In Vitro Production and Identification of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides Derived from Distilled Spent Grain Prolamin Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Prolamins from DDSG
2.3. Protein Content Analysis
2.4. Amino Acid Composition Analysis
2.5. Enzymatic Hydrolysis of DDSG-PI
2.6. Determination of the Degree of Hydrolysis (DH)
2.7. Determination of ACE Inhibitory Activity
2.8. Ultrafiltration of DDSG-PI Hydrolysates
2.9. Semi-Preparative RP–HPLC
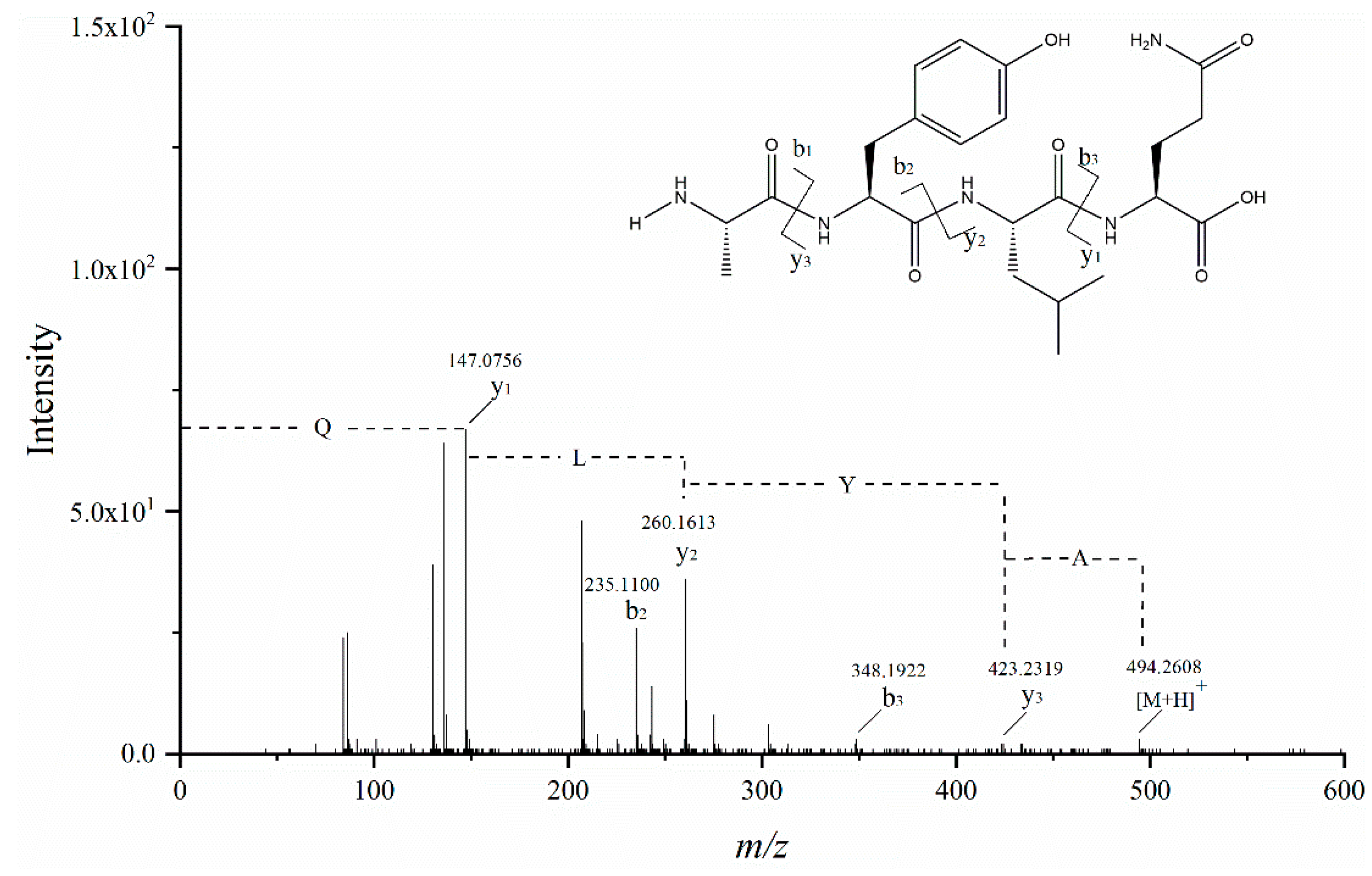
2.10. Peptide Identification by UPLC–Q–TOF–MS/MS
2.11. Peptide Synthesis
2.12. Quantification of Peptides with ACE Inhibitory Activity
2.13. Statistical Analysis
3. Results and Discussion
3.1. Prolamin Content of DDSG and Its Amino Acid Composition
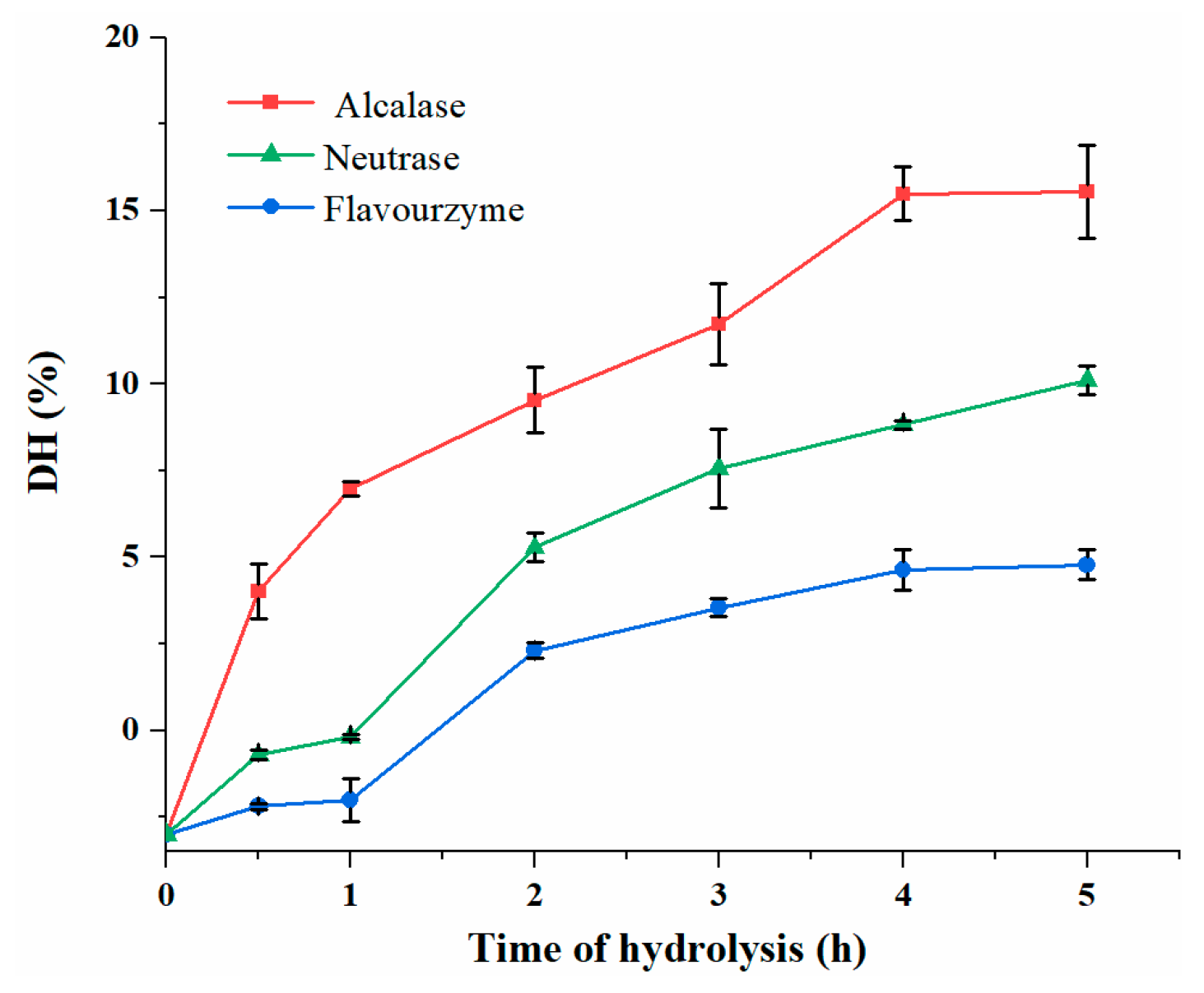
3.2. DH Analysis of DDSG-PI Hydrolysates
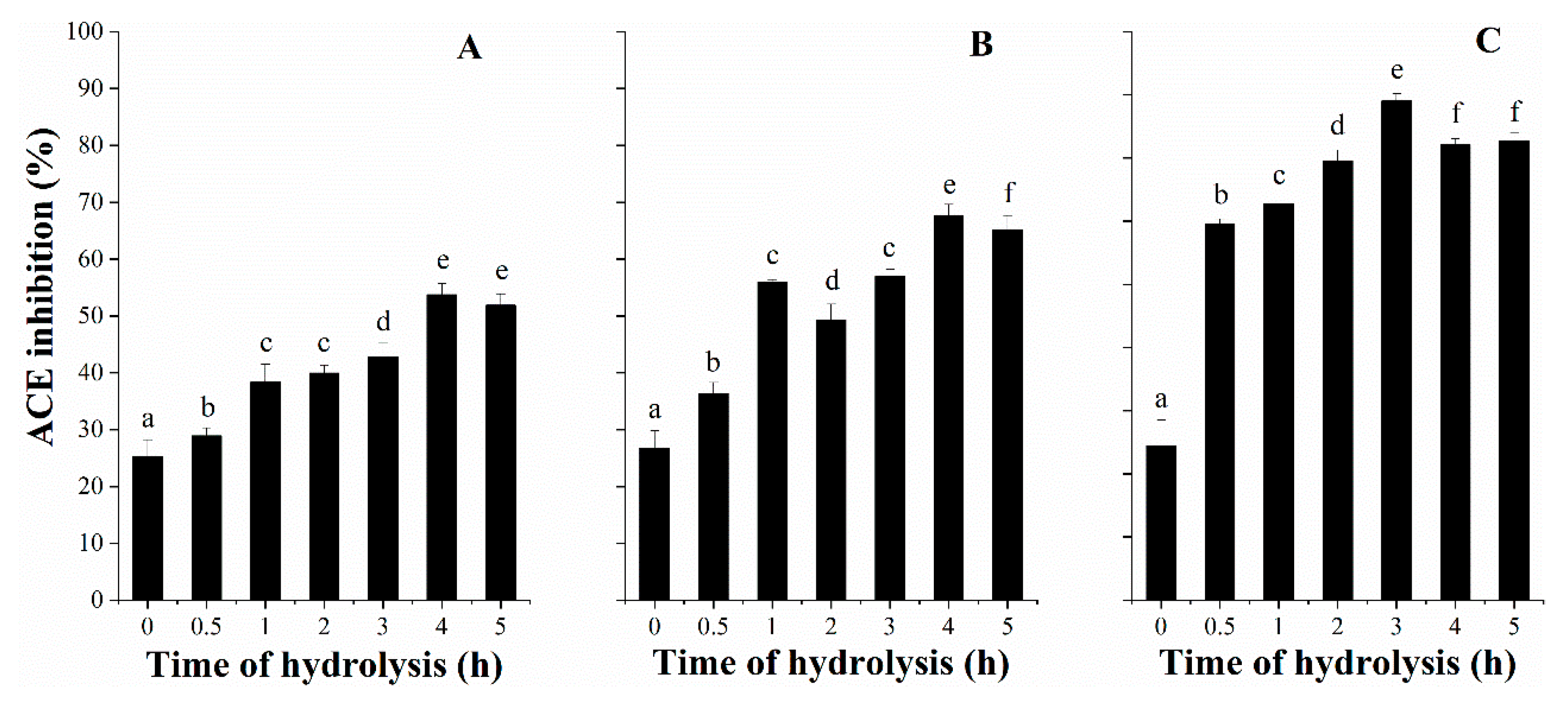
3.3. ACE Inhibitory Activity with Hydrolysis Time
3.4. ACE Inhibitory Activity of Ultrafiltration Fractions
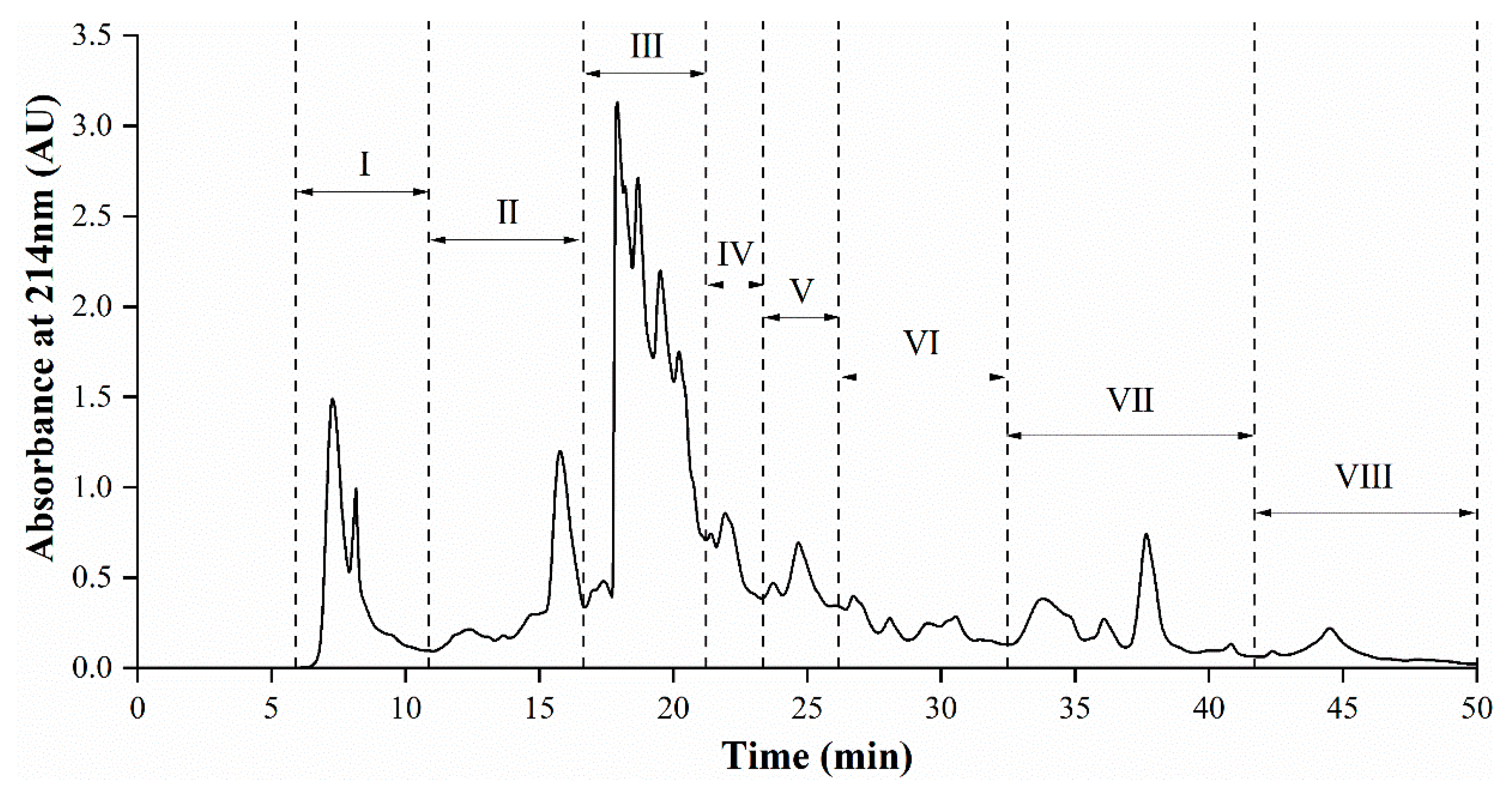
3.5. Semi-Preparative RP-HPLC Fractionation of AGH
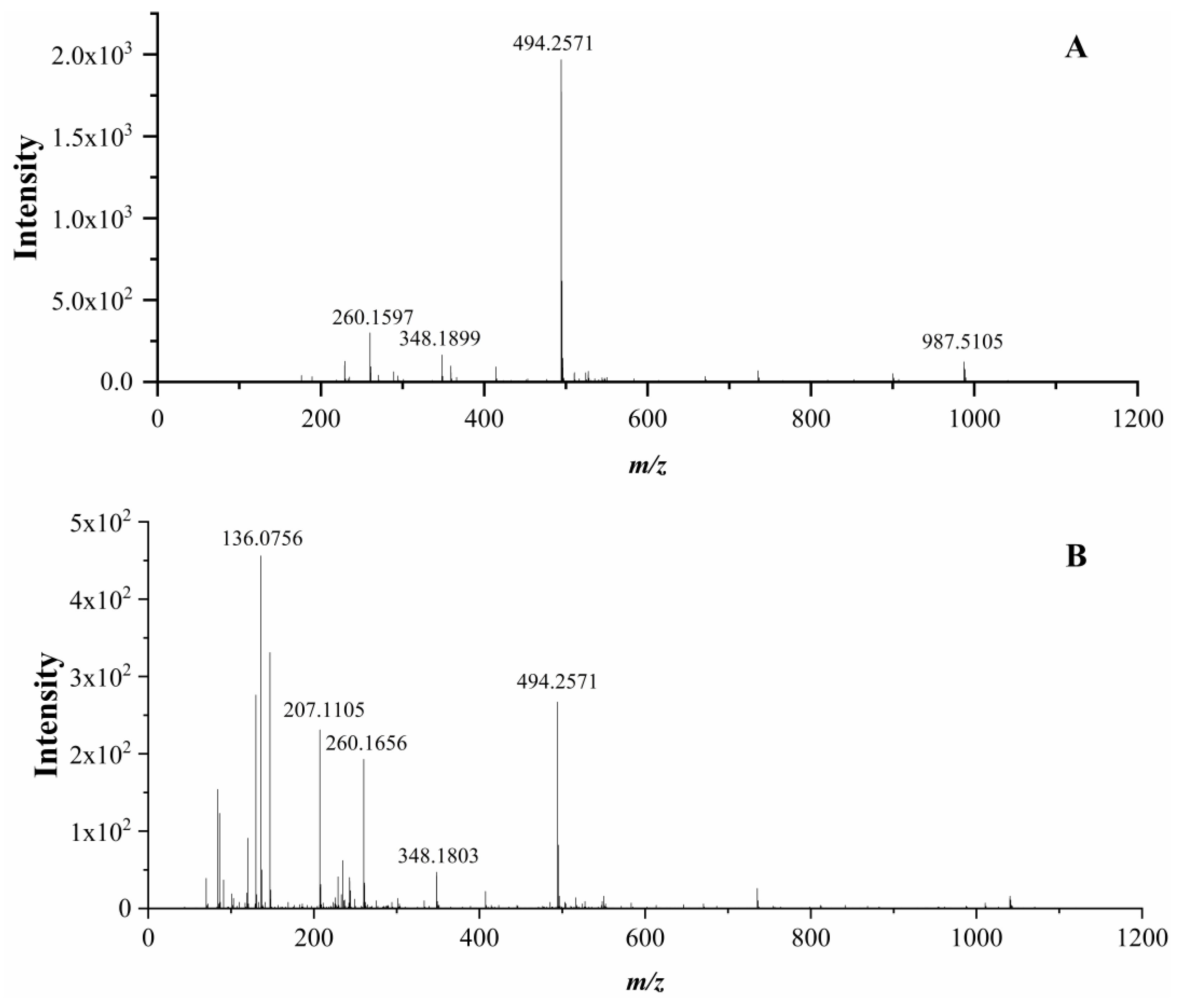
3.6. Identification of Peptides and Their ACE Inhibitory Activity
3.7. Quantification of Identified Peptides from DDSG-PI Hydrolysate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balti, R.; Bougatef, A.; Sila, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef] [PubMed]
