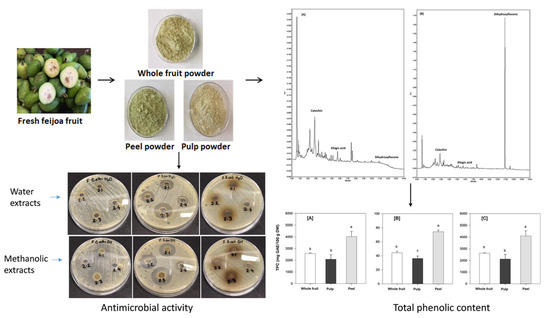
Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Analysis
2.3. Measurement of Physico-Chemical Parameters
2.4. Extraction and Analysis of Individual Polyphenols
2.4.1. Extraction of Free Phenolic Compounds
2.4.2. Extraction of Bound Phenolic Compounds
2.4.3. U(H)PLC-PDA-MS Analysis
2.5. Total Phenolic Content (TPC)
2.6. Analysis of Sugar
2.7. Analysis of Vitamin E (Alpha-Tocopherol)
2.8. Analysis of Vitamin C (Ascorbic Acid)
2.9. Antimicrobial Screening Test
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters
3.2. Proximate Analysis
3.3. Minerals and Heavy Metals
3.4. Sugar Components
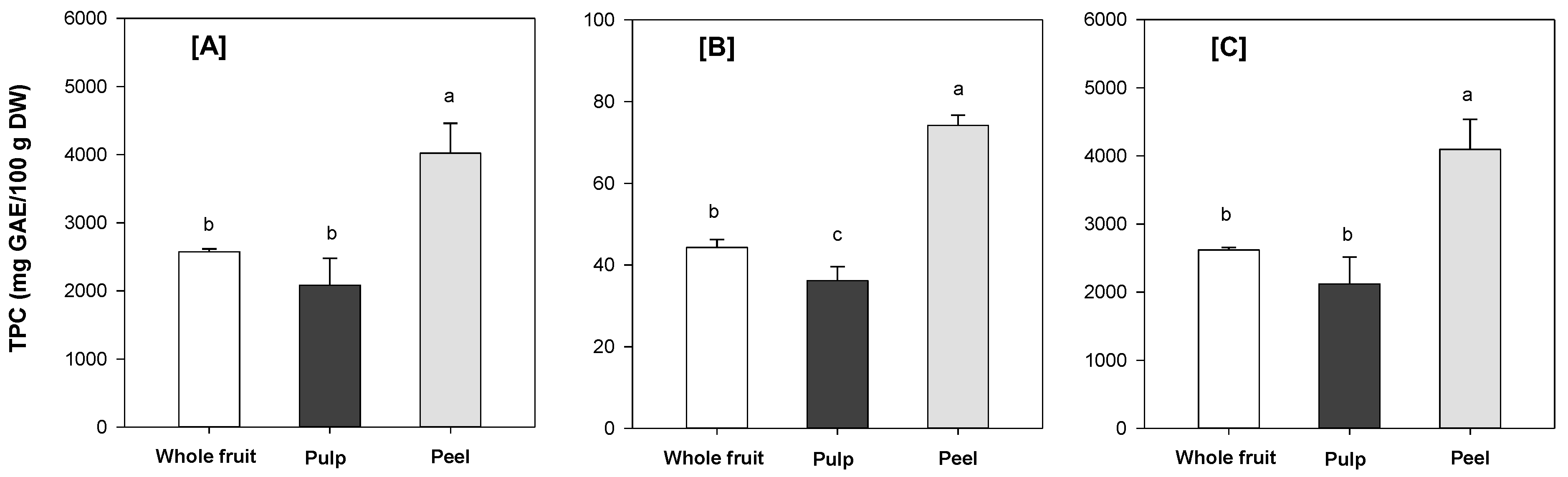
3.5. Total Phenolic Content (TPC)
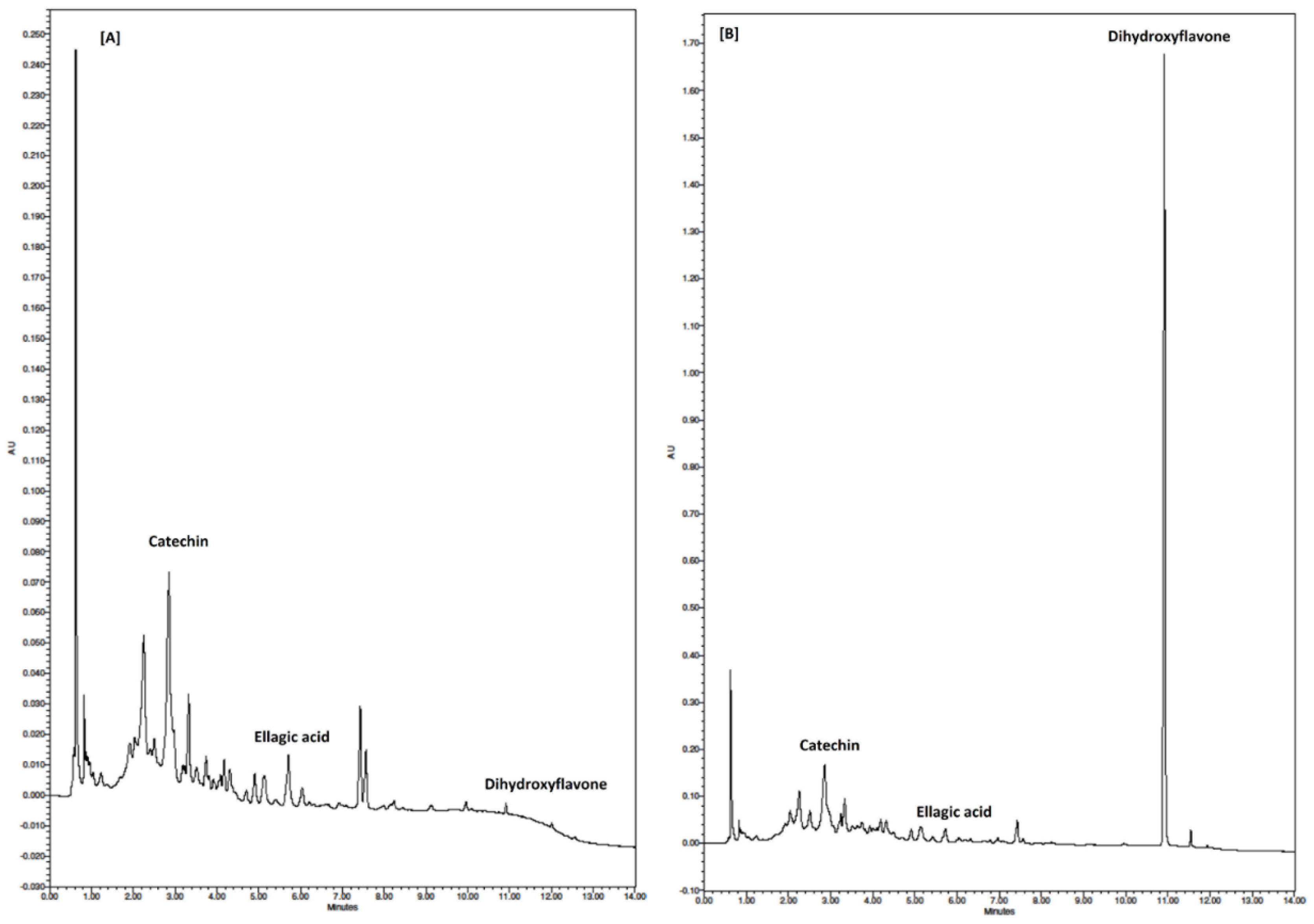
3.6. Individual Phenolic Compounds
3.7. Vitamins
3.8. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weston, R.J. Bioactive products from fruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chem 2010, 121, 923–926. [Google Scholar] [CrossRef]
- Kabiri, S.; Gheybi, F.; Jokar, M.; Basiri, S. Antioxidant acitvity and physicochemical properties of fresh, dried and infused herbal extract of Feijoa Fruit. Nat. Sci. 2016, 14, 7. [Google Scholar]
- Zhu, F. Chemical and biological properties of feijoa (Acca sellowiana). Trends Food Sci Technol. 2018, 81, 121–131. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wang, W.; Waterhouse, G.I.N.; Wadhwa, S.S. Utilisation Potential of Feijoa Fruit Wastes as Ingredients for Functional Foods. Food Bioprocess Technol. 2013, 6, 3441–3455. [Google Scholar] [CrossRef]
- Aoyama, H.; Sakagami, H.; Hatano, T. Three new flavonoids, proanthocyanidin, and accompanying phenolic constituents from Feijoa sellowiana. Biosci. Biotechnol. Biochem. 2018, 82, 31–41. [Google Scholar] [CrossRef]
