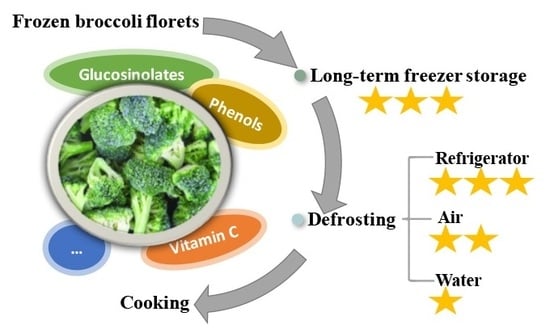
Main Health-Promoting Compounds Response to Long-Term Freezer Storage and Different Thawing Methods in Frozen Broccoli Florets
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.1.1. Freezer Storage
2.1.2. Defrosting Methods
- (1)
- Water defrosting: Samples were placed in 10 volumes of 18 °C water. After 5 min, the temperature of samples reached up to 10 °C, and defrosting was completed.
- (2)
- Air defrosting: Samples were wrapped by polyethylene films and placed in a 20 °C chamber with 60% relative humidity. After 1.5 h, the temperature of samples reached up to 10 °C, and defrosting was completed.
- (3)
- Refrigerator defrosting: The samples were wrapped by polyethylene films and placed in a 4 °C refrigerator. After 8 h, defrosting was stopped when the temperature of samples was higher than 4 °C.
2.2. Determination of Glucosinolate Contents
2.3. Determination of Vitamin C Contents
2.4. Determination of Total Phenol Contents
2.5. Statistical Analysis
3. Results
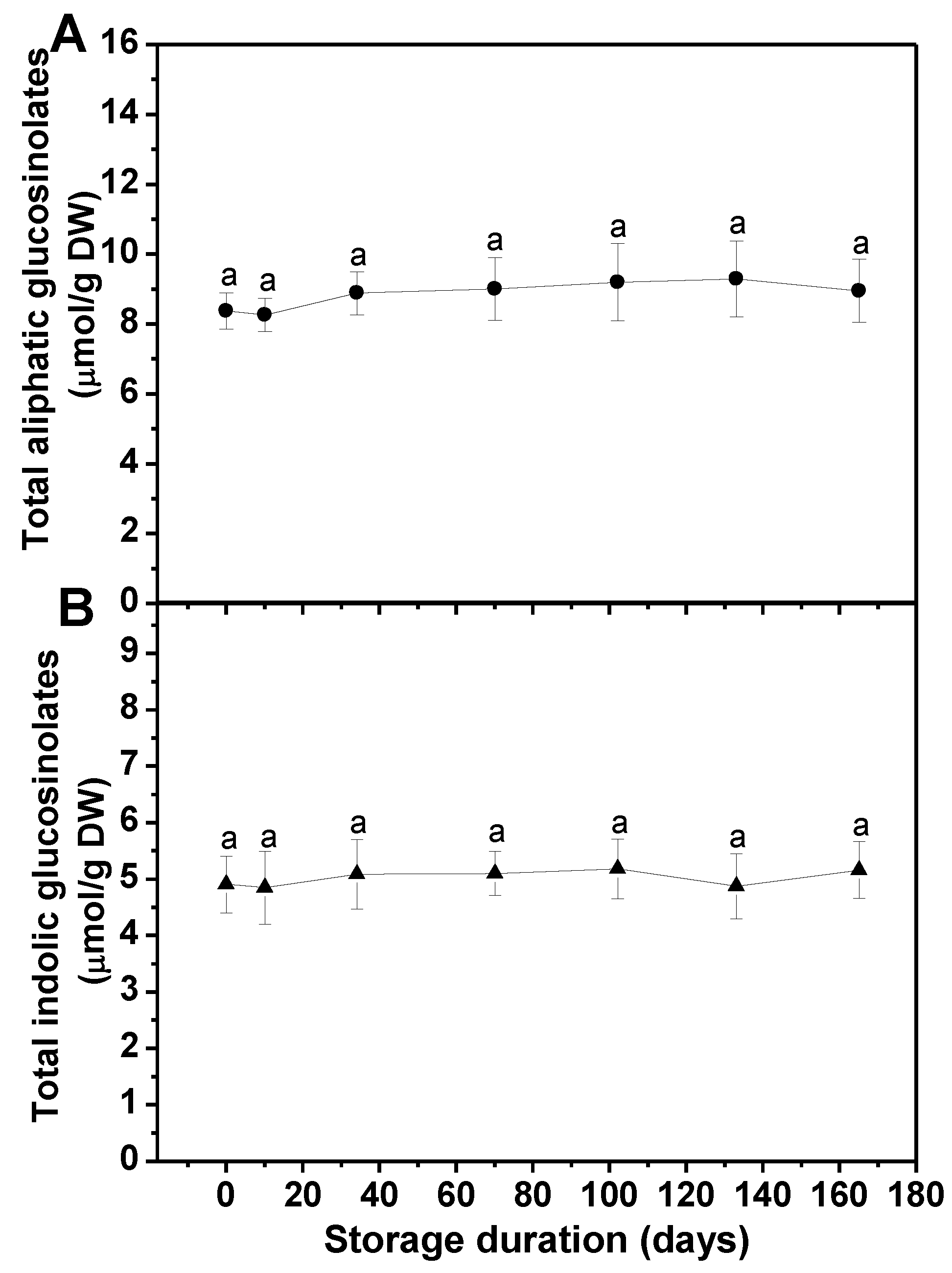
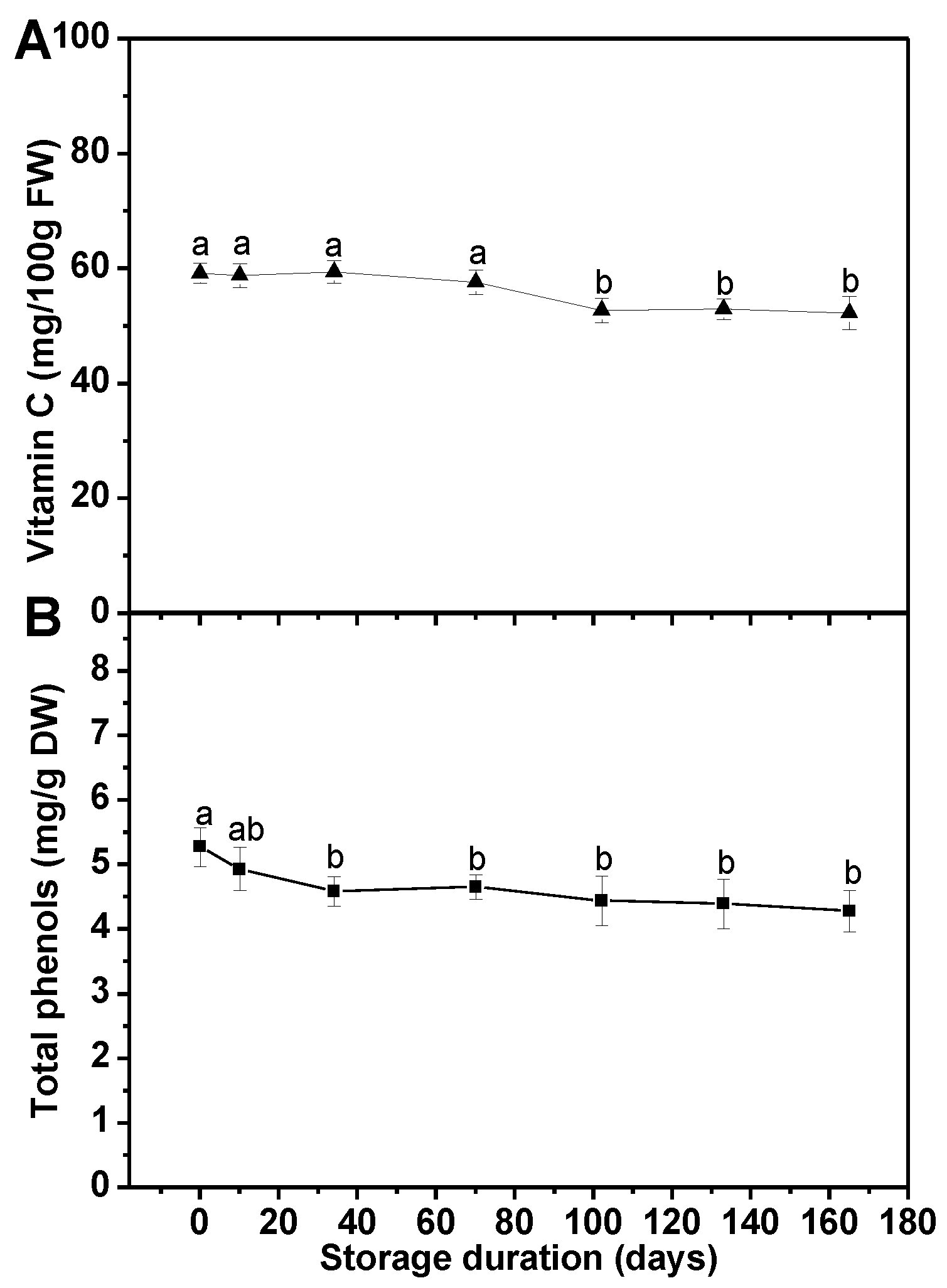
3.1. Effects of Long-Term Freezing Storage on Main Health-Promoting Compounds in Frozen Broccoli Florets
3.1.1. Glucosinolates
3.1.2. Vitamin C and Total Phenols
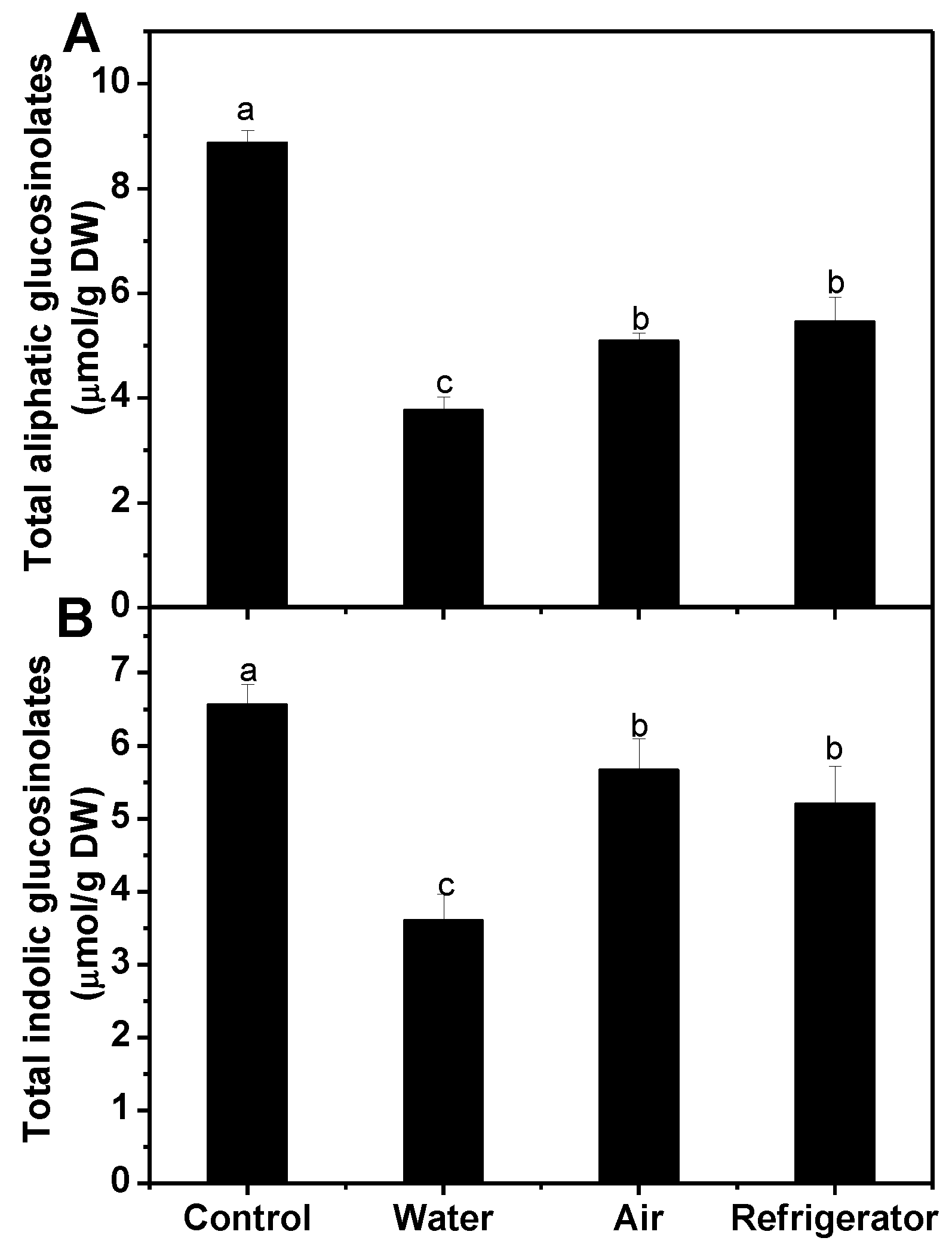
3.2. Effects of Different Defrosting Methods on Main Health-Promoting Compounds in Frozen Broccoli Florets
3.2.1. Glucosinolates
3.2.2. Vitamin C and Total Phenols
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alibabic, V.; Skender, A.; Bajramovic, M.; Sertovic, E.; Bajric, E. Evaluation of morphological, chemical, and sensory characteristics of raspberry cultivars grown in Bosnia and Herzegovina. Turk. J. Agric. For. 2018, 42, 67–74. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Marks, G.C. Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br. J. Nutr. 2003, 90, 687–697. [Google Scholar] [CrossRef]
