Fermented Sea Tangle (Laminaria japonica Aresch) Suppresses RANKL-Induced Osteoclastogenesis by Scavenging ROS in RAW 264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Preparation of FST
2.3. Cell Culture and Viability Analysis
2.4. Osteoclast Differentiation and TRAP Assay
2.5. F-Actin Ring Staining
2.6. Western Blot Analysis
2.7. Immunofluorescence Staining for NF-κB
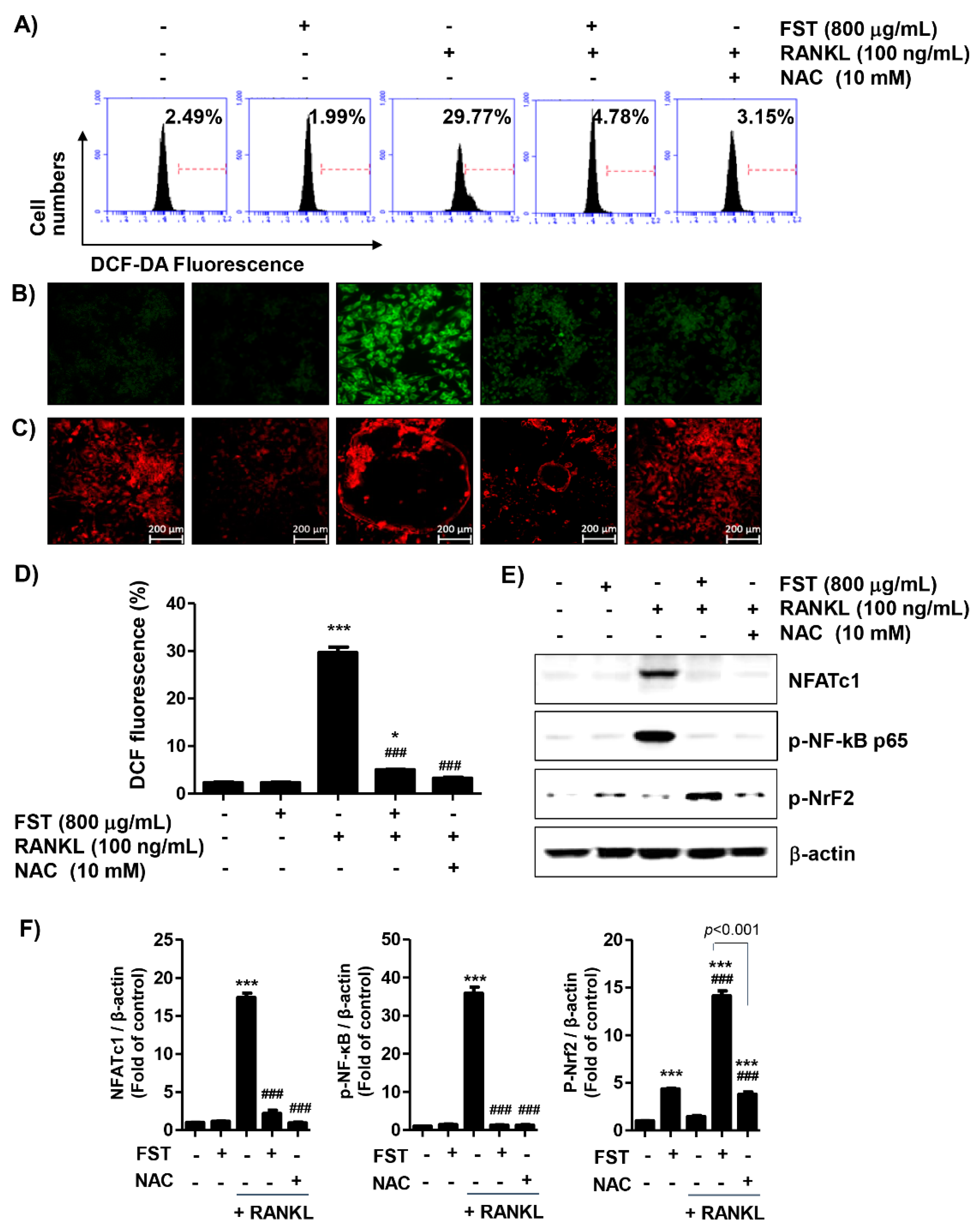
2.8. Measurement of Intracellular ROS Levels
2.9. Statistical Analysis
3. Results
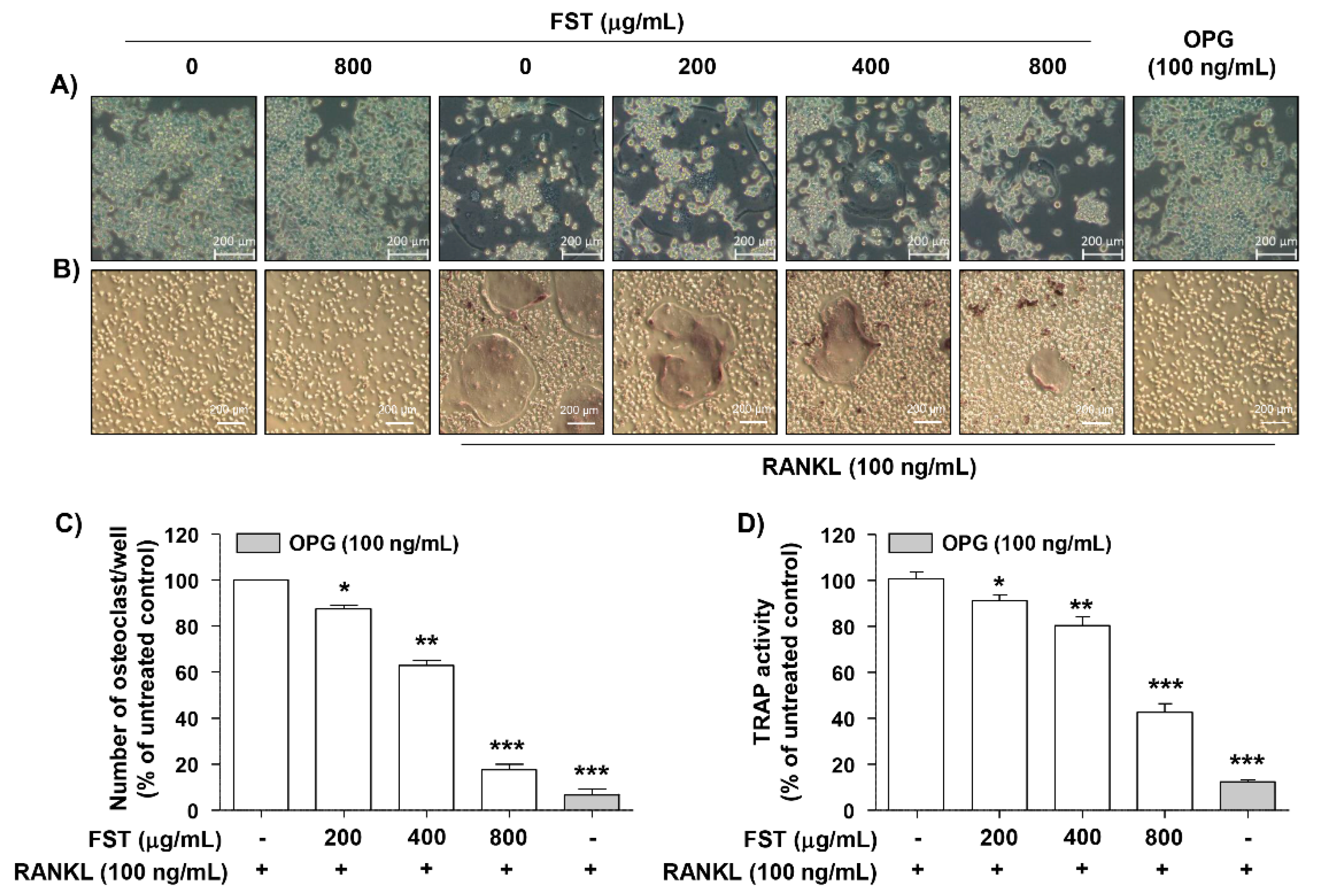
3.1. Effect of FST on Cell Viability in RAW 264.7 Cells
3.2. FST Suppresses RANKL-Induced Osteoclastogenesis in RAW 264.7 Cells
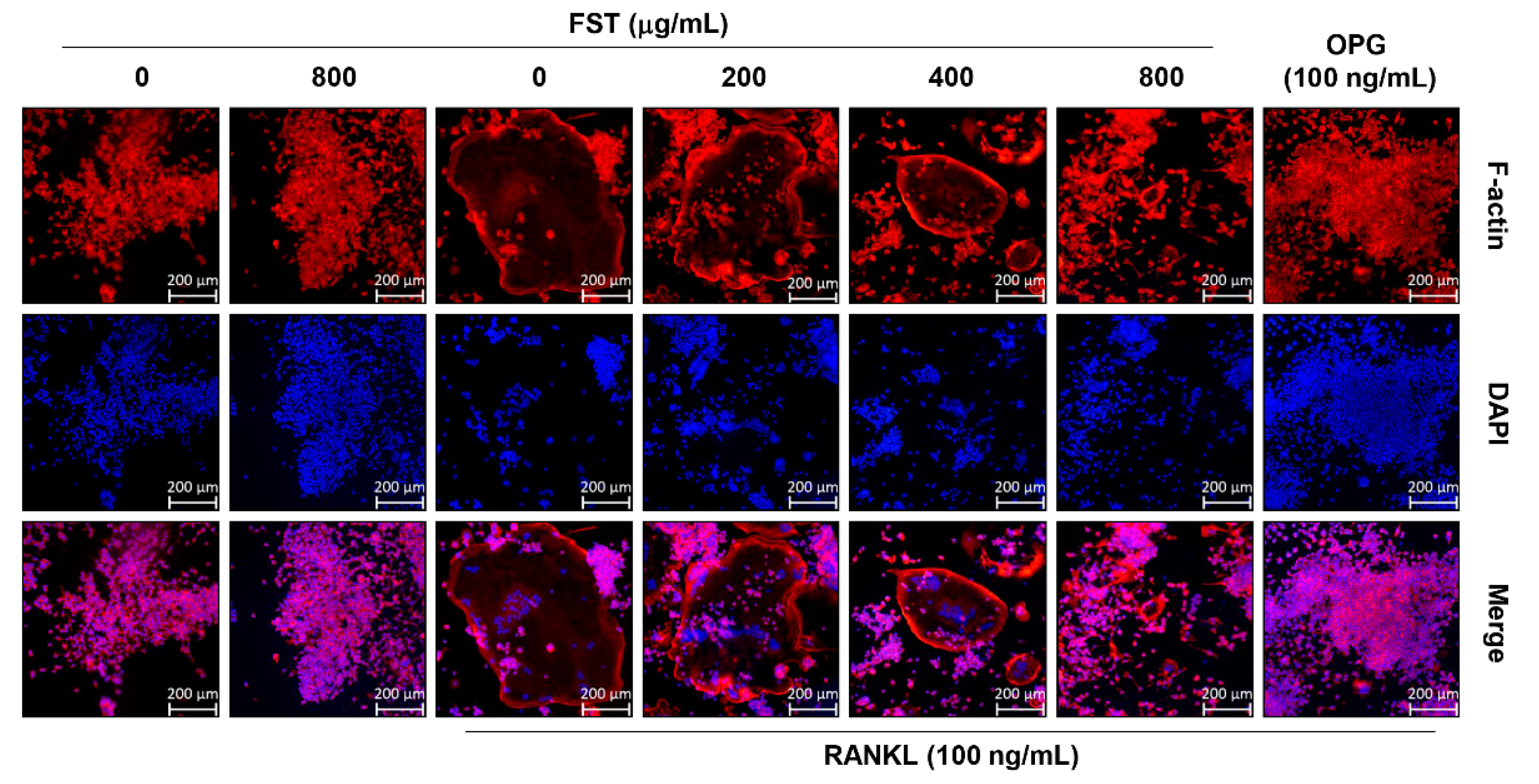
3.3. FST Disrupts RANKL-Induced Formation of F-Actin Rich Adhesive Structures in RAW 264.7 Mouse Macrophage-Like Cells
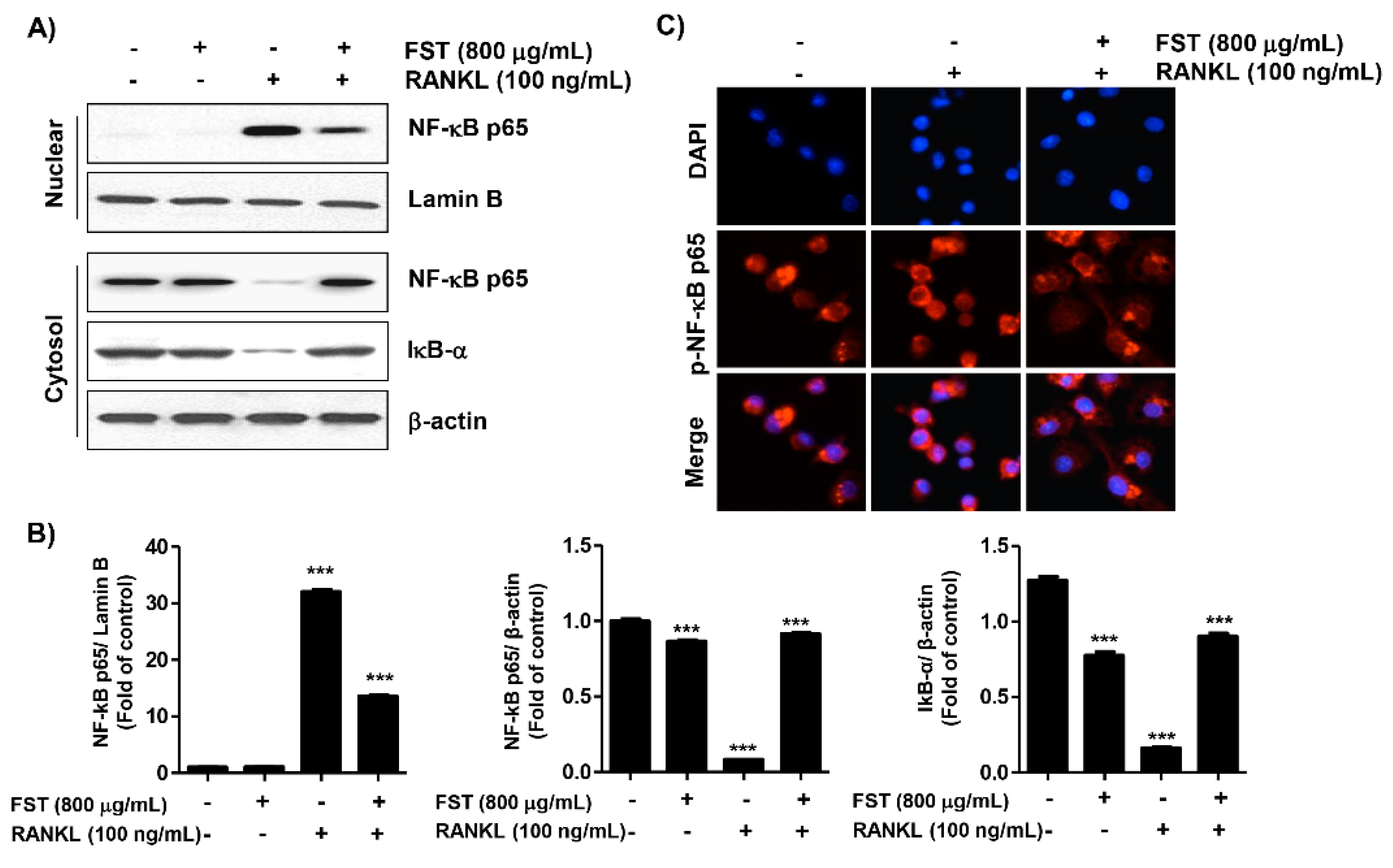
3.4. FST Inhibits the RANKL-Induced Nuclear Translocation of NF-κB and IκBα Degradation in RAW 264.7 Cells
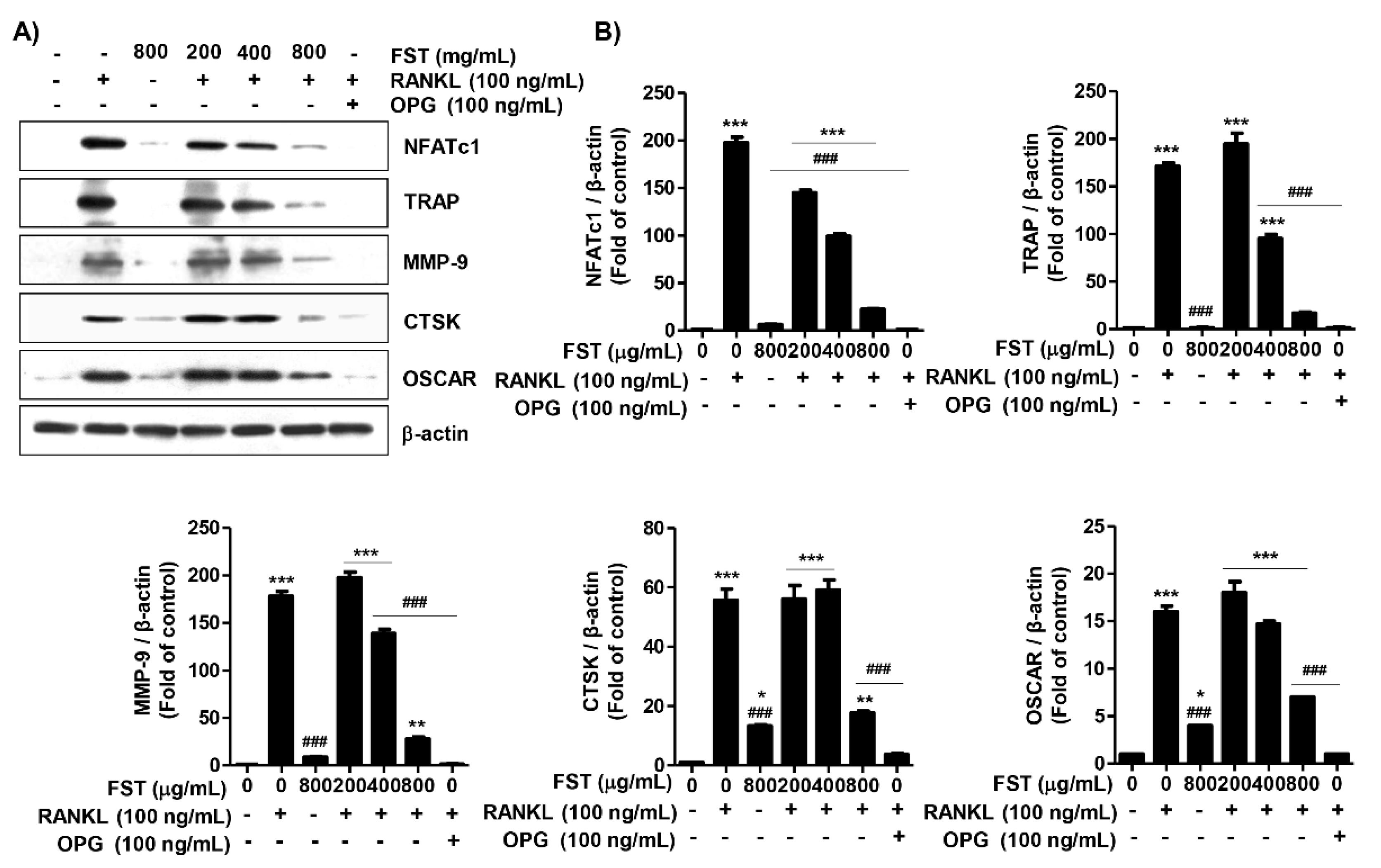
3.5. FST Down-Regulates RANKL-Induced Osteoclast-Associated Gene Expression in RAW 264.7 Cells
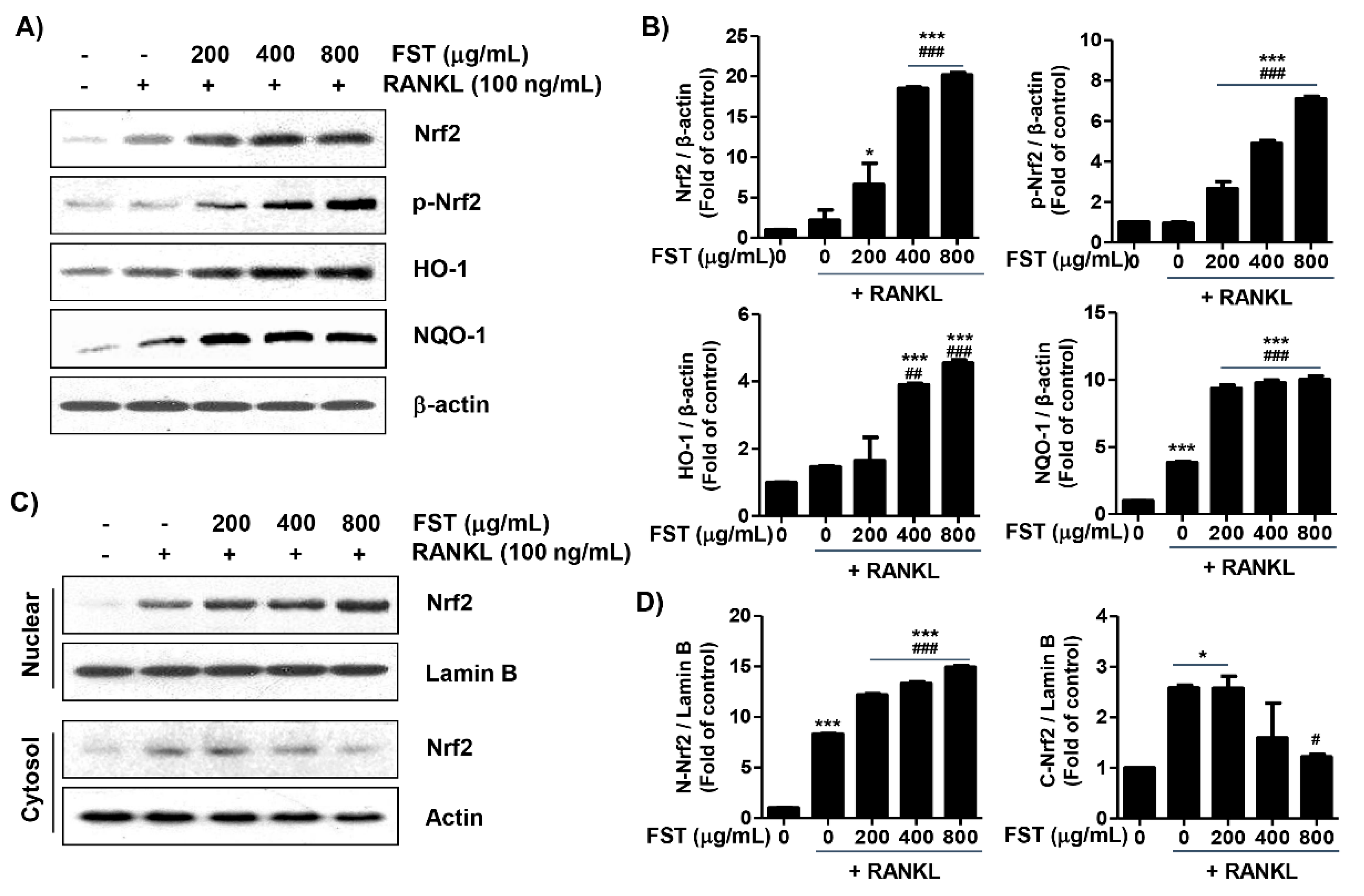
3.6. FST Attenuates RANKL-Induced Intracellular ROS Accumulation Associated with Activation of Nrf2 in RAW 264.7 Mouse Macrophage-Like Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kikuta, J.; Ishii, M. Osteoclast migration, differentiation and function: Novel therapeutic targets for rheumatic diseases. Rheumatology 2013, 52, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Galson, D.L.; Roodman, G.D. Pathobiology of Paget’s disease of bone. J. Bone Metab. 