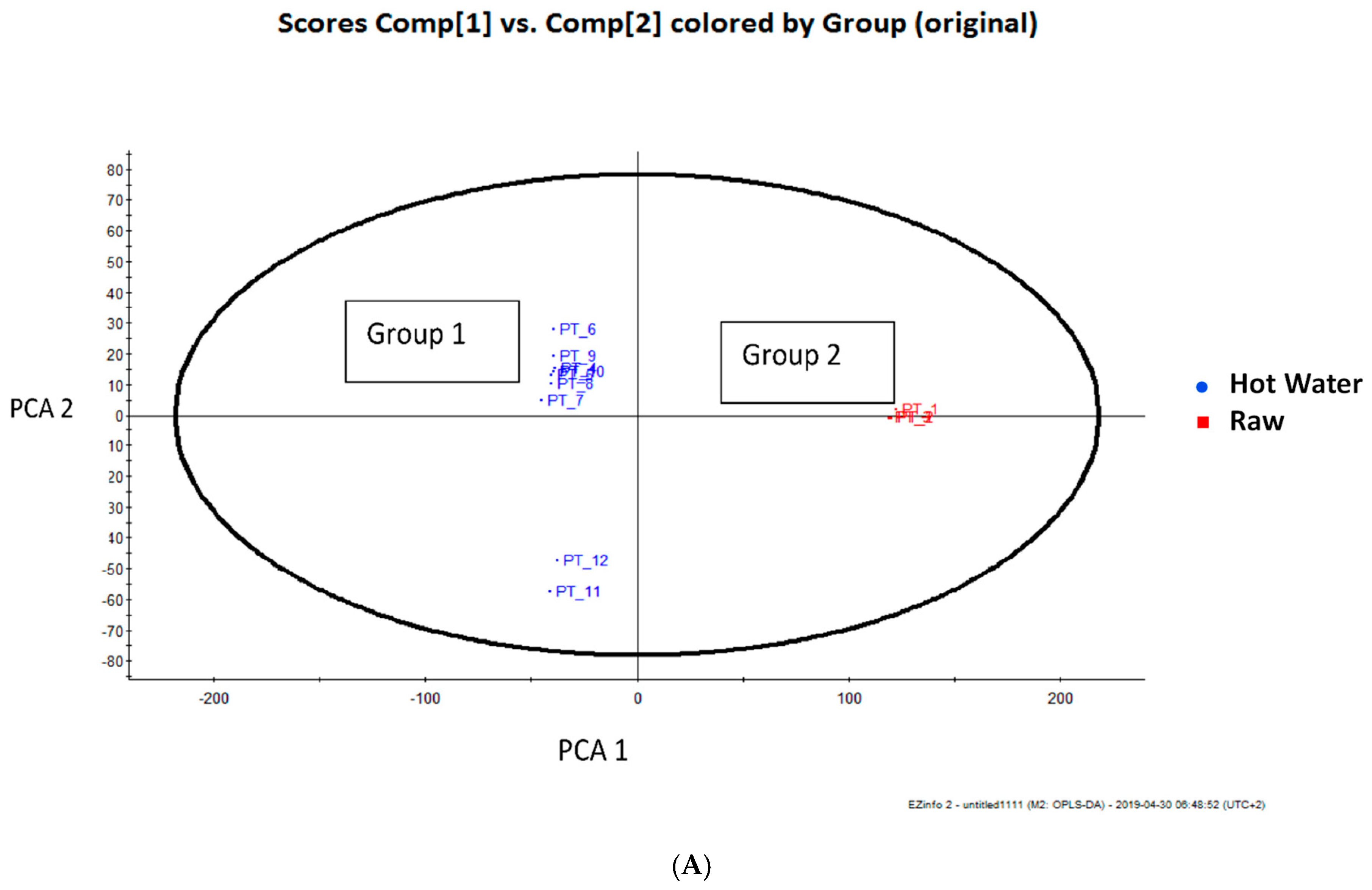
3.2. Total Phenolic Compounds and Phenolic Components
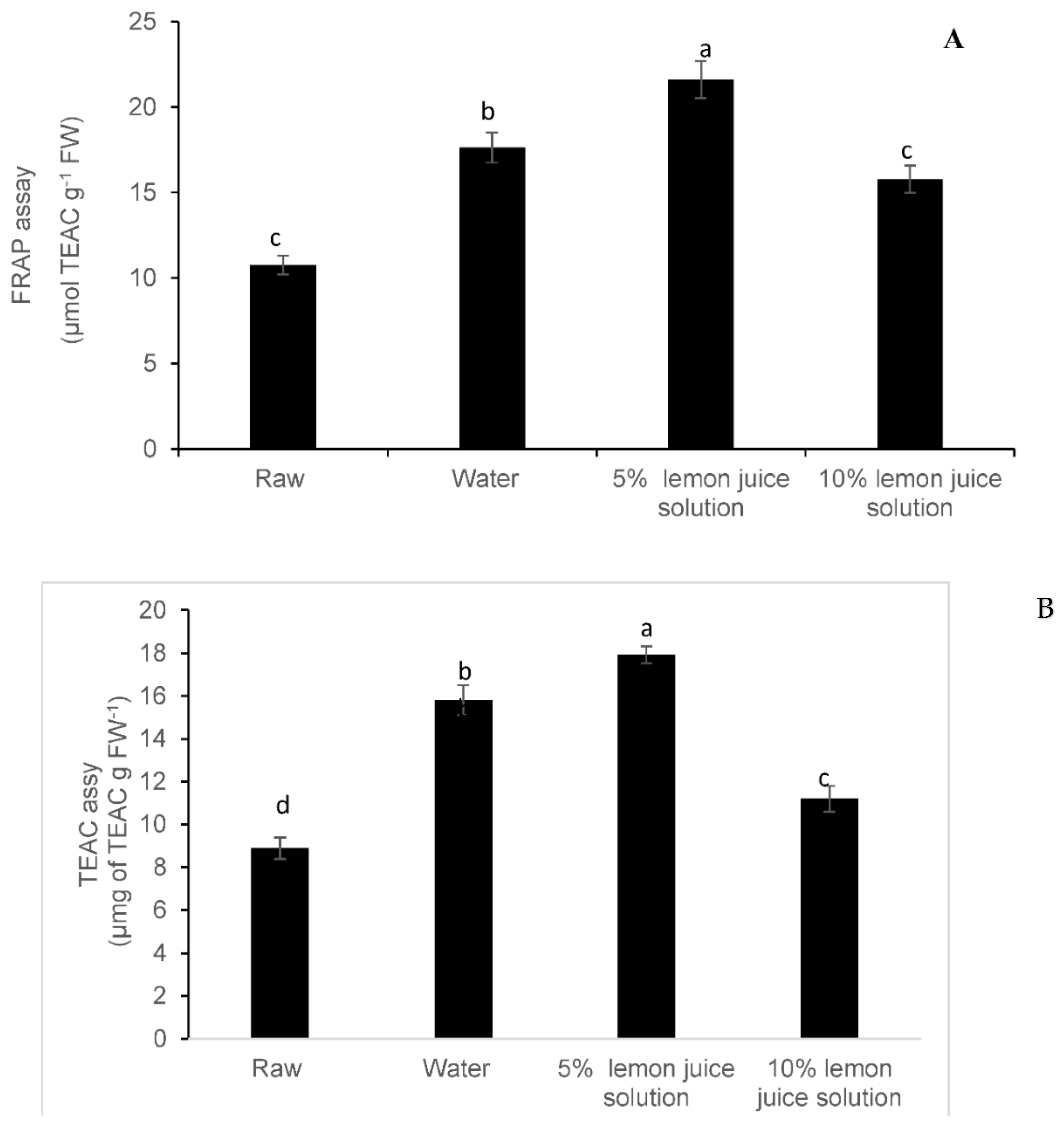
3.3. Antioxidant Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Averbeke, W.; Netshithuthuni, C. Effect of Irrigation Scheduling on Leaf Yield of Nonheading Chinese Cabbage (Brassica rapa L. subsp. chinensis). South Afr. J. Plant Soil 2010, 27, 322–327. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Sivakumar, D.; Beukes, M.; van Rensburg, J.W. Effect of modified atmosphere packaging on the quality and bioactive compounds of Chinese cabbage (Brasicca rapa L. ssp. chinensis). J. Sci. Food Agric. 2013, 93, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, Z.; Wang, Z.; Biao Zhu, S. Effects of storage temperature on the contents of carotenoids and glucosinolates in pakchoi (Brassica rapa L. ssp. var. Communis). J. Food Biochem. 2010, 34, 1186–1204. [Google Scholar] [CrossRef]
- Sturm, C.; Wagner, A.E. Brassica-derived plant bioactives as modulators of chemopreventive and inflammatory signaling pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, D.; Lehmann, C.; Florian, S.; Barknowitz, G.; Haack, M.; Mewis, I.; Kipp, A.P. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Herz, C.; Marton, M.R.; Tran, H.T.T.; Grundemann, C.; Schell, J.; Lamy, E. Benzyl isothiocyanate but not benzyl nitrile from Brassicales plants dually blocks the COX and LOX pathway in primary human immune cells. J. Funct. Foods. 2016, 23, 135–143. [Google Scholar] [CrossRef]
- Guzmán-Pérez, V.; Bumke-Vogt, C.; Schreiner, M.; Mewis, I.; Borchert, A.; Pfeiffer, A.F.H. Benzylglucosinolate derived isothiocyanate from Tropaeolum majus reduces gluconeogenic gene and protein expression in human cells. PLoS ONE 2016, 11, e0162397. [Google Scholar] [CrossRef] [PubMed]