- So, P.B.T.; Rubio, P.; Lirio, S.; Macabeo, A.P.; Huang, H.Y.; Corpuz, M.J.A.T.; Villaflores, O.B. In vitro angiotensin I converting enzyme inhibition by a peptide isolated from Chiropsalmus quadrigatus Haeckel (box jellyfish) venom. Toxicon 2016, 119, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fandino, R.; Otte, J.; van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Eriksson, U.; Danilczyk, U.; Penninger, J.M. Just the beginning: Novel functions for angiotensin-converting enzymes. Curr. Biol. 2002, 12, R745–R752. [Google Scholar] [CrossRef]
- Rabelo, E.R.; Rohde, L.E.; Schaan, B.D.; Rubira, M.C.; Ruschel, K.B.; Plentz, R.D.M.; Consolim-Colombo, F.M.; Irigoyen, M.C.; Moreno, H. Bradykinin or acetylcholine as vasodilators to test endothelial venous function in healthy subjects. Clinics 2008, 63, 677–682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beltrami, L.; Zingale, L.C.; Carugo, S.; Cicardi, M. Angiotensin-converting enzyme inhibitor-related angioedema: How to deal with it. Expert Opin. Drug Saf. 2006, 5, 643–649. [Google Scholar] [CrossRef]
- Maruyama, S.; Suzuki, H. A Peptide Inhibitor of Angiotensin I Converting Enzyme in the Tryptic Hydrolysate of Casein. Agric. Biol. Chem. 1982, 46, 1393–1394. [Google Scholar]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef]
- Corrons, M.A.; Liggieri, C.S.; Trejo, S.A.; Bruno, M.A. ACE-inhibitory peptides from bovine caseins released with peptidases from Maclura pomifera latex. Food Res. Int. 2017, 93, 8–15. [Google Scholar] [CrossRef]