- Peng, Y.; Bishop, K.S.; Quek, S.Y. Extraction Optimization, Antioxidant Capacity and Phenolic Profiling of Extracts from Flesh, Peel and Whole Fruit of New Zealand Grown Feijoa Cultivars. Antioxidants 2019, 8, 141. [Google Scholar] [CrossRef]
- Vidal Talamini do Amarante, C.; Goede de Souza, A.; Dal Toé Benincá, T.; Steffens, C. Fruit quality of Brazilian genotypes of feijoa at harvest and after storage. Pesqui. Agropecu. Bras. 2017, 52, 734–742. [Google Scholar] [CrossRef]
- Amarante, C.V.T.d.; Souza, A.G.d.; Benincá, T.D.T.; Steffens, C.A. Phenolic content and antioxidant activity of fruit of Brazilian genotypes of feijoa. Pesqui. Agropecu. Bras. 2017, 52, 1223–1230. [Google Scholar] [CrossRef]
- Motohashi, N.; Kawase, M.; Shirataki, Y.; Tani, S.; Saito, S.; Sakagami, H.; Kurihara, T.; Nakashima, H.; Wolfard, K.; Mucsi, I.; et al. Biological activity of Feijoa peel extracts. Anticancer Res. 2000, 20, 4323–4329. [Google Scholar]
- Shaw, G.J.; Allen, J.M.; Yates, M.K.; Franich, R.A. Volatile flavour constituents of feijoa (Feijoa sellowiana): Analysis of fruit flesh. J. Sci. Food Agric. 1990, 50, 357–361. [Google Scholar] [CrossRef]
- Keles, H.; Ince, S.; Kucukkurt, I.; Tatli, II.; Akkol, E.K.; Kahraman, C.; Demirel, H.H. The effects of Feijoa sellowiana fruits on the antioxidant defense system, lipid peroxidation, and tissue morphology in rats. Pharm. Biol. 2012, 50, 318–325. [Google Scholar] [CrossRef]
- Monforte, M.T.; Fimiani, V.; Lanuzza, F.; Naccari, C.; Restuccia, S.; Galati, E.M. Feijoa sellowiana Berg fruit juice: Anti-inflammatory effect and activity on superoxide anion generation. J. Med. Food 2014, 17, 455–461. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Mastrobuoni, F.; Di Patre, D.; Zampella, L.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Agronomic, nutraceutical and molecular variability of feijoa (Acca sellowiana (O. Berg) Burret) germplasm. Sci. Hortic. 2015, 191, 1–9. [Google Scholar] [CrossRef]