- Onyeoziri, I.O.; Kinnear, M.; de Kock, H.L. Relating sensory profiles of canned amaranth (Amaranthus. cruentus), cleome (Cleome gynandra), cowpea (Vigna. unguiculata) and Swiss chard (Beta vulgaris) leaves to consumer acceptance. J. Sci. Food Agric. 2018, 98, 2231–2242. [Google Scholar] [CrossRef]
- Schlotz, N.; Odongo, G.; Herz, C.; Waßmer, H.; Kühn, C.; Hanschen, F.S.; Neugart, S.; Binder, N.; Ngwene, B.; Schreiner, M.; et al. Are Raw Brassica Vegetables Healthier Than Cooked Ones? A Randomized, Controlled Crossover Intervention Trial on the Health-Promoting Potential of Ethiopian Kale. Nutrients 2018, 10, 1622. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef]
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A mechanistic perspective on process-induced changes in glucosinolate content in Brassica vegetables: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Eyarkai Nambi, V.; Gupta, R.K.; Kumar, S.; Sharma, P.C. Degradation kinetics of bioactive components, antioxidant activity, colour and textural properties of selected vegetables during blanching. J. Food Sci. Technol. 2016, 53, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Ndou, A.; Tinyani, P.P.; Slabbert, R.M.; Sultanbawa, Y.; Sivakuma, D. An integrated approach for harvesting Natal plum (Carissa macrocarpa) for quality and functional compounds related to maturity stage. Food Chem. 2019, 293, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Stander, M.; Van Wyk, B.E.; Taylor, M.J.C.; Long, H.S. Analysis of Phenolic Compounds in Rooibos Tea (Aspalathus linearis) with a Comparison of Flavonoid-Based Compounds in Natural Populations of Plants from Different Regions. J. Agric. Food Chem. 2017, 65, 10270–10281. [Google Scholar] [CrossRef] [PubMed]
- Mpai, S.; du Preez, R.; Sultanbawa, Y.; Sivakumar, D. Phytochemicals and nutritional composition in accessions of Kei-apple (Dovyalis caffra): Southern African indigenous fruit. Food Chem. 2018. 253, 37–45. [CrossRef]