2014, 21, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, N.K.; Lee, S.Y. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol. Cells 2017, 40, 706–713. [Google Scholar] [PubMed]
- Bi, H.; Chen, X.; Gao, S.; Yu, X.; Xiao, J.; Zhang, B.; Liu, X.; Dai, M. Key triggers of osteoclast-related diseases and available strategies for targeted therapies: A review. Front. Med. 2017, 4, 234. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Matsuo, K. Molecular mechanisms of triggering, amplifying and targeting RANK signaling in osteoclasts. World J. Orthop. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef]
- Lee, S.H.; Jang, H.D. Scoparone attenuates RANKL-induced osteoclastic differentiation through controlling reactive oxygen species production and scavenging. Exp. Cell Res. 2015, 331, 267–277. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, C.; Zhu, X.; Li, Y.; Yu, R.; Xu, W. Glycyrrhizin suppresses RANKL-Induced osteoclastogenesis and oxidative stress through inhibiting NF-κB and MAPK and activating AMPK/Nrf2. Calcif. Tissue Int. 2018, 103, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Thummuri, D.; Naidu, V.G.M.; Chaudhari, P. Carnosic acid attenuates RANKL-induced oxidative stress and osteoclastogenesis via induction of Nrf2 and suppression of NF-κB and MAPK signalling. J. Mol. Med. 2017, 95, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, C.; Kim, G.Y.; Park, E.K.; Jeon, Y.J.; Kim, S.; Hwang, H.J.; Choi, Y.H. Sargassum serratifolium attenuates RANKL-induced osteoclast differentiation and oxidative stress through inhibition of NF-κB and activation of the Nrf2/HO-1 signaling pathway. Biosci. Trends 2018, 12, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Cheung, A.M.; Khan, A.A. Drug-related adverse events of osteoporosis therapy. Endocrinol. Metab. Clin. 2017, 46, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T. Extracts of marine algae show inhibitory activity against osteoclast differentiation. Adv. Food Nutr. Res. 2011, 64, 443–454. [Google Scholar] [PubMed]
- Venkatesan, J.; Kim, S.K. Osteoporosis treatment: Marine algal compounds. Adv. Food Nutr. Res. 2011, 64, 417–427. [Google Scholar] [PubMed]
- De Jesus Raposo, M.F.; De Morais, A.M.; De Morais, R.M. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, E27. [Google Scholar] [CrossRef] [PubMed]
- Shirosaki, M.; Koyama, T. Laminaria japonica as a food for the prevention of obesity and diabetes. Adv. Food Nutr. Res. 2011, 64, 199–212. [Google Scholar]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. A comparison study on polysaccharides extracted from Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Jang, M.S. Anti-obesity effects of Laminaria japonica fermentation on 3T3-L1 adipocytes are mediated by the inhibition of C/EBP-α/β and PPAR-γ. Cell. Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Kim, S.C.; Lee, J.H.; Lee, J.R.; Kim, I.K.; Baek, S.Y.; Kim, Y.W. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress. BMC Complement. Altern. Med. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Je, J.Y.; Park, S.Y.; Ahn, C.B. Antioxidant and cytoprotective activities of enzymatic extracts from Rhizoid of Laminaria japonica. Prev. Nutr. Food Sci. 2017, 22, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wu, X.; Li, F.; She, W.; Zhou, L.; Pi, B.; Xu, Z.; Huang, X. Laminaria Japonica polysaccharides effectively inhibited the growth of nasopharyngeal carcinoma cells in vivo and in vitro study. Exp. Toxicol. Pathol. 2017, 69, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Senevirathne, M.; Kim, J.S.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Kang, Y.M.; Kim, J.I.; Nam, B.H.; Ahn, C.B.; et al. Protective effect of fermented sea tangle against ethanol and carbon tetrachloride-induced hepatic damage in Sprague-Dawley rats. Food Chem. Toxicol. 2010, 48, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Lee, B.J.; Kim, J.I.; Nam, B.H.; Cha, J.Y.; Kim, Y.M.; Ahn, C.B.; Choi, J.S.; Choi, I.S.; Je, J.Y. Antioxidant effects of fermented sea tangle (Laminaria japonica) by Lactobacillus brevis BJ20 in individuals with high level of γ-GT: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. 2012, 50, 1166–1169. [Google Scholar] [CrossRef]