- Sheih, I.C.; Fang, T.J.; Wu, T.K. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Wang, X.M.; Chen, H.X.; Fu, X.G.; Li, S.Q.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Connolly, A.; O’Keeffe, M.B.; Piggott, C.O.; Nongonierma, A.B.; FitzGerald, R.J. Generation and identification of angiotensin converting enzyme (ACE) inhibitory peptides from a brewers’ spent grain protein isolate. Food Chem. 2015, 176, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M.A.; Hashim, S.N.; Yea, C.S.; Ebrahimpour, A.; Zarei, M.; Muhammad, K.; Abdul-Hamid, A.; Saari, N. High angiotensin-I converting enzyme (ACE) inhibitory activity of Alcalase-digested green soybean (Glycine max) hydrolysates. Food Res. Int. 2018, 106, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Suzuki, N.; Tanue, Y.; Kai, N.; Nakazawa, Y.; Nagashima, T. Angiotensin I-converting enzyme inhibitory activities of enzymatic hydrolysates from lees as by-product produced during sake making using fugu muscle and fin. J. Food Agric. Environ. 2008, 6, 19–22. [Google Scholar]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides from Sake and Sake Lees. Biosci. Biotechnol. Biochem. 1994, 58, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- De, L. Present Situations of Comprehensive Utilization of Distillers Grains and Study of Multistage Chain Development Technology. Liq Mak. Sci. Technol. 2018, 4, 101–105. (In Chinese) [Google Scholar]
- Taylor, J.R.N.; Schüssler, L. The protein compositions of the different anatomical parts of sorghum grain. J. Cereal Sci. 1986, 4, 361–369. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Chiozzi, R.Z.; Lagana, A. Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 2018, 410, 3425–3444. [Google Scholar] [CrossRef]