- Egea, I.; Sánchez-Bel, P.; Romojaro, F.; Pretel, M.T. Six edible wild fruits as potential antioxidant additives or nutritional supplements. Plant Foods Hum. Nutr. 2010, 65, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Al-Dabbas, M.; Saleh, M.; Hamad, H.; Hamadeh, W. Chlorophyll color retention in green pepper preserved in natural lemon juice. J. Food Process. Pres. 2016, 41, 1–6. [Google Scholar] [CrossRef]
- Gunawan, M.I.; Barringer, S.A. Green color degradation of blanched broccoli (Brassica oleracea) due to acid and microbial growth. J. Food Process. Pres. 2000, 24, 253–263. [Google Scholar] [CrossRef]
- Minguez-mosquera, M.I.; Garrido-Fernandez, J.; Gandul-Rojas, B. Pigment changes in olives during fermentation and brine storage. J. Agric. Food Chem. 1989, 37, 8–11. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and color loss in blanched green peas. Food Chem. 2007, 100, 609–615. [Google Scholar] [CrossRef]
- Youssef, K.M.; Mokhtar, S.M. Effect of Drying Methods on the Antioxidant Capacity, Color and Phytochemicals of Portulaca oleracea L. Leaves. Int. J. Food Sci. Nutr. 2014, 4, 4–6. [Google Scholar] [CrossRef]
- Ahmed, J.; Shivhare, U.S.; Singh, G. Drying characteristics and product quality of coriander leaves. Food Bioprod. Process. 2011, 79, 103–106. [Google Scholar] [CrossRef]
- Devece, C.; Rodríguez-López, J.N.; Fenoll, L.G.; Tudela, J.; Catalá, J.M.; de los Reyes, E.; García-Cánovas, F. Enzyme inactivation analysis for industrial blanching applications: comparison of microwave, conventional, and combination heat treatments on mushroom polyphenoloxidase activity. J. Agric. Food Chem. 1999, 47, 4506–4511. [Google Scholar] [CrossRef] [PubMed]