- Cha, J.Y.; Jeong, J.J.; Yang, H.J.; Lee, B.J.; Cho, Y.S. Effect of fermented sea tangle on the alcohol dehydrogenase and acetaldehyde dehydrogenase in Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2011, 21, 791–795. [Google Scholar] [CrossRef]
- You, J.S.; Sung, M.J.; Chang, K.J. Evaluation of 8-week body weight control program including sea tangle (Laminaria japonica) supplementation in Korean female college students. Nutr. Res. Pract. 2009, 3, 307–314. [Google Scholar] [CrossRef][Green Version]
- Choi, W.C.; Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jo, Y.H.; Jeon, B.H. Effects of γ-aminobutyric acid-enriched fermented sea tangle (Laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae 2016, 31, 175–187. [Google Scholar] [CrossRef]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jeon, B.H. GABA-enriched fermented Laminaria japonica improves cognitive impairment and neuroplasticity in scopolamine- and ethanol-induced dementia model mice. Nutr. Res. Pract. 2018, 12, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jeon, B.H. The effects of fermented Laminaria japonica on short-term working memory and physical fitness in the elderly. Evid. Based Complement. Altern. Med. 2018, 2018, 8109621. [Google Scholar]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C. Osteoprotegerin ligand and osteoprotegerin: Novel implications for osteoclast biology and bone metabolism. Eur. J. Endocrinol. 