- Bhandari, S.; Kwak, J.-H. Chemical composition and antioxidant activity in different tissues of Brassica vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Mena, P.; Garcia-Viguera, C.; Moreno, D.A. Brassica foods as a dietary source of vitamin C: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1076–1091. [Google Scholar] [CrossRef]
- Kmiecik, W.; Lisiewska, Z.; Korus, A. Retention of mineral constituents in frozen brassicas depending on the method of preliminary processing of the raw material and preparation of frozen products for consumption. Eur. Food Res. Technol. 2007, 224, 573–579. [Google Scholar] [CrossRef]
- Mahn, A.; Reyes, A. An overview of health-promoting compounds of broccoli (Brassica oleracea var. italica) and the effect of processing. Food Sci. Technol. Int. 2012, 18, 503–514. [Google Scholar] [CrossRef]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar] [CrossRef]
- Higdon, J.; Delage, B.; Williams, D.; Dashwood, R. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]
- Brennan, P.S.; Shewfelt, R.L. Effect of coooling delay at harvest on broccoli quality during postharvest storage. J. Food Qual. 1989, 12, 13–22. [Google Scholar] [CrossRef]
- Miao, H.; Wang, J.; Cai, C.; Chang, J.; Zhao, Y.; Wang, Q. Accumulation of glucosinolates in broccoli. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 133–162. [Google Scholar]
- Tosun, B.N.; Yücecan, S. Influence of commercial freezing and storage on vitamin C content of some vegetables. Int. J. Food Sci. Technol. 2008, 43, 316–321. [Google Scholar] [CrossRef]
- Alanís-Garza, P.A.; Becerra-Moreno, A.; Mora-Nieves, J.L.; Mora-Mora, J.P.; Jacobo-Velázquez, D.A. Effect of industrial freezing on the stability of chemopreventive compounds in broccoli. Int. J. Food Sci. Nutr. 2015, 66, 282–288. [Google Scholar] [CrossRef]
- Cai, C.; Miao, H.; Qian, H.; Yao, L.; Wang, B.; Wang, Q. Effects of industrial pre-freezing processing and freezing handling on glucosinolates and antioxidant attributes in broccoli florets. Food Chem. 2016, 210, 451–456. [Google Scholar] [CrossRef]
