Assessment of a New GC-MS/MS System for the Confirmatory Measurement of PCDD/Fs and (N)DL-PCBs in Food under EU Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Chemicals
2.2. Samples and Certified Reference Material
2.3. Sample Preparation
2.4. Instrumentation and Measurements
2.5. Method Validation Criteria
3. Results and Discussion
3.1. Gas Chromatography-Triple Quadrupole Mass Spectrometry (GC-QQQMS/MS) Method Development
3.2. Method Validation and Regulatory EU 2017/664-771 Compliance
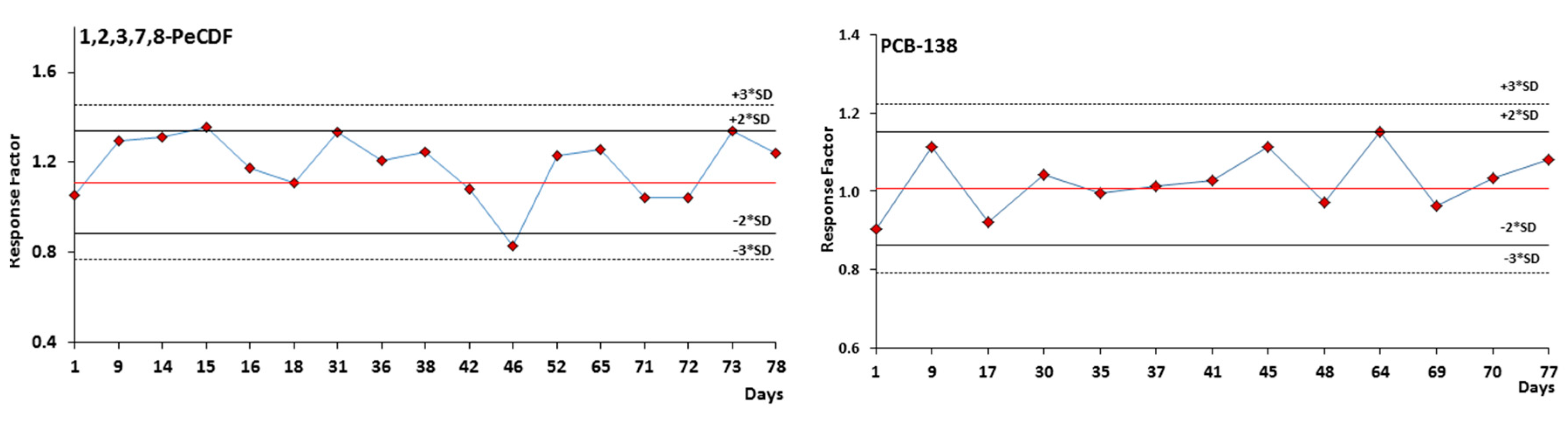
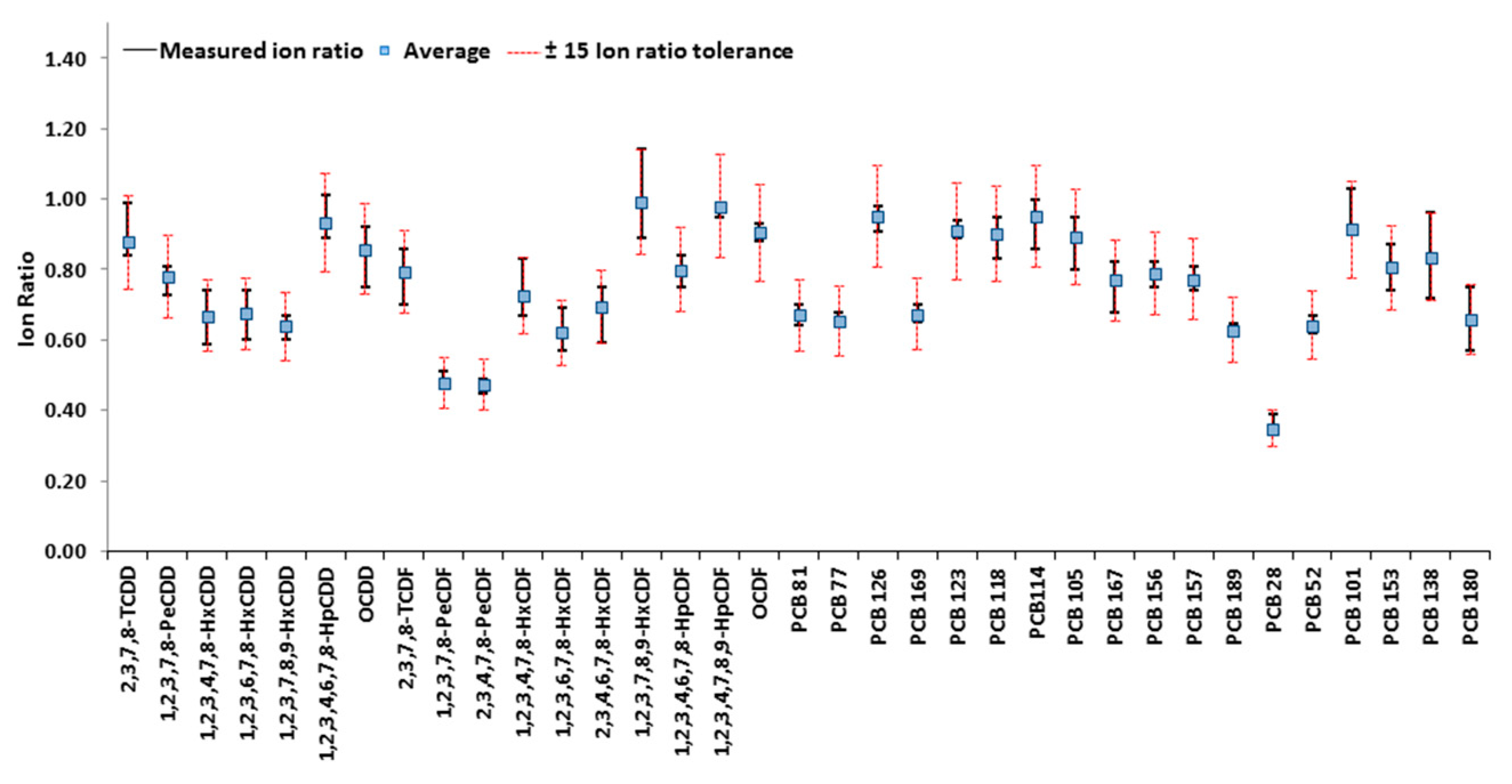
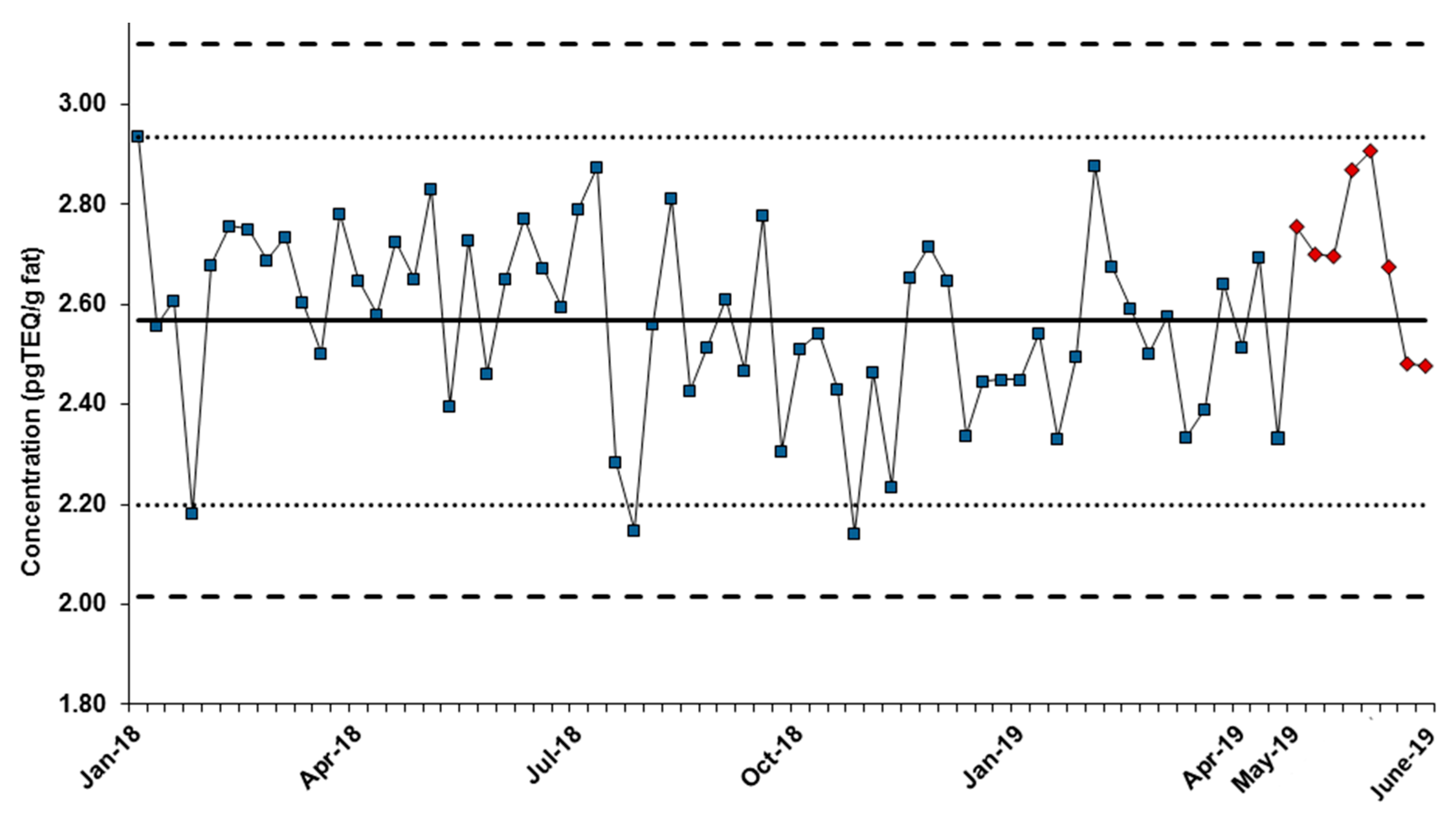
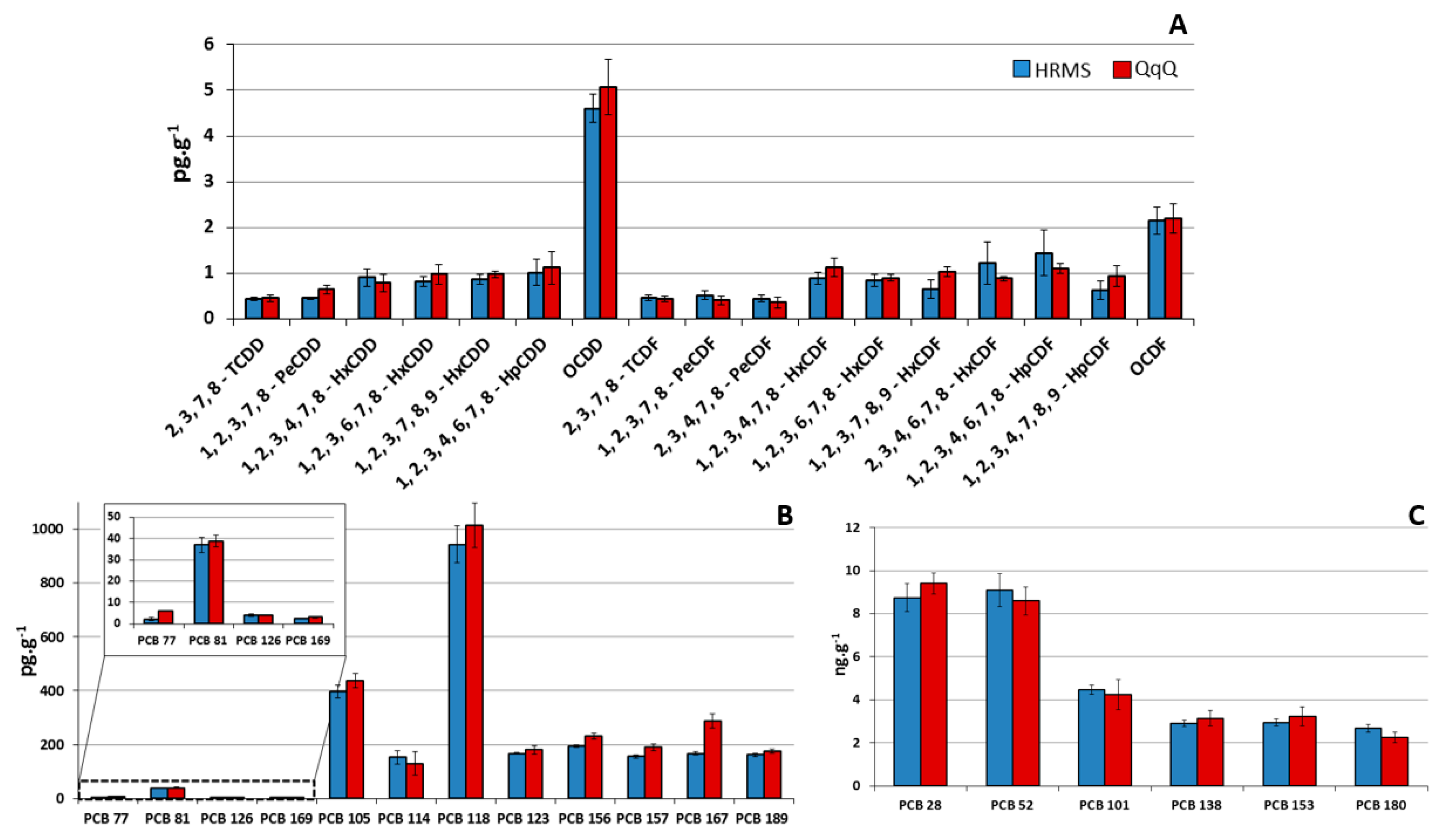
3.3. Quality Controls and Performance Comparison with a Routine Gas Chromatography-High Resolution Mass Spectrometry (GC-HRMS) Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNEP. Report of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants on the work of its sixth meeting; UNEP: Geneva, Switzerland, 2013. [Google Scholar]
- Zheng, G.J.; Leung, A.O.W.; Jiao, L.P.; Wong, M.H. Polychlorinated dibenzo-p-dioxins and dibenzofurans pollution in China: Sources, environmental levels and potential human health impacts. Environ. Int. 2008, 34, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Parzefall, W. Risk assessment of dioxin contamination in human food. Food Chem. Toxicol. 2002, 40, 1185–1189. [Google Scholar] [CrossRef]