1999, 141, 195–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kong, X.; Yang, Y.; Wu, W.; Wan, H.; Li, X.; Zhong, M.; Su, X.; Jia, S.; Lin, N. Triterpenoid saponin W3 from Anemone flaccida suppresses osteoclast differentiation through inhibiting activation of MAPKs and NF-κB pathways. Int. J. Biol. Sci. 2015, 11, 1204–1214. [Google Scholar] [CrossRef]
- Hong, S.; Huh, J.E.; Lee, S.Y.; Shim, J.K.; Rhee, S.G.; Jeong, W. TRP14 inhibits osteoclast differentiation via its catalytic activity. Mol. Cell. Biol. 2014, 34, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Alles, N. Osteoclast function and bone-resorbing activity: An overview. Biochem. Biophys. Res. Commun. 2016, 476, 115–120. [Google Scholar] [CrossRef]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef]
- Kim, Y.W.; Baek, S.H.; Lee, S.H.; Kim, T.H.; Kim, S.Y. Fucoidan, a sulfated polysaccharide, inhibits osteoclast differentiation and function by modulating RANKL signaling. Int. J. Mol. Sci. 2014, 15, 18840–18855. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Kou, J.; Wang, C.; Wang, K. Role of reactive oxygen species in angiotensin II: Induced receptor activator of nuclear factor-κB ligand expression in mouse osteoblastic cells. Mol. Cell. Biochem. 