- Mosbah, H.; Louati, H.; Boujbiha, M.A.; Chahdoura, H.; Snoussi, M.; Flamini, G.; Ascrizzi, R.; Bouslema, A.; Achour, L.; Selmi, B. Phytochemical characterization, antioxidant, antimicrobial and pharmacological activities of Feijoa sellowiana leaves growing in Tunisia. Ind. Crop. Prod. 2018, 112, 521–531. [Google Scholar] [CrossRef]
- Basile, A.; Conte, B.; Rigano, D.; Senatore, F.; Sorbo, S. Antibacterial and antifungal properties of acetonic extract of Feijoa sellowiana fruits and its effect on Helicobacter pylori growth. J. Med. Food 2010, 13, 189–195. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Washington, DC, USA, 1997. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Lekala, C.S.; Madani, K.S.H.; Phan, A.D.T.; Maboko, M.M.; Fotouo, H.; Soundy, P.; Sultanbawa, Y.; Sivakumar, D. Cultivar-specific responses in red sweet peppers grown under shade nets and controlled-temperature plastic tunnel environment on antioxidant constituents at harvest. Food Chem. 2019, 275, 85–94. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Mattivi, F.; Vrhovsek, U. Overall dietary polyphenol intake in a bowl of strawberries: The influence of Fragaria spp. in nutritional studies. J. Funct. Foods 2015, 18, 1057–1069. [Google Scholar] [CrossRef]
- Netzel, M.; Fanning, K.; Netzel, G.; Zabaras, D.; Karagianis, G.; Treloar, T.; Russell, D.; Stanley, R. Urinary excretion of antioxidants in healthy humans following queen garnet plum juice ingestion: A new plum variety rich in antioxidant compounds. J. Food Biochem. 2012, 36, 159–170. [Google Scholar] [CrossRef]