- Focant, J.F.; Pirard, C.; Eppe, G.; De Pauw, E. Recent advances in mass spectrometric measurement of dioxins. J. Chromatogr. A 2005, 1067, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ingelido, A.M.; Brambilla, G.; Abballe, A.; di Domenico, A.; Fulgenzi, A.R.; Iacovella, N.; Valentini, S.; De Felip, E. PCDD, PCDF, and DL-PCB analysis in food: Performance evaluation of the high-resolution gas chromatography/low-resolution tandem mass spectrometry technique using consensus-based samples. Rapid Commun. Mass Spectrom. 2012, 26, 236–242. [Google Scholar] [CrossRef] [PubMed]
- García-Bermejo, Á.; Ábalos, M.; Sauló, J.; Abad, E.; González, M.J.; Gómara, B. Triple quadrupole tandem mass spectrometry: A real alternative to high resolution magnetic sector instrument for the analysis of polychlorinated dibenzo-p-dioxins, furans and dioxin-like polychlorinated biphenyls. Anal. Chim. Acta 2015, 189, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Franchina, F.A.; Zoccali, M.; Pantò, S.; Sciarrone, D.; Dugo, P.; Mondello, L. Untargeted and targeted comprehensive two-dimensional GC analysis using a novel unified high-speed triple quadrupole mass spectrometer. J. Chromatogr. A 2013, 1278, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Franchina, F.A.; Machado, M.E.; Tranchida, P.Q.; Zini, C.A.; Caramão, E.B.; Mondello, L. Determination of aromatic sulphur compounds in heavy gas oil by using (low-)flow modulated comprehensive two-dimensional gas chromatography-triple quadrupole mass spectrometry. J. Chromatogr. A 2015, 1387, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Tranchida, P.Q.; Franchina, F.A.; Zoccali, M.; Bonaccorsi, I.; Cacciola, F.; Mondello, L. A direct sensitivity comparison between flow-modulated comprehensive 2D and 1D GC in untargeted and targeted MS-based experiments. J. Sep. Sci. 2013, 36, 2746–2752. [Google Scholar] [CrossRef] [PubMed]
- L’Homme, B.; Scholl, G.; Eppe, G.; Focant, J.F. Validation of a gas chromatography-triple quadrupole mass spectrometry method for confirmatory analysis of dioxins and dioxin-like polychlorobiphenyls in feed following new EU Regulation 709/2014. J. Chromatogr. A 2015, 1376, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Fürst, P.; Bernsmann, T.; Baumeister, D. Optimization