- Cieslik, E.; Leszczynska, T.; Filipiak-Florkiewicz, A.; Sikora, E.; Pisulewski, P.M. Effects of some technological processes on glucosinolate contents in cruciferous vegetables. Food Chem. 2007, 105, 976–981. [Google Scholar] [CrossRef]
- Dosz, E.B.; Jeffery, E.H. Modifying the processing and handling of frozen broccoli for increased sulforaphane formation. J. Food Sci. 2014, 78, H1459–H1463. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Abreu, M.; Brandão, T.R.S.; Silva, C.L.M. Degradation kinetics of colour, vitamin C and drip loss in frozen broccoli (Brassica oleracea L. ssp. Italica) during storage at isothermal and non-isothermal conditions. Int. J. Refrig. 2011, 34, 2136–2144. [Google Scholar]
- Nugrahedi, P.Y.; Verkerk, R.; Widianarko, B.; Dekker, M. A mechanistic perspective on process-induced changes in glucosinolate content in Brassica vegetables: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 823–838. [Google Scholar] [CrossRef]
- Yuan, G.F.; Sun, B.; Yuan, J.; Wang, Q.M. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Rosa, E.A.S. Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 1999, 79, 1028–1032. [Google Scholar] [CrossRef]
- Barnes, B.; Tressler, D.K.; Fenton, F. Effect of different cooking methods on the vitamin C content of quick-frozen broccoli. J. Food Sci. 2010, 8, 13–26. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Caruso, M.; Pietrafesa, A.; Natella, S. The influence of processing and preservation on the retention of health-promoting compounds in broccoli. J. Food Sci. 2007, 72, S130–S135. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Pinheiro, J.; Abreu, M.; Fundo, J.; Brandão, T.R.S.; Silva, C.L.M. Quality changes of frozen broccoli stored at different temperatures. In Proceedings of the 3rd CIGR Section VI International Symposium on Food and Agricultural Products: Processing and Innovations, Naples, Italy, 24–26 September 2007. [Google Scholar]