- Wei, D.; Fan, W.L.; Xu, Y. Identification of water-soluble peptides in distilled spent grains by UPLC-Q-TOF-MS. Food Ferment. Ind. 2019, 45, 203–207. (In Chinese) [Google Scholar]
- Emmambux, N.M.; Taylor, J.R.N. Sorghum kafirin interaction with various phenolic compounds. J. Sci. Food Agric. 2003, 83, 402–407. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int. J. Food Sci. Technol. 2013, 48, 1670–1681. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using o-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins1. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Rudolph, S.; Lunow, D.; Kaiser, S.; Henle, T. Identification and quantification of ACE-inhibiting peptides in enzymatic hydrolysates of plant proteins. Food Chem. 2017, 224, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar]
- Jiang, J.; Chen, S.; Ren, F.; Luo, Z.; Zeng, S.S. Yak milk casein as a functional ingredient: Preparation and identification of angiotensin-I-converting enzyme inhibitory peptides. J. Dairy Res. 2007, 74, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.D.; Tsou, M.J.; Tsai, Z.Y.; Tsai, T.C. Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor. Food Chem. 2006, 98, 725–732. [Google Scholar] [CrossRef]
- Guerard, F.; Guimas, L.; Binet, A. Production of tuna waste hydrolysates by a commercial neutral protease preparation. J. Mol. Catal. B-Enzym. 2002, 19, 489–498. [Google Scholar] [CrossRef]