- Rangel, J.C.; Benavides Lozano, J.; Heredia, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990. [Google Scholar] [CrossRef]
- Ogiwara, I.; Ohtsuka, Y.; Yoneda, Y.; Sakurai, K.; Hakoda, N.; Shimura, I. Extraction Method by Water followed by Microwave Heating for Analyzing Sugars in Strawberry Fruits. J. Jpn. Soc. Hortic. Sci. 1999, 68, 949–953. [Google Scholar] [CrossRef][Green Version]
- Shanmugavelan, P.; Kim, S.Y.; Kim, J.B.; Kim, H.W.; Cho, S.M.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; Kim, H.R. Evaluation of sugar content and composition in commonly consumed Korean vegetables, fruits, cereals, seed plants, and leaves by HPLC-ELSD. Carbohydr. Res. 2013, 380, 112–117. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Campos, F.M.; Ribeiro, S.M.R.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Stringheta, P.C. Optimization of methodology to analyze ascorbic and dehydroascorbic acid in vegetables. Química Nova. 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Spinola, V.; Mendes, B.; Camara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef]
- Sánchez-Mora, F.D.; Saifert, L.; Ciotta, M.N.; Ribeiro, H.N.; Petry, V.S.; Rojas-Molina, A.M.; Lopes, M.E.; Lombardi, G.G.; dos Santos, K.L.; Ducroquet, J.P.H.J.; et al. Characterization of Phenotypic Diversity of Feijoa Fruits of Germplasm Accessions in Brazil. Agrosyst. Geosci. Environ. 2019, 2. [Google Scholar] [CrossRef]
- Parra-Coronado, A.; Fischer, G.; Camacho-Tamayo, J.H. Development and quality of pineapple guava fruit in two locations with different altitudes in Cundinamarca, Colombia. Bragantia 2015, 74, 359–366. [Google Scholar] [CrossRef][Green Version]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand. Available online: https://www.nrv.gov.au/ (accessed on 5 May 2019).