- Yuan, G.; Sun, B.; Yuan, J.; Wang, Q. Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 2010, 118, 774–781. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, l-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.S.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Bahçeci, K.S.; Acar, J. Reversible degradation kinetics of vitamin C in peas during frozen storage. Eur. Food Res. Technol. 2007, 224, 749–753. [Google Scholar] [CrossRef]
- Bulut, M.; Bayer, Ö.; Kırtıl, E.; Bayındırlı, A. Effect of freezing rate and storage on the texture and quality parameters of strawberry and green bean frozen in home type freezer. Int. J. Refrig. 2018, 88, 360–369. [Google Scholar] [CrossRef]
- Martins, R.C.; Silva, C.L.M. Kinetics of frozen stored green bean (Phaseolus vulgaris L.) quality changes: Texture, vitamin C, reducing sugars, and starch. J. Food Sci. 2010, 68, 2232–2237. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of l-ascorbic acid, sugar, pectin and freeze–thaw treatment on polyphenol content of frozen strawberries. LWT Food Sci. Technol. 2009, 42, 581–586. [Google Scholar] [CrossRef]
- Van Buggenhout, S.; Messagie, I.; Maes, V.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Minimizing texture loss of frozen strawberries: Effect of infusion with pectinmethylesterase and calcium combined with different freezing conditions and effect of subsequent storage/thawing conditions. Eur. Food Res. Technol. 2006, 223, 395. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur. Food Res. Technol. 2002, 215, 310–316. [Google Scholar] [CrossRef]
- Dosz, E.B.; Jeffery, E.H. Commercially produced frozen broccoli lacks the ability to form sulforaphane. J. Funct. Foods 2013, 5, 987–990. [Google Scholar] [CrossRef]
- Rungapamestry, V.; Duncan, A.J.; Fuller, Z.; Ratcliffe, B. Effect of cooking brassica vegetables on the subsequent hydrolysis and metabolic fate of glucosinolates. Proc. Nutr. Soc. 2007, 66, 69–81. [Google Scholar] [CrossRef]