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yan, B.; Li, J.; Jiang, Y. Effects of various temperatures and pH values on the extraction yield of phenolics from litchi Fruit pericarp tissue and the antioxidant activity of the extracted anthocyanins. Int. J. Mol. Sci. 2008, 9, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Brandão, T.R.S.; Silva, C.L.M. Carrot (Daucus carota L.) peroxidase inactivation, phenolic content and physical changes kinetics due to blanching. J. Food Eng. 2010, 97, 574–581. [Google Scholar]
- Sikora, E.; Cieslik, E.; Leszczynska, T.; Filipiak Florkiewicz, A.; Pisulewski, P. The antioxidant activity of selected cruciferous vegetables subjected to aqua thermal processing. Food Chem. 2008, 107, 55–59. [Google Scholar] [CrossRef]
- Ewalda, C.; Fjelkner-Modig, S.; Johansson, K.; Sjöholm, I.; Åkesso, B. Effect of processing on major flavonoids in processed onions, green beans, and peas. Food Chem. 1999, 64, 231–235. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mizobuchi, T.; Kajikawa, R.; Kawashima, H.; Miyabe, F.; Terao, J. Radical-scavenging activity of vegetables and the effect of cooking on their activity. Food Sci. Technol. Res. 2001, 7, 250–257. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Gahler, S.; Otto, K.; Bohm, V. Alterations of vitamin C, total phenolics, and antioxidant capacity as affected by processing tomatoes to different products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Wong, K.W.; Benzie, I.F.F. The effect of cooking on Brassica vegetables. Food Chem. 2008, 110, 706–710. [Google Scholar] [CrossRef]
- Korus, A.; Lisiewska, Z. Effect of preliminary processing and method of preservation on the content of selected antioxidative compounds in kale (Brassica oleracea L. var. acephala) leaves. Food Chem. 2011, 129, 149–154. [Google Scholar] [CrossRef]
- Sun, H.S.; Mu, T.H.; Xi, L.S. Effect of pH, heat, and light treatments on the antioxidant activity of sweet potato leaf polyphenols. Int. J. Food Prop. 2017, 20, 318–332. [Google Scholar] [CrossRef]