- Otten, J.; Hellwig, J.; Meyers, L. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academic Press: Washington, DC, USA, 2006. [Google Scholar]
- Cheung Chung, S.W.; Kwong, K.P.; Yau, J.C.; Wong, W.W. Dietary exposure to antimony, lead and mercury of secondary school students in Hong Kong. Food Addit. Contam. Part A 2008, 25, 831–840. [Google Scholar] [CrossRef]
- EFSA_Panel_on_Contaminants_in_the_Food_Chain_(CONTAM). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar]
- European_Food_Safety_Authority. Safety of aluminium from dietary intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 754, 1–34. [Google Scholar]
- Oksana, B.; Magomed, O.; Zuchra, O. Chemical composition of fruits of a feijoa (F. sellowiana) in the conditions of subtropics of russia. Potravin. Slovak J. Food Sci. 2014, 8, 119–123. [Google Scholar]
- Liu, H.-F.; Wu, B.-H.; Fan, P.-G.; Li, S.-H.; Li, L.-S. Sugar and acid concentrations in 98 grape cultivars analyzed by principal component analysis. J. Sci. Food Agric. 2006, 86, 1526–1536. [Google Scholar] [CrossRef]
- Wilson, C.W.; Shaw, P.E.; Campbell, C.W. Determination of organic acids and sugars in guava (Psidium guajava L.) cultivars by high-performance liquid chromatography. J. Sci. Food Agric. 1982, 33, 777–780. [Google Scholar] [CrossRef]
- Silveira, A.C.; Oyarzún, D.; Rivas, M.; Záccari, F. Postharvest quality evaluation of feijoa fruits (Acca sellowiana (Berg) Burret). Agrociencia (Montev.) 2016, 20, 14–21. [Google Scholar]
- Bontempo, P.; Mita, L.; Miceli, M.; Doto, A.; Nebbioso, A.; De Bellis, F.; Conte, M.; Minichiello, A.; Manzo, F.; Carafa, V.; et al. Feijoa sellowiana derived natural flavone exerts anti-cancer action displaying HDAC inhibitory activities. Int. J. Biochem. Cell Biol. 2007, 39, 1902–1914. [Google Scholar]
- He, J.; Xiang, Z.; Zhu, X.; Ai, Z.; Shen, J.; Huang, T.; Liu, L.; Ji, W.; Li, T. Neuroprotective Effects of 7, 8-dihydroxyflavone on Midbrain Dopaminergic Neurons in MPP(+)-treated Monkeys. Sci. Rep. 2016, 6, 34339. [Google Scholar] [CrossRef]
- Krishna, G.; Agrawal, R.; Zhuang, Y.; Ying, Z.; Paydar, A.; Harris, N.G.; Royes, L.F.F.; Gomez-Pinilla, F. 7,8-Dihydroxyflavone facilitates the action exercise to restore plasticity and functionality: Implications for early brain trauma recovery. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1204–1213. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, W.-E.; Yan, J.-Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.-L.; Feng, X.-S.; Yang, J.; Li, G.-H. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.C.; Barone, A.; Frusciante, L.; Rigano, M.M. Vitamin E Content and Composition in Tomato Fruits: Beneficial Roles and Bio-Fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef]
- Ruberto, G.; Tringali, C. Secondary metabolites from the leaves of Feijoa sellowiana Berg. Phytochemistry 2004, 65, 2947–2951. [Google Scholar] [CrossRef]