- Villarreal-García, D.; Alanís-Garza, P.A.; Cuéllar-Villarreal, M.d.R.; Redondo-Gil, M.; Mora-Nieves, J.L.; Jacobo-Velázquez, D.A. Effects of different defrosting methods on the stability of bioactive compounds and consumer acceptability of frozen broccoli. CyTA J. Food 2015, 13, 312–320. [Google Scholar] [CrossRef]
- Reis, L.C.R.; Pechina, M.; Oliveira, V.R.; Hagen, M.E.K.; Jablonski, A.; Flôres, S.H.; Oliveira Rios, A. Effect of different thawing conditions on the concentration of bioactive substances in broccoli (Brassica oleracea var. Avenger). J. Food Process. Preserv. 2015, 39, 2673–2679. [Google Scholar] [CrossRef]

| Duration of Storage (days) | Aliphatic Glucosinolates (μmol/g DW) | Indolic Glucosinolates (μmol/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GIB | PRO | SIN | GRA | GNA | 4–OH GBS | GBS | 4–OM GBS | NGBS | |
| 0 | 0.57 ± 0.05 b | 2.29 ± 0.19 b | 0.46 ± 0.04 a | 4.89 ± 0.33 a | 0.18 ± 0.03 a | 0.24 ± 0.02 b | 2.57 ± 0.17 a | 0.43 ± 0.01 a | 1.68 ± 0.23 a |
| 10 | 0.67 ± 0.06 ab | 2.18 ± 0.30 b | 0.48 ± 0.08 a | 4.76 ± 0.37 ab | 0.18 ± 0.03 a | 0.32 ± 0.01 a | 2.62 ± 0.25 a | 0.43 ± 0.02 a | 1.55 ± 0.20 a |
| 34 | 0.80 ± 0.08 a | 2.78 ± 0.33 ab | 0.51 ± 0.10 a | 4.62 ± 0.31 ab | 0.18 ± 0.05 a | 0.32 ± 0.02 a | 2.64 ± 0.22 a | 0.44 ± 0.03 a | 1.76 ± 0.22 a |
| 70 | 0.77 ± 0.06 a | 2.83 ± 0.36 ab | 0.51 ± 0.03 a | 4.70 ± 0.51 ab | 0.21 ± 0.01 a | 0.31 ± 0.02 a | 2.63 ± 0.15 a | 0.45 ± 0.05 a | 1.78 ± 0.21 a |
| 102 | 0.75 ± 0.09 a | 3.05 ± 0.51 ab | 0.53 ± 0.08 a | 4.69 ± 0.42 ab | 0.18 ± 0.04 a | 0.31 ± 0.03 a | 2.66 ± 0.26 a | 0.46 ± 0.04 a | 1.82 ± 0.13 a |
| 133 | 0.78 ± 0.03 a | 3.09 ± 0.49 ab | 0.50 ± 0.08 a | 4.74 ± 0.52 ab | 0.18 ± 0.05 a | 0.32 ± 0.01 a | 2.54 ± 0.17 a | 0.45 ± 0.02 a | 1.65 ± 0.16 a |
| 165 | 0.80 ± 0.05 a | 3.15 ± 0.47 a | 0.51 ± 0.13 a | 4.31 ± 0.14 b | 0.19 ± 0.03 a | 0.30 ± 0.02 a | 2.54 ± 0.20 a | 0.44 ± 0.04 a | 1.94 ± 0.19 a |