- Iwai, K.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In Vitro Antioxidative Effects and Tyrosinase Inhibitory Activities of Seven Hydroxycinnamoyl Derivatives in Green Coffee Beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Baldermann, S.; Ngwene, B.; Wesonga, J.; Schrein, M. Indigenous leafy vegetables of Eastern Africa—A source of extraordinary secondary plant metabolites. Food Res. Int. 2017, 100, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.A.; Ali, R.F.M. Bioactive compounds and antioxidant activity of fresh and processed white cauliflower. Biomed Res Int. 2013, 367819, 1–9. [Google Scholar] [CrossRef]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.P. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar]
- Klopsch, R.; Baldermann, S.; Voss, A.; Rohn, S.; Schreiner, M.; Neugart, S. Bread enriched with legume microgreens and leaves—ontogenetic and baking-driven changes in the profile of secondary plant metabolites. Front. Chem. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Riedl, K.M.; Schwartz, S.J. Effects of food formulation and thermal processing on flavones in celery and chamomile. Food Chem. 2013, 141, 1406–1411. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Hansen, M.; Wicklund, T.; Bengtsson, G.B. Processing (blanching, boiling, steaming) effects on the content of glucosinolates and antioxidant-related parameters in cauliflower (Brassica oleracea L. ssp. botrytis). LWT—Food Sci. Technol. 2009, 42, 63–73. [Google Scholar] [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [Google Scholar] [CrossRef]
- Sarvan, I.; Valerio, F.; Lonigr, O.; de Candia, S.; Verkerk, R.; Dekker, M.; Lavermicocca, P. Glucosinolate content of blanched cabbage (Brassica oleracea var. capitata) fermented by the probiotic strain Lactobacillus paracasei LMG-P22043. Food Res. Int. 2013, 54, 706–710. [Google Scholar] [CrossRef]
