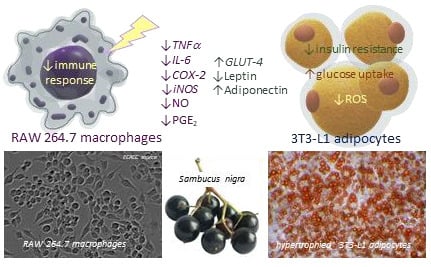
Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Elderberry Fruit Extract
2.2. Determination of Individual Phenolic Compounds Using HPLC-DAD-MSn Analysis
2.3. T3-L1 Cell Culture, Differentiation, and Treatment
2.4. Macrophage Cell Culture and Anti-Inflammatory Experiment Procedure
2.5. Cell Viability Assay
2.6. Measurement of Reactive Oxygen Species in Adipocytes
2.7. Measurement of Intracellular Triglyceride Content in Adipocytes
2.8. Glucose Uptake Measurement in Adipocytes
2.9. Determination of Adipokine Production in 3T3-L1 Adipocytes
2.10. Determination of IL-6, TNF-α, and PGE2 Production in RAW 264.7 Macrophages
2.11. Determination of NO Production in RAW 264.7 Macrophages
2.12. Quantification of Gene Expression Using Real-Time PCR
2.13. Digestive Enzyme Inhibition Assays
2.13.1. Measurement of Pancreatic Lipase Inhibition
2.13.2. Measurement of α-Amylase Inhibition
2.13.3. Measurement of α-Glucosidase Inhibition
2.13.4. Data Analysis
2.14. Statistical Analysis
3. Results
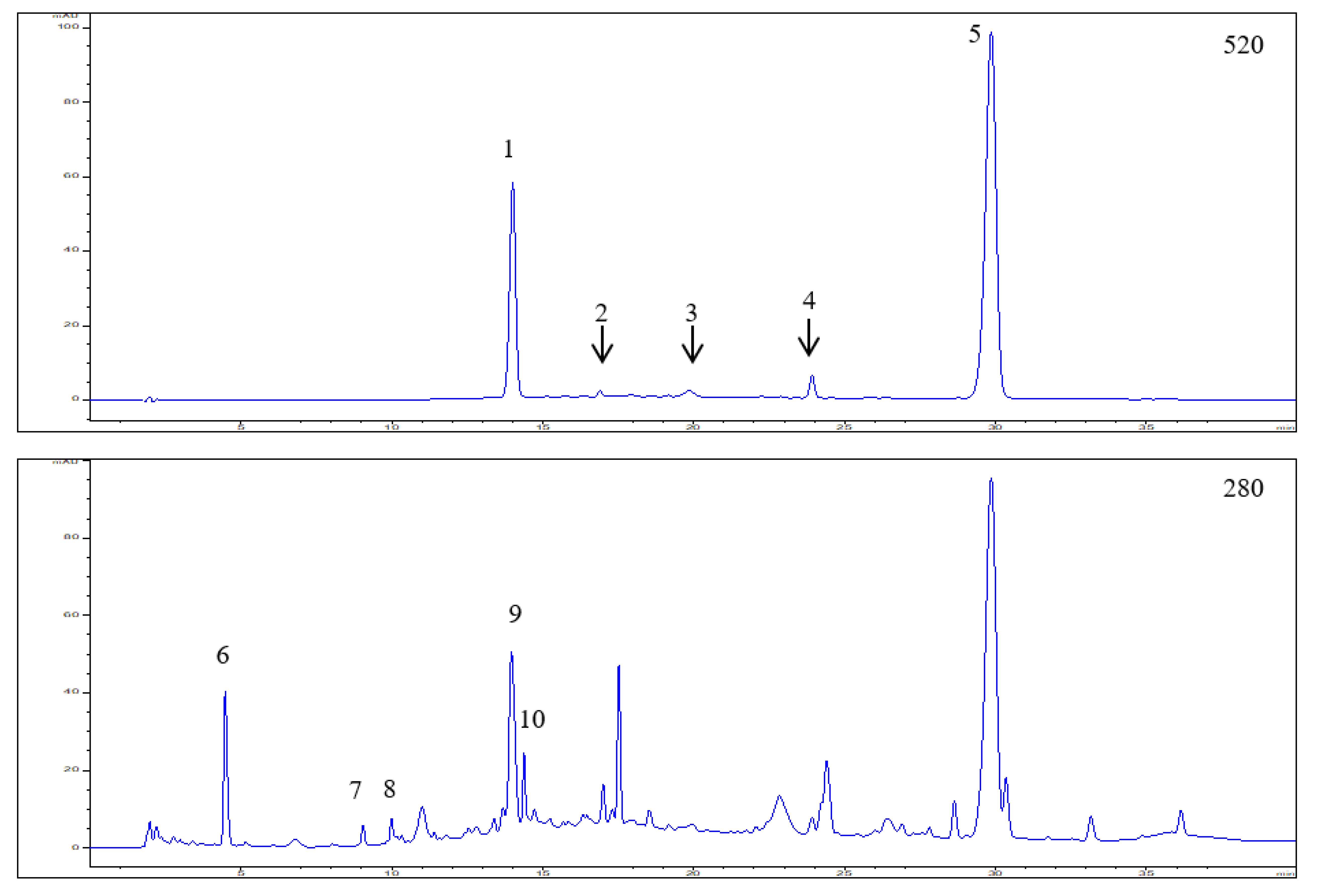
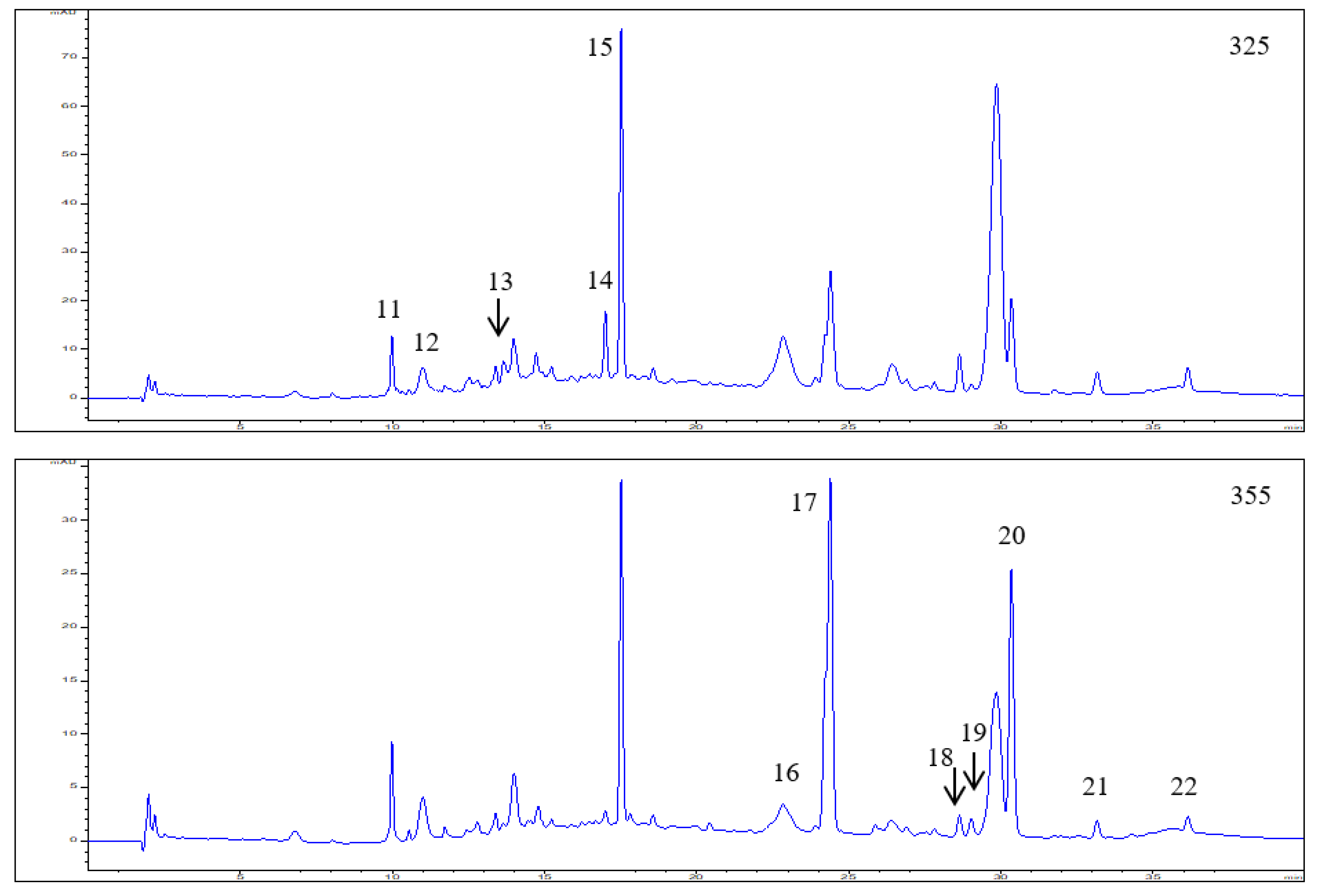
3.1. Polyphenol Composition in the Elderberry Fruit Extract
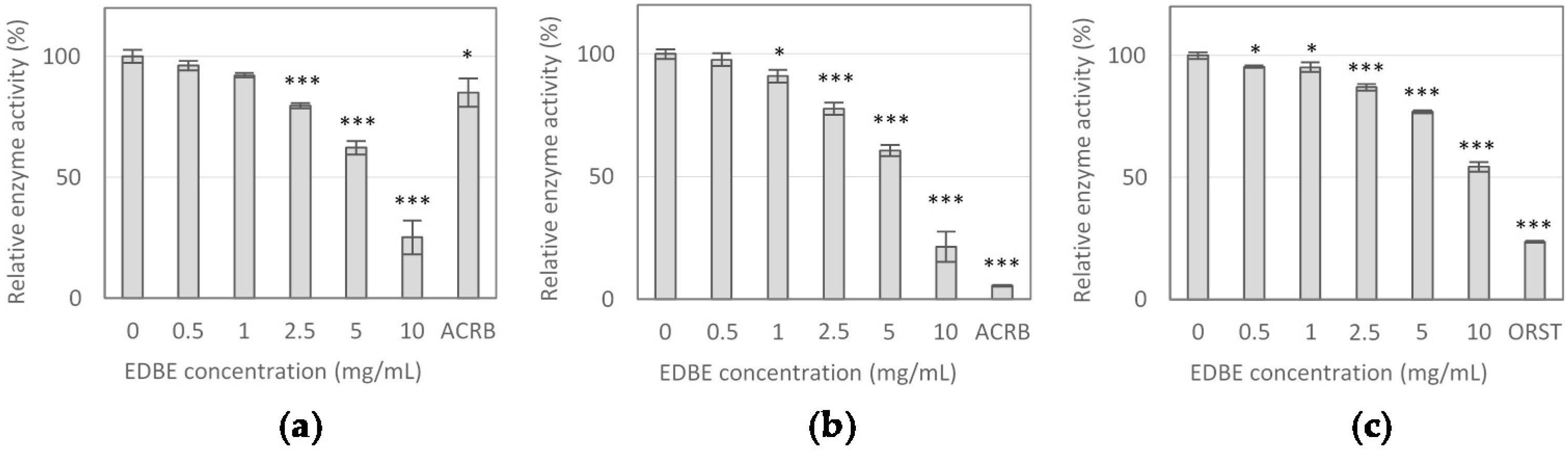
3.2. Digestive Enzyme Activity Inhibition by Elderberry Fruit Extract
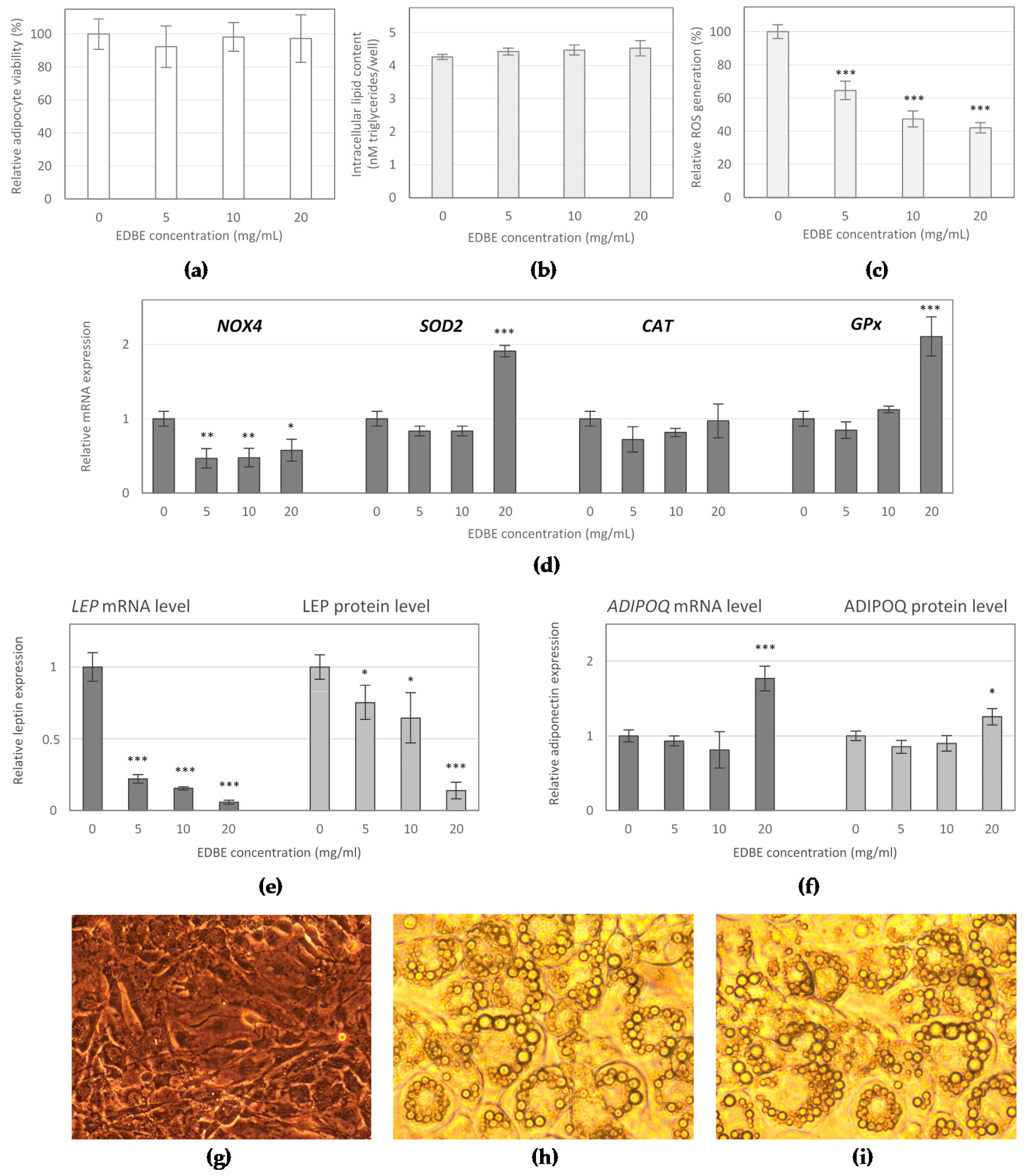
3.3. The Effect of Elderberry Fruit Extract on Hypertrophied Adipocytes
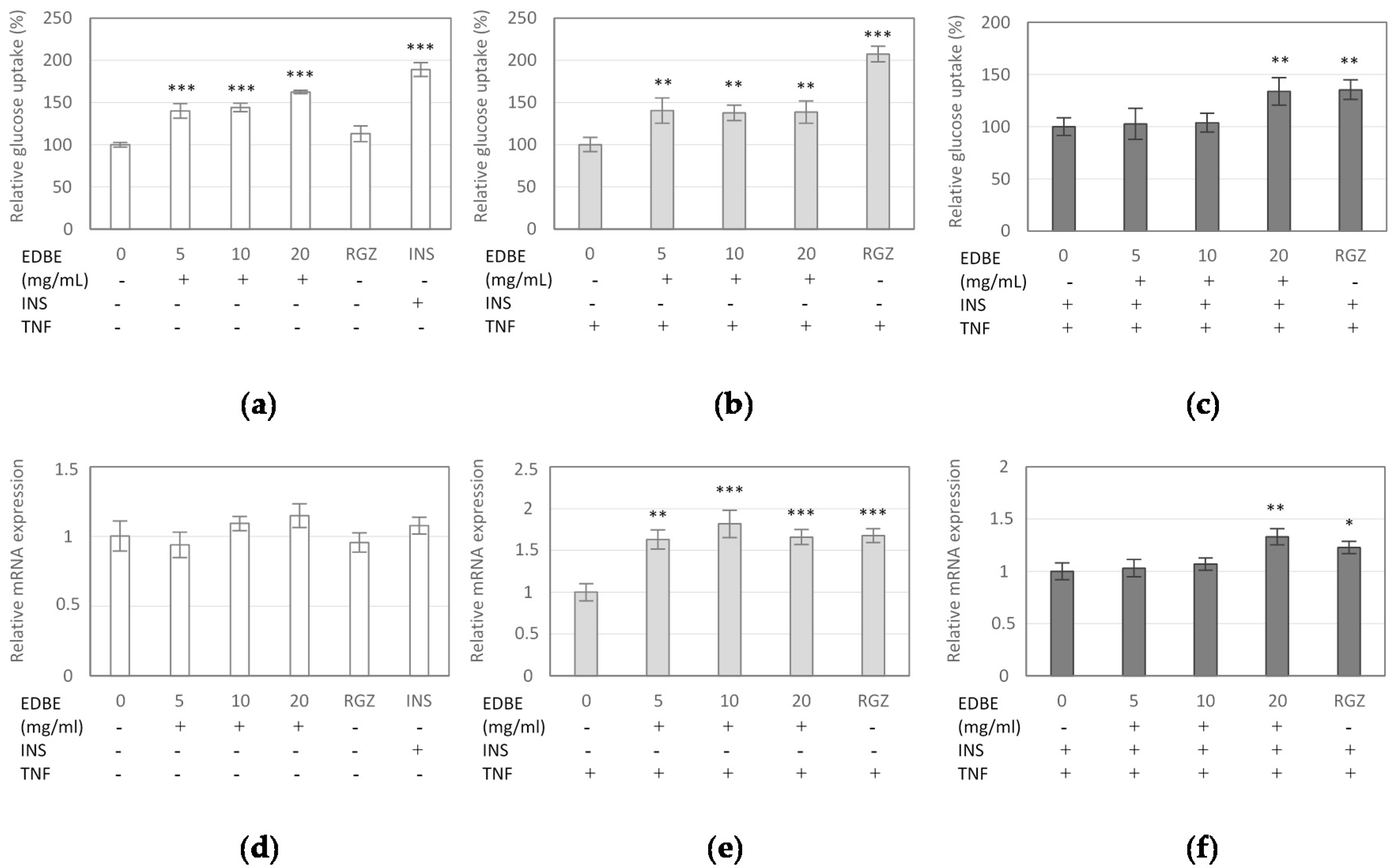
3.4. The Effect of Elderberry Fruit Extract on Glucose Uptake in Mature 3T3-L1 Adipocytes