- Hwang, E.S.; Stacewicz-Sapuntzakis, M.; Bowen, P.E. Effects of heat treatment on the carotenoid and tocopherol composition of tomato. J. Food Sci. 2012, 77, C1109–C1114. [Google Scholar] [CrossRef]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. GMR 2003, 2, 63–76. [Google Scholar]
- Aperis, G.; Myriounis, N.; Spanakis, E.K.; Mylonakis, E. Developments in the treatment of candidiasis: More choices and new challenges. Expert Opin. Investig. Drugs 2006, 15, 1319–1336. [Google Scholar] [CrossRef]
- Richardson, M.D. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 2005, 56 (Suppl. 1), i5–i11. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]


| Parameters | Fresh Whole Fruit Puree * | Literature Data |
|---|---|---|
| TSS (%) | 13.9 ± 0.1 | 10.08–12.89 [13] |
| 9.3–12.5 [29] | ||
| 11.19–13.35 [30] | ||
| pH | 3.1 ± 0.03 | 2.45–3.68 [7] |
| 3.2–3.4 [29] | ||
| TA (g citric acid Eq/100 g) | 2.0 ± 0.05 | 4.05–6.7 [13] |
| 0.9–1.5 [29] | ||
| 1.58–1.93 [30] | ||
| TSS: TA | 7.2 ± 0.2 | 1.9–3.35 [13] |
| 8.5–12.1 [29] | ||
| Moisture content (%) | 80.3 ± 0.8 | 83.3 [3] |
| Proximate Composition | Quantity per 100 g DW | Quantity per 100 g FW * | Literature Data [3] per 100 g FW | |
|---|---|---|---|---|
| Energy | 1203 kJ | 237 kJ | n/a | |
| 288 Cal | 57 Cal | 61 Cal | ||
| Protein | 3.7 g | 0.73 g | 0.71 g | |
| Fat | Total fat content | 2.2 g | 0.43 g | 0.42 g |
| Saturated fatty acids | 0.6 g | 0.12 g | 0.104 g | |
| Monounsaturated fatty acids | 0.3 g | 0.058 g | 0.056 g | |
| Polyunsaturated fatty acids | 1.3 g | 0.26 g | 0.136 g | |
| Trans fatty acids | <0.1 g | <0.02 g | 0 g | |
| Dietary fibre | Total dietary fibre Crude fibre | 34.6 g 20.4 g | 6.8 g 4.01 g | 6.4 g n/a |
| Ash | 3.5% | 0.01 g | n/a | |
| Minerals and Heavy Metals | Quantity per kg DW | Quantity per kg FW * | Relative Percentage per 100 g FW ≠ | Nutrition Information ** | |
|---|---|---|---|---|---|
| Minerals | Sodium (Na) | 96 mg | 18.87 mg | 0.15 | 1.3 g/day AI [32] |
| Potassium (K) | 13,000 mg | 2,556 mg | 5.4 | 4.7 g/day AI [32] | |
| Iron (Fe) | 12.8 mg | 2.5 mg | 3.1 | 8 mg/day RDA [32] | |
| Calcium (Ca) | 940 mg | 185 mg | 1.5 | 1200 mg/day AI [32] | |
| Magnesium (Mg) | 614 mg | 121 mg | 3.5 | 350 mg/day EAR [32] | |
| Zinc (Zn) | 4.3 mg | 0.9 mg | 0.82 | 11 mg/day RDA [32] | |
| Iodine (I) | 0.4 mg | 0.08 mg | 8.4 | 95 µg/day EAR [32] | |
| Heavy metals | Mercury (Hg) | <0.01 mg | <0.002 mg | 5 µg/kg BW/week UL [33] | |
| Lead (Pb) | 0.11 mg | 0.022 mg | 25 µg/kg BW/week UL [33] | ||
| Cadmium (Cd) | <0.01 mg | <0.002 mg | 2.5 µg/kg BW/week UL [34] | ||
| Arsenic (As) | <0.025 mg | <0.005 mg | - | ||
| Aluminium (Al) | 1.29 mg | 0.25 mg | 1.0 mg/kg BW/week UL [35] | ||
| Chromium (Cr) | <0.025 mg | <0.005 mg | 25-35 µg/kg day AI [32] | ||