- Mao, X.Y.; Ni, J.R.; Sun, W.L.; Hao, P.P.; Fan, L. Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides. Food Chem. 2007, 103, 1282–1287. [Google Scholar] [CrossRef]
- Lee, S.H.; Qian, Z.J.; Kim, S.K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Liu, C.; Yu, Z.X.; Wang, F.J.; Zhong, X.; Jiang, L.; Zhang, F.F.; Tang, Y.X.; Yan, Z.H.; Zeng, S.; Pu, T. Isolation and Structural Elucidation of an Unknown Impurity in Prasugrel by Semi-Preparative Liquid Chromatography. J. Chromatogr. Sci. 2015, 53, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Boye, J.I.; Simpson, B.K.; Prasher, S.O. Purification and characterization of angiotensin I-converting enzyme inhibitory peptides of small red bean (Phaseolus vulgaris) hydrolysates. J. Funct. Food. 2013, 5, 1116–1124. [Google Scholar] [CrossRef]
- Megias, C.; Yust, M.D.; Pedroche, J.; Lquari, H.; Giron-Calle, J.; Alaiz, M.; Millan, F.; Vioque, J. Purification of an ACE inhibitory peptide after hydrolysis of sunflower (Helianthus annuus L.) protein isolates. J. Agric. Food Chem. 2004, 52, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Huo, J.Y.; Huang, M.Q.; Zhao, M.M.; Luo, X.L.; Sun, B.G. Structural Characterization of a Tetrapeptide from Sesame Flavor-Type Baijiu and Its Preventive Effects against AAPH-Induced Oxidative Stress in HepG2 Cells. J. Agric. Food Chem. 2017, 65, 10495–10504. [Google Scholar] [CrossRef] [PubMed]
- Hsi, K.Y. Peptide Identification of Tandem Mass Spectrometry from Quadrupole Time-of-Flight Mass Spectrometers. Ph.D. Thesis, UC San Diego, San Diego, CA, USA, 2009. [Google Scholar]