2014, 396, 249–255. [Google Scholar] [CrossRef]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Hyun, J.W. Oxidative stress, Nrf2, and epigenetic modification contribute to anticancer drug resistance. Toxicol. Res. 2017, 33, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in bone metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, S.; Lee, H.; Yang, Y.; Jeong, W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013, 65, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Shinohara, F.; Kajiya, M.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Nuclear Nrf2 induction by protein transduction attenuates osteoclastogenesis. Free Radic. Biol. Med. 2014, 77, 239–248. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Manufacturer | Item No. |
|---|---|---|
| β-actin | Santa Cruz | sc-1615 |
| CTSK | Santa Cruz | sc-48353 |
| HO-1 | Millipore | 374090 |
| IkBα | Santa Cruz | sc-371 |
| Lamin B | Santa Cruz | sc-6216 |
| MMP-9 | Abcam | 38898 |
| NFATc1 | Santa Cruz | sc-7294 |
| NF-κB p65 | Santa Cruz | sc-109 |
| Phospho- NF-κB p65 | Cell signaling | 3033 |
| Nrf2 | Santa Cruz | sc-13032 |
| phospho-Nrf2 | Abcam | 76026 |
| NQO-1 | Novus | NB200-209 |
| OSCAR | R&D system | MAB1633 |
| TRAP | Thermo Fisher Scientific | PA5-42729 |
| Goat anti-mouse IgG-HRP | Santa Cruz | sc-2005 |
| Goat anti-rabbit IgG-HRP | Santa Cruz | sc-2004 |
| Bovine anti-goat IgG-HRP | Santa Cruz | sc-2350 |