- Chen, X.; Hanschen, F.S.; Neugart, S.; Schreiner, M.; Vargas, S.A.; Gutschmann, B.; Baldermann, S. Boiling and steaming induced changes in secondary metabolites in three different cultivars of pak choi (Brassica rapa subsp. chinensis). J. Food Compos. Anal. 2019, 82, 103232. [Google Scholar] [CrossRef]
- Hanschen1, F.S.; Klopsch1, R.; Oliviero, T.; Schreiner, M.; Verkerk, R.; Dekker, M. Optimizing isothiocyanate formation during enzymatic glucosinolate breakdown by adjusting pH value, temperature and dilution in Brassica vegetables and Arabidopsis thaliana. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ludikhuyze, L.; Rodrigo, L.; Hendrickx, M. The activity of myrosinase from broccoli (Brassica oleracea L. cv. italica): Influence of intrinsic and extrinsic factors. J. Food Prot. 2000, 63, 400–403. [Google Scholar] [CrossRef] [PubMed]
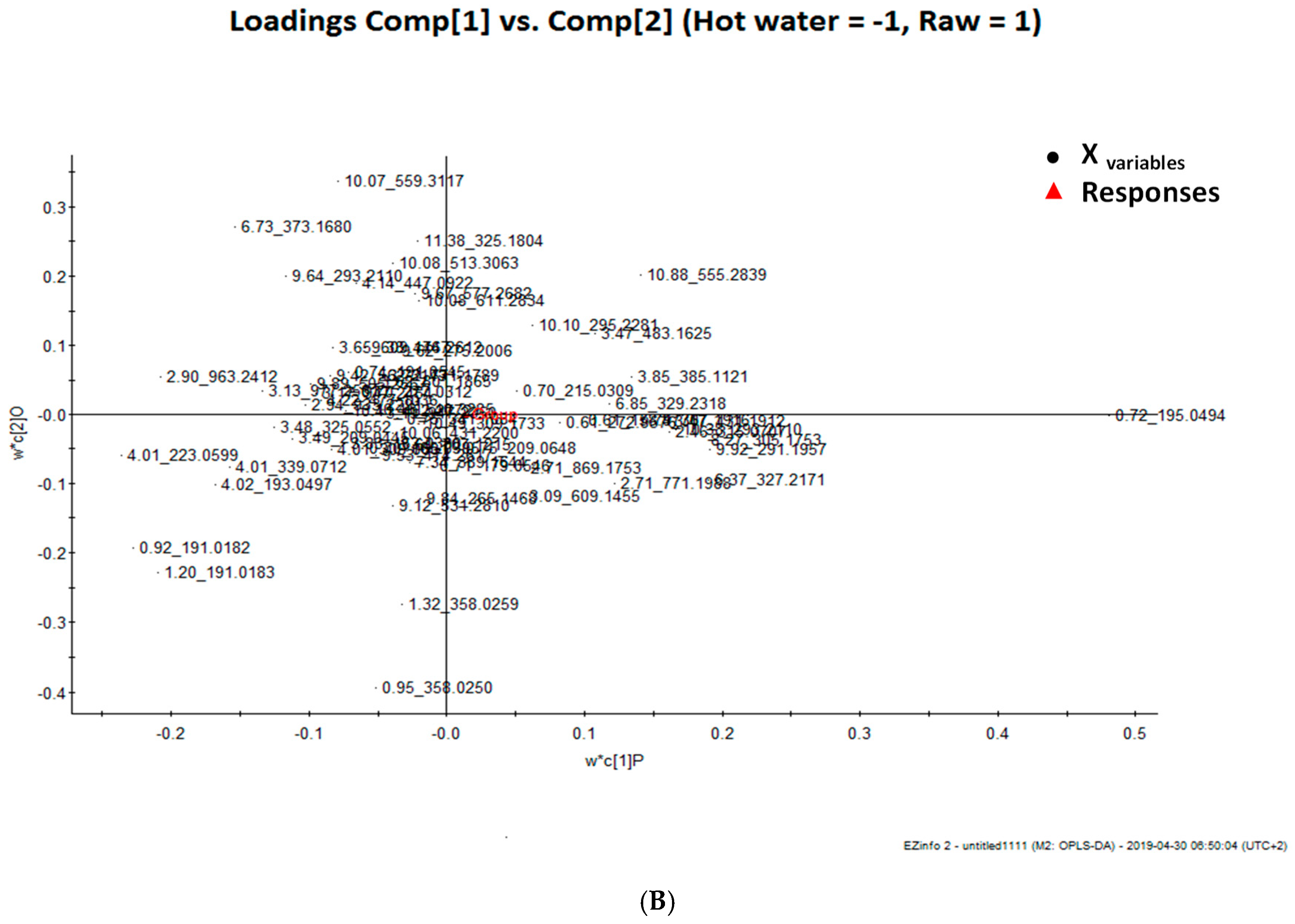
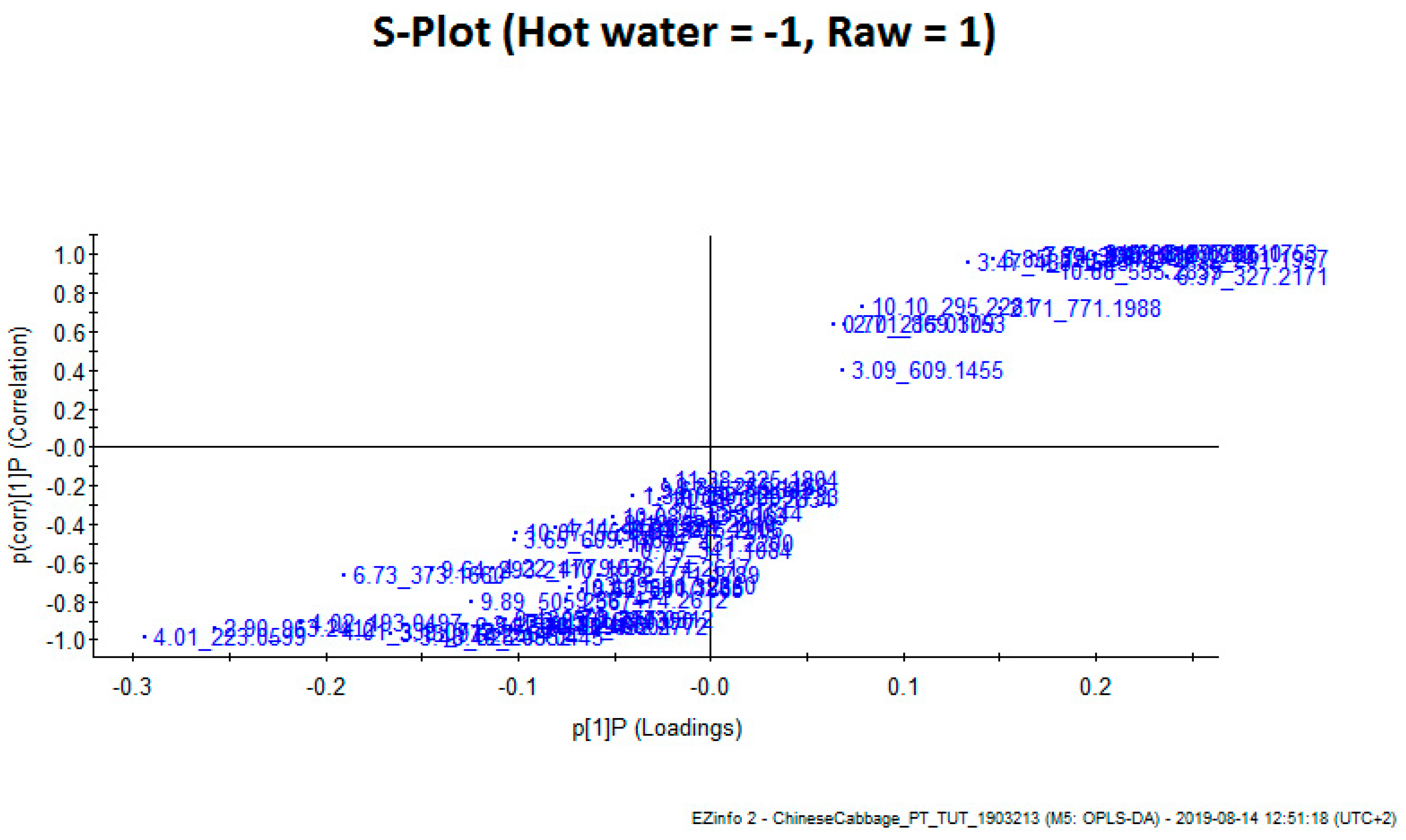
| Retention Time | M−H | M−H Formula | Error (ppm) | MSE Fragments | UV | Tentative Identification |
|---|---|---|---|---|---|---|
| 0.8 | 195.0493 | C6H11O7 | −6.2 | 227 | Gluconic acid | |
| 0.8 | 133.0127 | C4H4O5 | −7.5 | Malic acid | ||
| 0.92 | 191.0181 | C6H7O7 | 0.6 | 155.127,111 | 280 | Quinic acid |
| 2.464 | 315.0707 | C13H15O9 | −2.9 | 153.109 | 306 | Protocatechuoyl-hexose |
| 2.72 | 771.1898 | C33H39O21 | −0.6 | 609.285 | 265.347 | Kaempferol 3-O-sophoroside 7-O-hexoside |
| 3.08 | 609.1463 | C27H29O16 | 1.1 | 447.285 | 265.341 | kaempferol-dihexoside |
| 3.47 | 431.1916 | C20H31O10 | −0.2 | 385.153,97 | 330 | Unknown |
| 3.6 | 609.1488 | C27H29O16 | 5.3 | 285.255 | 264.340 | Kaempferol 3-O-sophoroside |
| 4.14 | 447.0947 | C21H19O11 | 4.2 | 285.255,99 | 264.350 | Kaempferol hexoside |
| 4.3 | 449.0743 | C20H17O12 | 5.1 | 363.157,97 | 364.350 | Myricetin 3-O-arabinoside |