| Defrosting Methods | Aliphatic Glucosinolates (μmol/g DW) | Indolic Glucosinolates (μmol/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GIB | PRO | SIN | GRA | GNA | 4–OH GBS | GBS | 4–OM GBS | NGBS | |
| Control | 0.80 ± 0.02 a | 3.16 ± 0.10 a | 0.49 ± 0.02 a | 4.21 ± 0.09 a | 0.21 ± 0.01 a | 0.33 ± 0.02 a | 2.94 ± 0.08 a | 0.56 ± 0.03 a | 2.74 ± 0.14 a |
| Water | 0.29 ± 0.02 c | 1.20 ± 0.08 c | 0.24 ± 0.02 b | 1.92 ± 0.13 c | 0.11 ± 0.02 b | 0.11 ± 0.01 d | 1.64 ± 0.16 c | 0.35 ± 0.07 b | 1.50 ± 0.11 b |
| Air | 0.33 ± 0.05 c | 1.73 ± 0.09 b | 0.27 ± 0.01 b | 2.63 ± 0.08 b | 0.14 ± 0.02 b | 0.18 ± 0.01 b | 2.23 ± 0.11 b | 0.44 ± 0.05 b | 2.80 ± 0.16 a |
| Refrigerator | 0.48 ± 0.05 b | 1.68 ± 0.18 b | 0.28 ± 0.04 b | 2.88 ± 0.21 b | 0.14 ± 0.02 b | 0.14 ± 0.01 c | 1.92 ± 0.17 c | 0.38 ± 0.06 b | 2.56 ± 0.17 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, H.; Lin, J.; Zeng, W.; Wang, M.; Yao, L.; Wang, Q. Main Health-Promoting Compounds Response to Long-Term Freezer Storage and Different Thawing Methods in Frozen Broccoli Florets. Foods 2019, 8, 375. https://doi.org/10.3390/foods8090375
Miao H, Lin J, Zeng W, Wang M, Yao L, Wang Q. Main Health-Promoting Compounds Response to Long-Term Freezer Storage and Different Thawing Methods in Frozen Broccoli Florets. Foods. 2019; 8(9):375. https://doi.org/10.3390/foods8090375
Chicago/Turabian StyleMiao, Huiying, Jiayao Lin, Wei Zeng, Mengyu Wang, Leishuan Yao, and Qiaomei Wang. 2019. "Main Health-Promoting Compounds Response to Long-Term Freezer Storage and Different Thawing Methods in Frozen Broccoli Florets" Foods 8, no. 9: 375. https://doi.org/10.3390/foods8090375
APA StyleMiao, H., Lin, J., Zeng, W., Wang, M., Yao, L., & Wang, Q. (2019). Main Health-Promoting Compounds Response to Long-Term Freezer Storage and Different Thawing Methods in Frozen Broccoli Florets. Foods, 8(9), 375. https://doi.org/10.3390/foods8090375