| Sugar Components | Whole Fruit | Pulp | Peel | Literature Data (Whole Fresh Fruit) |
|---|---|---|---|---|
| Fructose | 11.9 ± 0.4 a * | 12.3 ± 0.6 a | 12.2 ± 0.1 a | |
| (2.3) ** | (2.3) | (2.3) | 1.4–4.3 g/100 g FW [36] | |
| Glucose | 13.4 ± 0.5 a | 13.7 ± 0.4 a | 13.2 ± 0.3 a | |
| (2.6) | (2.7) | (2.6) | 0.07–1.5 g/100 g FW [36] | |
| Sucrose | 25.9 ± 1.0 b | 29.0 ± 0.9 a | 11.5 ± 0.3 c | |
| (5.1) | (5.7) | (2.3) | 2.15–5.9 g/100 g FW [36] | |
| Total sugars | 51.2 ± 1.3 b | 55.0 ± 1.6 a | 36.9 ± 1.5 c | |
| (10.1) | (10.8) | (7.3) |
| Vitamins | Quantity (per 100 g) * | Nutrition Information [31] (RDI for Adults) | |
|---|---|---|---|
| Whole Fruit (DW) | Whole Fruit (FW) | ||
| B1 (Thiamin) | <5.0 µg | <1 µg | 1.1–1.2 mg/day |
| B2 (Riboflavin) | <5.0 µg | <1 µg | 1.6 mg/day |
| B3 (Niacin) | 270 µg | 53.1 µg | 14–16 mg/day |
| B5 (Pantothenic acid) | 1100 µg | 216.3 µg | 4–6 mg/day |
| B6 (Pyridoxine) | 190 µg | 37.4 µg | 1.7 mg/day |
| B7 (Biotin) | <5.0 µg | <1 µg | 25–30 µg/day |
| B12 (Cyanocobalamin) | <5.0 µg | <1 µg | 2.4 µg/day |
| Sample | Vitamin C (L-AA + DHAA) | Literature Data (mg/100 g FW) | Vitamin E (α-Tocopherol) | Literature Data (mg/100 g FW) | ||
|---|---|---|---|---|---|---|
| (mg/100 g DW) * | (mg/100 g FW) * | (mg/100 g DW) * | (mg/100 g FW) * | |||
| Whole fruit powder | 319.2 ± 2.5 b | 62.8 ± 0.4 b | 32.9 [43] | 1.41 ± 0.11 b | 0.28 ± 0.02 b | 0.16 [43] |
| 27.9–39.9 [7] | ||||||
| Pulp powder | 281.1 ± 0.6 c | 51.8 ± 0.1 c | 38.7–92.5 [13] | 0.27 ± 0.03 c | 0.05 ± 0.01 c | n/a |
| Peel powder | 469.4 ± 4.3 a | 95 ± 0.6 a | 63.5–101 [13] | 2.27 ± 0.14 a | 0.45 ± 0.03 a | n/a |
| Samples | E. coli | S. aureus | C. albicans | |||
|---|---|---|---|---|---|---|
| MeOH | Water | MeOH | Water | MeOH | Water | |
| Whole fruit powder | 11.9 ± 0.2 b * | - | 23.1 ± 0.8 b | 20.1 ± 0.1 b | 15.5 ± 1.2 a | - |
| Pulp powder | - | - | 22.7 ± 0.3 b | 18.9 ± 0.2 c | - | - |
| Peel powder | 14.7 ± 1.1 a | - | 26.5 ± 0.2 a | 23.4 ± 0 a | 15.6 ± 3.2 a | - |
| Antibiotic control | 29.2 | 55.8 | 27.1 | |||
| Methanol (20%, v/v) | - | - | - | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, A.D.T.; Chaliha, M.; Sultanbawa, Y.; Netzel, M.E. Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana). Foods 2019, 8, 376. https://doi.org/10.3390/foods8090376
Phan ADT, Chaliha M, Sultanbawa Y, Netzel ME. Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana). Foods. 2019; 8(9):376. https://doi.org/10.3390/foods8090376
Chicago/Turabian StylePhan, Anh Dao Thi, Mridusmita Chaliha, Yasmina Sultanbawa, and Michael E. Netzel. 2019. "Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana)" Foods 8, no. 9: 376. https://doi.org/10.3390/foods8090376
APA StylePhan, A. D. T., Chaliha, M., Sultanbawa, Y., & Netzel, M. E. (2019). Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana). Foods, 8(9), 376. https://doi.org/10.3390/foods8090376