- Kumar, R.; Chaudhary, K.; Sharma, M.; Nagpal, G.; Chauhan, J.S.; Singh, S.; Gautam, A.; Raghava, G.P. AHTPDB: A comprehensive platform for analysis and presentation of antihypertensive peptides. Nucleic Acids Res. 2015, 43, D956–D962. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [PubMed]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Salampessy, J.; Reddy, N.; Phillips, M.; Kailasapathy, K. Isolation and characterization of nutraceutically potential ACE-Inhibitory peptides from leatherjacket (Meuchenia sp.) protein hydrolysates. LWT Food Sci. Technol. 2017, 80, 430–436. [Google Scholar] [CrossRef]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Roytrakul, S.; Yongsawatdigul, J. Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1-3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem. 2017, 220, 190–197. [Google Scholar] [CrossRef]
- Rho, S.J.; Lee, J.S.; Chung, Y.I.; Kim, Y.W.; Lee, H.G. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem. 2009, 44, 490–493. [Google Scholar] [CrossRef]
- Wu, J.P.; Ding, X.L. Hypotensive and physiological effect of angiotensin converting enzyme inhibitory peptides derived from soy protein on spontaneously hypertensive rats. J. Agric. Food Chem. 2001, 49, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Vallabha, V.S.; Tiku, P.K. Antihypertensive Peptides Derived from Soy Protein by Fermentation. Int. J. Pept. Res. Ther. 2014, 20, 161–168. [Google Scholar] [CrossRef]
- Giacometti, J.; Buretic-Tomljanovic, A. Peptidomics as a tool for characterizing bioactive milk peptides. Food Chem. 2017, 230, 91–98. [Google Scholar] [CrossRef] [PubMed]