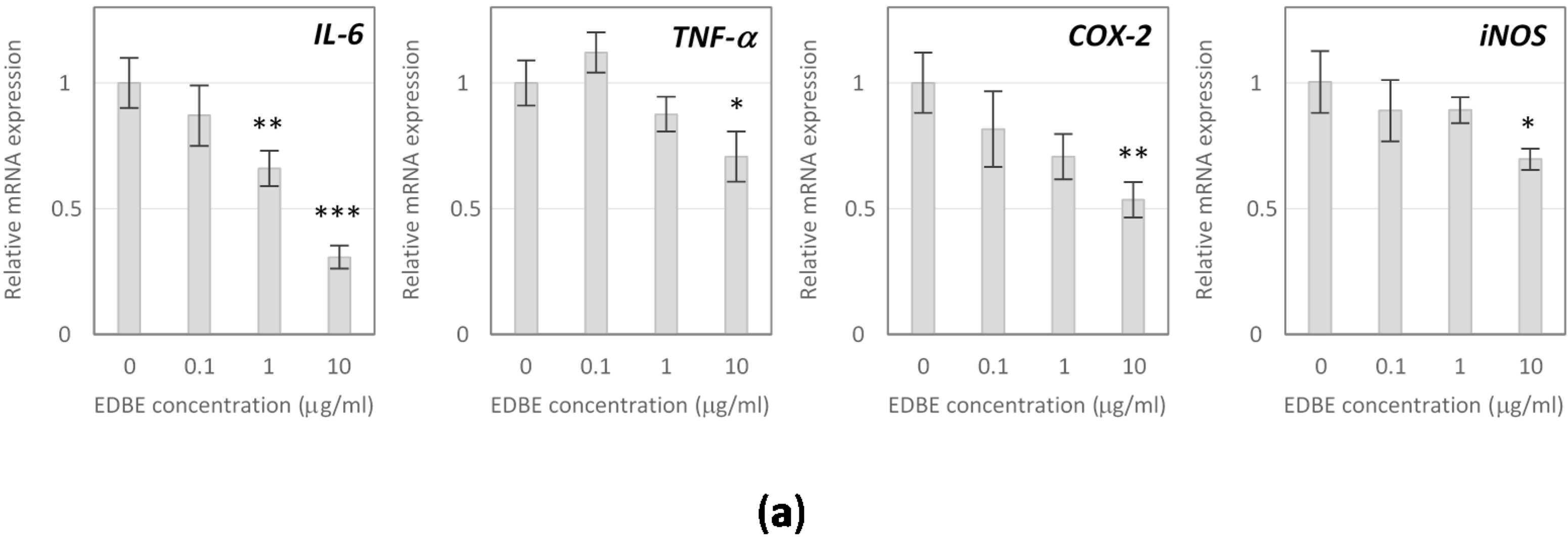
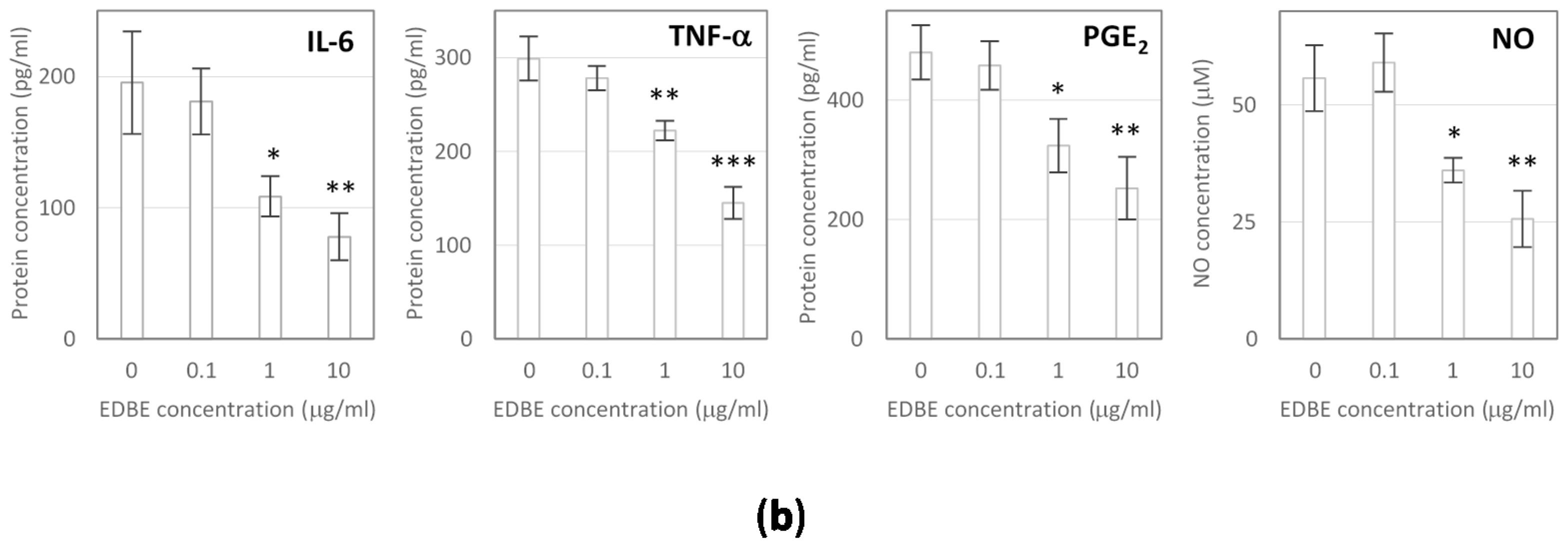
3.5. Anti-Inflammatory Effects of Elderberry Fruit Extract
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.P. Obesity and the development of type 2 diabetes: The effects of fatty tissue inflammation. Diabetes Metab. Syndr. Obes. 2010, 3, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Tateya, S.; Kim, F.; Tamori, Y. Recent advances in obesity-induced inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A. Current evidence on the health-beneficial effects of berry fruits in the prevention and treatment of metabolic syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Rychlik, J.; Juzwa, W.; Myszka, K.; Dembczyński, R.; Białas, W. Anti-inflammatory effects of gastrointestinal digested Sambucus nigra L. fruit extract analysed in co-cultured intestinal epithelial cells and lipopolysaccharide-stimulated macrophages. J. Funct. Foods 2015, 19, 649–660. [Google Scholar] [CrossRef]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczyński, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Neves, D.; Valentao, P.; Bernardo, J.; Oliveira, M.C.; Ferreira, J.M.G.; Pereira, D.M.; Andrade, P.B.; Videira, R.A. A new insight on elderberry anthocyanins bioactivity: Modulation of mitochondrial redox chain functionality and cell redox state. J. Funct. Foods 2019, 56, 145–155. [Google Scholar] [CrossRef]