| 5.89 | 269.0488 | C15H10O5 | −0.2 | 151.133,119,97 | 262 | Apigenin |
| 6.37 | 327.2166 | C18H31O5 | 1.5 | 229. 211,171,97 | weak | unknown |
| 6.85 | 329.2328 | C18H33O5 | 0.2 | 211.171,97 | 270 | unknown |
| 7.73 | 307.191 | C18H27O4 | 0.3 | 235.121 | 311 | unknown |
| 7.91 | 307.1913 | C18H27O4 | 1.3 | 235.220,121,99 | 240 | unknown |
| 8.25 | 305.1747 | C18H25O4 | −2.6 | 249. 135 | 319 | unknown |
| 9.90 | 291.1958 | C18H27O3 | −0.7 | 277.265,121 | 280 | unknown |
| 10.37 | 293.211 | C18H29O3 | −2.4 | 255.185,143 | 280 | unknown |
| 12.07 | 591.2595 | C34H39O9 | 0.2 | 515.325,183,149 | 409 | unknown |
| Retention Time | Mass | P(1)P | p(corr)(1)P | |
|---|---|---|---|---|
| 9.92_291.1957 | 9.92 | 291.1957 | 0.237452 | 0.971414 |
| 10.38_293.2110 | 10.38 | 293.211 | 0.2061 | 0.995988 |
| 8.27_305.1753 | 8.27 | 305.1753 | 0.231893 | 0.998607 |
| 7.74_307.1916 | 7.74 | 307.1916 | 0.168101 | 0.991243 |
| 2.46_315.0707 | 2.46 | 315.0707 | 0.2004 | 0.998301 |
| 6.37_327.2171 | 6.37 | 327.2171 | 0.237674 | 0.87965 |
| 6.85_329.2318 | 6.85 | 329.2318 | 0.146947 | 0.969433 |
| 3.85_385.1121 | 3.85 | 385.1121 | 0.166316 | 0.981166 |
| 3.47_431.1912 | 3.47 | 431.1912 | 0.19889 | 0.989573 |
| 3.47_483.1625 | 3.47 | 483.1625 | 0.13382 | 0.953158 |
| 10.88_555.2839 | 10,88 | 555.2839 | 0.175476 | 0.902287 |
| Primary ID | Retention Time | Mass | P(1)P | p(corr)(1)P |
|---|---|---|---|---|
| 4.02_193.0497 | 4.02 | 193.0497 | −0.21239 | −0.912351 |
| 4.01_223.059 | 4.01 | 223.0599 | −0.29435 | −0.984697 |
| 3.48_325.0552 | 3.48 | 325.0552 | −0.15576 | −0.984697 |
| 4.01_339.0712 | 4.01 | 339.0712 | −0.19615 | −0.984697 |
| 2.90_963.2412 | 2.90 | 963.2412 | −0.25714 | −0.984697 |
| 3.13_977.2561 | 3.13 | 977.2561 | −0.16666 | −0.984697 |
| Quinic Acid | Protocatechuoyl Hexose | Kaempferol O-Sophoroside-O-Hexoside (mg kg−1) | Kaempferol-Dihexoside | Ferulic Acid | Kaempferol-Sophoroside | Kaempferol Hexoside | Myrectin-O-Arabinoside | |