| Items | MS Mode 1 | MS/MS Mode 2 |
|---|---|---|
| Capillary voltage | 3500 eV | 3500 eV |
| Mass range | 20–2000 m/z | 60–1000 m/z |
| Acquisition rate | 4 spectra/s | 2 spectra/s |
| Cone voltage | 20 eV | 20 eV |
| Source temp. | 100 °C | 100 °C |
| Desolvation temp. | 400 °C | 400 °C |
| Desolvation gas flow | 700 L/h | 700 L/h |
| Cone gas flow | 50 L/h | 50 L/h |
| Collision energy 3 | 6/20 eV | 10–25 eV |
| Amino Acid | Content (mg/g DDSG-PI DW) 1 |
|---|---|
| Ala | 98.50 ± 2.3 |
| Cys | <0.50 |
| Asp | 55.40 ± 0.20 |
| Glu | 250.8 ± 22.7 |
| Phe | 56.20 ± 0.10 |
| Gly | 13.00 ± 0.30 |
| His | 10.50 ± 0.30 |
| Ile | 40.90 ± 2.7 |
| Lys | <0.50 |
| Leu | 154.3 ± 33.2 |
| Met | 12.80 ± 0.50 |
| Pro | 88.50 ± 11.6 |
| Arg | 13.50 ± 0.10 |
| Ser | 36.20 ± 5.6 |
| Thr | 18.80 ± 0.80 |
| Val | 47.20 ± 6.4 |
| Tyr | 37.50 ± 0.70 |
| Total | 940.0 |
| Fractions | IC50 (μg/mL) | Yield (%) |
|---|---|---|
| AGH | 11.92 ± 0.18 | 100.0 |
| >5 kDa | 24.58 ± 1.43 * | 3.920 |
| 3–5 kDa | 10.41 ± 0.79 * | 2.550 |
| 1–3 kDa | 26.74 ± 1.02 * | 9.350 |
| <1 kDa | 6.750 ± 0.37 * | 84.18 |
| Peptide Sequence | No. of Amino Acid Residues | Theoretical Mass (Da) | Observed Molecular Ion, m/z (Charge) | IC50 (μM) |
|---|---|---|---|---|
| AVQ | 3 | 316.1747 | 317.1808 | 181.0 ± 6.17 a |
| NQL | 3 | 373.1961 | 374.2036 | 704.6 ± 16.06 b |
| YPQ | 3 | 406.1852 | 407.1905 | 220.0 ± 9.56 c |
| AYLQ | 3 | 493.2536 | 494.2571 | 228.6 ± 9.63 c |
| VLPVLS | 3 | 626.4003 | 627.4010 | 248.1 ± 7.15 d |