- Badescu, M.; Badulescu, O.; Badescu, L.; Ciocoiu, M. Effects of Sambucus nigra and Aronia melanocarpa extracts on immune system disorders within diabetes mellitus. Pharm. Biol. 2015, 53, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Salvador, Â.C.; Król, E.; Lemos, V.C.; Santos, S.A.O.; Bento, F.P.M.S.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczyński, B.; Krejpcio, Z.; et al. Effect of Elderberry (Sambucus nigra L.) Extract Supplementation in STZ-Induced Diabetic Rats Fed with a High-Fat Diet. Int. J. Mol. Sci. 2017, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.; Norris, G.; Lee, S.G.; Porter, C.M.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.; Wangensteen, H.; Barsett, H. Elderberry and elderflower extracts, phenolic compounds, and metabolites and their effect on complement, RAW 264.7 macrophages and dendritic cells. Int. J. Mol. Sci. 2017, 18, 584. [Google Scholar] [CrossRef] [PubMed]
- Simonyi, A.; Chen, Z.; Jiang, J.; Zong, Y.; Chuang, D.Y.; Gu, Z.; Lu, C.H.; Fritsche, K.L.; Greenlief, C.M.; Rottinghaus, G.E.; et al. Inhibition of microglial activation by elderberry extracts and its phenolic components. Life Sci. 2015, 128, 30–38. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Zielińska-Wasielica, J.; Olkowicz, M. Inhibitory effects of lingonberry (Vaccinium vitis-idaea L.) fruit extract on obesity-induced inflammation in 3T3-L1 adipocytes and RAW 264.7 macrophages. J. Funct. Foods 2019, 54, 371–380. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, J.W.; Cha, Y.-N.; Kim, C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J. Immunoass. Immunoch. 