| Mouse anti-rabbit igG-TR | Santa Cruz | Sc-3917 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-W.; Ji, S.Y.; Lee, H.; Hong, S.H.; Kim, G.-Y.; Park, C.; Lee, B.-J.; Park, E.K.; Hyun, J.W.; Jeon, Y.-J.; et al. Fermented Sea Tangle (Laminaria japonica Aresch) Suppresses RANKL-Induced Osteoclastogenesis by Scavenging ROS in RAW 264.7 Cells. Foods 2019, 8, 290. https://doi.org/10.3390/foods8080290
Jeong J-W, Ji SY, Lee H, Hong SH, Kim G-Y, Park C, Lee B-J, Park EK, Hyun JW, Jeon Y-J, et al. Fermented Sea Tangle (Laminaria japonica Aresch) Suppresses RANKL-Induced Osteoclastogenesis by Scavenging ROS in RAW 264.7 Cells. Foods. 2019; 8(8):290. https://doi.org/10.3390/foods8080290
Chicago/Turabian StyleJeong, Jin-Woo, Seon Yeong Ji, Hyesook Lee, Su Hyun Hong, Gi-Young Kim, Cheol Park, Bae-Jin Lee, Eui Kyun Park, Jin Won Hyun, You-Jin Jeon, and et al. 2019. "Fermented Sea Tangle (Laminaria japonica Aresch) Suppresses RANKL-Induced Osteoclastogenesis by Scavenging ROS in RAW 264.7 Cells" Foods 8, no. 8: 290. https://doi.org/10.3390/foods8080290
APA StyleJeong, J.-W., Ji, S. Y., Lee, H., Hong, S. H., Kim, G.-Y., Park, C., Lee, B.-J., Park, E. K., Hyun, J. W., Jeon, Y.-J., & Choi, Y. H. (2019). Fermented Sea Tangle (Laminaria japonica Aresch) Suppresses RANKL-Induced Osteoclastogenesis by Scavenging ROS in RAW 264.7 Cells. Foods, 8(8), 290. https://doi.org/10.3390/foods8080290