|---|---|---|---|---|---|---|---|---|
| Raw | 209.8 ± 0.02 c,* | 46.0 ± 0.07 a | 50.4 ± 0.01 a | 27.7 ± 0.05 b | 8.0 ± 0.03 c | 20.8 ± 0.02 b | 20.3 ± 0.06 b | 13.4 ± 0.03 a |
| Water | 709.7 ± 0.04 b | 0.0 ± 0.00 b | 31.8 ± 0.06 c | 19.9 ± 0.08 c | 462.9 ± 0.10 a | 28.9 ± 0.05 b | 28.9 ± 0.03 b | 8.5 ± 0.01 b |
| 5% lemon juice solution | 765.9 ± 0.01 b | 0.0 ± 0.00 b | 46.4 ± 0.04 b | 37.1 ± 0.0l a | 463.9 ± 0.04 a | 73.6 ± 0.08 a | 69.9 ± 0.02 a | 6.9 ± 0.07 c |
| 10% lemon juice solution | 1067.2 ± 0.05 a | 0.0 ± 0.00 b | 17.2 ± 0.03 d | 12.9 ± 0.04 d | 101.4 ± 0.03 b | 1.7 ± 0.03 c | 0.8 ± 0.12 c | 2.1 ± 0.02 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Managa, M.G.; Remize, F.; Garcia, C.; Sivakumar, D. Effect of Moist Cooking Blanching on Colour, Phenolic Metabolites and Glucosinolate Content in Chinese Cabbage (Brassica rapa L. subsp. chinensis). Foods 2019, 8, 399. https://doi.org/10.3390/foods8090399
Managa MG, Remize F, Garcia C, Sivakumar D. Effect of Moist Cooking Blanching on Colour, Phenolic Metabolites and Glucosinolate Content in Chinese Cabbage (Brassica rapa L. subsp. chinensis). Foods. 2019; 8(9):399. https://doi.org/10.3390/foods8090399
Chicago/Turabian StyleManaga, Millicent G., Fabienne Remize, Cyrielle Garcia, and Dharini Sivakumar. 2019. "Effect of Moist Cooking Blanching on Colour, Phenolic Metabolites and Glucosinolate Content in Chinese Cabbage (Brassica rapa L. subsp. chinensis)" Foods 8, no. 9: 399. https://doi.org/10.3390/foods8090399
APA StyleManaga, M. G., Remize, F., Garcia, C., & Sivakumar, D. (2019). Effect of Moist Cooking Blanching on Colour, Phenolic Metabolites and Glucosinolate Content in Chinese Cabbage (Brassica rapa L. subsp. chinensis). Foods, 8(9), 399. https://doi.org/10.3390/foods8090399