2006, 27, 31–44. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Salazar-Olivo, L.A. The anti-diabetic properties of Guazuma ulmifolia Lam are mediated by the stimulation of glucose uptake in normal and diabetic adipocytes without inducing adipogenesis. J. Ethnopharmacol. 2008, 118, 252–256. [Google Scholar] [CrossRef]
- Boath, A.S.; Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit Digestive Enzymes: A Source of Potential Health Benefits? Food Dig. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K.C.; Zhang, Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017, 214, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; González-Campoy, J.M.; Bray, G.A.; Kitabchi, A.E.; Bergman, D.A.; Schorr, A.B.; Rodbard, H.W.; Henry, R.R. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev. Cardiovasc. Ther. 2008, 6, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E.; Morse, S.; Borne, D.M.; Aguilar, E.A.; Reisin, E. Leptin and hypertension in obesity. Vasc. Health Risk Manag. 2006, 2, 163–169. [Google Scholar] [CrossRef]
- Zeyda, M.; Stulnig, T.M. Obesity, inflammation, and insulin resistance—A mini-review. Gerontology 2009, 55, 379–386. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Nguyen, T.K.Y.; Kase, E.T.; Tadesse, M.; Barsett, H.; Wangensteen, H. Enhanced Glucose Uptake in Human Liver Cells and Inhibition of Carbohydrate Hydrolyzing Enzymes by Nordic Berry Extracts. Molecules 2017, 22, 1806. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic Elderberry Extracts, Anthocyanins, Procyanidins, and Metabolites Influence Glucose and Fatty Acid Uptake in Human Skeletal Muscle Cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Jayarathne, S.; Koboziev, I.; Park, O.-H.; Oldewage-Theron, W.; Shen, C.-L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef]
- Aderem, A.; Ulevitch, R.J. Toll-like receptors in the induction of the innate immune response. Nature 2000, 406, 782–787. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and Inflammation: Epidemiology, Risk Factors, and Markers of Inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15, 120–122. [Google Scholar]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- García-Alonso, V.; Titos, E.; Alcaraz-Quiles, J.; Rius, B.; Lopategi, A.; López-Vicario, C.; Jakobsson, P.J.; Delgado, S.; Lozano, J.; Clària, J. Prostaglandin E2 Exerts Multiple Regulatory Actions on Human Obese Adipose Tissue Remodeling, Inflammation, Adaptive Thermogenesis and Lipolysis. PLoS ONE 2016, 11, e0153751. [Google Scholar] [CrossRef]
- Hsieh, P.-S.; Jin, J.-S.; Chiang, C.-F.; Chan, P.-C.; Chen, C.-H.; Shih, K.-C. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity 2009, 17, 1150–1157. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Juan, S.-H.; Shen, S.-C.; Hsu, F.-L.; Chen, Y.-C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003, 66, 1821–1832. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals: Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
| Gene | Accession | No. Sequence (5′–3′) | Amplicon (bp) |