| VLPSLN | 3 | 641.3748 | 642.3737 | 1246 ± 9.92 e |
| Compound | Precursor ion (m/z) | Cone Voltage (V) | Product ion (m/z) | Collision Energy (V) | Content (mg/g DDSG-PI DW) |
|---|---|---|---|---|---|
| AVQ | 317.1 | 16 | 147.2 | 12 | 4.070 ± 0.12 |
| NQL | 374.3 | 18 | 242.8 | 14 | 5.820 ± 0.85 |
| YPQ | 407.1 | 26 | 244.1 | 20 | 5.070 ± 0.18 |
| AYLQ | 494.5 | 26 | 135.5 | 40 | 0.1700 ± 0.02 |
| VLPVLS | 627.4 | 28 | 169.2 | 40 | 16.96 ± 1.71 |
| VLPSLN | 642.4 | 28 | 430.3 | 22 | 1.140 ± 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Fan, W.; Xu, Y. In Vitro Production and Identification of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides Derived from Distilled Spent Grain Prolamin Isolate. Foods 2019, 8, 390. https://doi.org/10.3390/foods8090390
Wei D, Fan W, Xu Y. In Vitro Production and Identification of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides Derived from Distilled Spent Grain Prolamin Isolate. Foods. 2019; 8(9):390. https://doi.org/10.3390/foods8090390
Chicago/Turabian StyleWei, Dong, Wenlai Fan, and Yan Xu. 2019. "In Vitro Production and Identification of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides Derived from Distilled Spent Grain Prolamin Isolate" Foods 8, no. 9: 390. https://doi.org/10.3390/foods8090390
APA StyleWei, D., Fan, W., & Xu, Y. (2019). In Vitro Production and Identification of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides Derived from Distilled Spent Grain Prolamin Isolate. Foods, 8(9), 390. https://doi.org/10.3390/foods8090390