|---|---|---|---|
| Mm LEP | NM-008493 | F: GGA TCA GGT TTT GTG GTG CT | 187 |
| R: TTG TGG CCC ATA AAG TCC TC | |||
| Mm GLUT-4 | NM-001359114.1 | F: TGC TGG GCA CAG CTA CCC | 162 |
| R: CGG TCA GGC GCT TTA GAC | |||
| Mm ADIPOQ | NM-009605 | F: CTG GCC ACT TTC TCC TCA TT TC | 120 |
| R: GGC ATG ACT GGG CAG GAT TA | |||
| Mm IL-6 | NM-031168.1 | F: TCT GAA GGA CTC TGG CTT TG | 142 |
| R: GAT GGA TGC TAC CAA ACT GGA | |||
| Mm NOS-2 | NM-010927.3 | F: TGA AGA AAA CCC CTT GTG CT | 100 |
| R: TTC TGT GCT GTC CCA GTG AG | |||
| Mm PTGS2 | NM-011198.3 | F: GGC GCA GTT TAT GTT GTC TGT | 107 |
| R: CAA GAC AGA TCA TAA GCG AGG A | |||
| Mm TNF-α | NM-001278601.1 | F: AGG GTC TGG GCC ATA GAA CT | 103 |
| R: CCA CCA CGC TCT TCT GTC TAC | |||
| Mm NOX-4 | NM-015760.5 | F: GAT CAC AGA AGG TCC CTA GCA G | 134 |
| R: GTT GAG GGC ATT CAC CAA GT | |||
| Mm SOD2 | NM-013671.3 | F: CGT GTC TGT GGG AGT CCA AGG TTC AG | 139 |
| R: GTC AAT CCC CAG CAG CGG AAT AAG | |||
| Mm CATALASE | NM-009804.2 | F: CCT CCT CGT TCA GGA TGT GGT T | 243 |
| R: CGA GGG TCA CGA ACT GTG TCA G | |||
| Mm GPx | NM-008160.6 | F: GGG CAA GGT GCT GCT CAT TG | 269 |
| R: AGA GCG GGT GAG CCT TCT CA | |||
| Mm ACTB | NM-007393 | F: CCA CAG CTG AGA GGG AAA TC | 193 |
| R: AAG GAA GGC TGG AAA AGA GC |
| Peak No. | RT (min) | UV λ max (nm) | [M]+/[M + H]+ (m/z) | [M − H]− (m/z) | MS/MS (m/z) | Tentative Identification | Concentration (mg/g) * |
|---|---|---|---|---|---|---|---|
| 1 | 14.01 | 280, 520 | 611.1651 | - | 287.0583 | Cyanidin-3,5-O-diglucoside | 3.27 ± 0.25 |
| 743.2095 | 287.0579 | Cyanidin-3-O-sambubiosyl-5-O-glucoside (co-elution) | |||||
| 2 | 16.90 | 280,520 | 449.1133 | 287.0632 | Cyanidin-3-O-glucoside | Trace amounts | |
| 3 | 19.84 | 280,520 | 595.1734 | 287.0578 | Cyanidin-3-O-rutinoside | Trace amounts | |
| 4 | 23.92 | 280,520 | 433.1187 | 271.0640 | Pelargonidin-3-O-glucoside | 0.31 ± 0.04 | |
| 5 | 29.85 | 280,520 | 581.1635 | 287.0633 | Cyanidin-3-O-sambubioside | 9.76 ± 0.68 | |
| 6 | 4.48 | 275 | 299.2506 | — | 4-Hydroxybenzoic acid glucoside | 1.60 ± 0.12 | |
| 7 | 9.05 | 280 | 289.1139 | 245.1203 | (+)/(−)-Catechin | 1.07 ± 0.06 | |
| 8 | 9.99 | 280 | 289.1140 | 245.1210 | (+)/(−)-Epicatechin | 1.41 ± 0.08 | |
| 9 | 13.96 | 268 | 597.4616 | — | Hydrolysable tannin | 3.45 ± 0.22 | |
| 10 | 14.38 | 268 | 597.4628 | — | Hydrolysable tannin | 0.92 ± 0.06 | |
| 11 | 9.99 | 299,325 | 353.2873 | 191.1737 | Neochlorogenic acid | 0.50 ± 0.03 | |
| 12 | 11.00 | 299,325 | 353.2886 | 191.1749 | Chlorogenic acid | 0.59 ± 0.04 | |
| 13 | 12.54 | 300,325 | 353.2865 | 191.1729 | Cryptochlorogenic acid | 0.26 ± 0.02 | |
| 14 | 17.00 | 310,234 | 337.0917 | 173.0443 | P-coumaroylquinic acid | 0.78 ± 0.06 | |
| 15 | 17.52 | 316,234 | 371.3016 | 163.0396 | P-Coumaric acid hexoside | 2.85 ± 0.18 | |
| 16 | 22.83 | 268,354 | 463.0882 | 301.0354 | Quercetin-3-O-glucoside | 0.29 ± 0.01 | |
| 17 | 24.39 | 255,355 | 609.1461 | 301.0349 | Quercetin-3-O-rutinoside | 2.27 ± 0.19 | |
| 18 | 28.63 | 255,358 | 505.0872 | 301.0366 | Quercetin 3-O-(6”-acetyl-glucoside) | 0.09 ± 0.01 | |
| 19 | 29.02 | 266,348 | 447.0935 | 285.0540 | Kaempferol-3-O-glucoside | 0.09 ± 0.01 | |
| 20 | 30.34 | 255,352 | 593.1515 | 285.0542 | Kaempferol-3-O-rutinoside | 1.36 ± 0.12 | |
| 21 | 33.15 | 255,370 | 301.0356 | 151.0031 | Quercetin | 0.09 ± 0.02 | |
| 22 | 36.14 | 255,352 | 623.1044 | 315.0449 | Isorhamnetin-3-O-rutinoside | 0.07 ± 0.01 |
| Enzyme Inhibitor | α-Glucosidase | α-Amylase | Lipase | |||
|---|---|---|---|---|---|---|
| IC10 | IC50 | IC10 | IC50 | IC10 | IC50 | |
| EDBE (mg/mL) | 1.25 ± 0.09 | 6.70 ± 0.56 | 1.25 ± 0.10 | 6.38 ± 0.44 | 2.09 ± 0.30 | 10.98 ± 0.47 |
| ACRB (μg/mL) | 85.12 ± 0.63 | > 100 | 1.30 ± 0.06 | 8.23 ± 0.16 | - | - |
| ORST (μg/mL) | - | - | - | - | 0.2 ± 0.02 | 1.43 ± 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. https://doi.org/10.3390/foods8080326
Zielińska-Wasielica J, Olejnik A, Kowalska K, Olkowicz M, Dembczyński R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods. 2019; 8(8):326. https://doi.org/10.3390/foods8080326
Chicago/Turabian StyleZielińska-Wasielica, Joanna, Anna Olejnik, Katarzyna Kowalska, Mariola Olkowicz, and Radosław Dembczyński. 2019. "Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages" Foods 8, no. 8: 326. https://doi.org/10.3390/foods8080326
APA StyleZielińska-Wasielica, J., Olejnik, A., Kowalska, K., Olkowicz, M., & Dembczyński, R. (2019). Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods, 8(8), 326. https://doi.org/10.3390/foods8080326