of GC-MS/MS for the determination of dioxins and PCBs in feed and food and comparison of results with GC-HRMS. In Dioxin and Related Compounds; Springer: New York, NY, USA, 2016; Volume 49. [Google Scholar]
- Kotz, A.; Malisch, R.; Focant, J.; Eppe, G.; Cederberg, T.; Rantakokko, P.; Fürst, P.; Bernsmann, T.; Lovász, C.; Scortichini, G.; et al. Analytical Criteria for Use of MS/MS for Determination of Dioxins and Dioxin-like PCBs in Feed and Food. Organohalogen Comp. 2012, 74, 156–159. [Google Scholar]
- Commision Regulation (EU) No 709/2014 of 20 June 2014 amending Regulation (EC) No 152/2009 as regards the determination of the levels of dioxins and polychlorinated biphenyls. Off. J. Eur. Union 2014, 188, 1–18.
- Commission Regulation (EU) No 589/2014 of 2 June 2014 laying down methods of sampling and analysis for the control of levels of dioxins, dioxin-like PCBs and non-dioxin-like PCBs in certain foodstuffs and repealing Regulation (EU) No 252/2012. Off. J. Eur. Union 2014, 164, 18–40.
- Commission Regulation (EU) 2017/644 of 5 April 2017 laying down methods of sampling and analysis for the control of levels of dioxins, dioxin-like PCBs and non-dioxin-like PCBs in certain foodstuffs and repealing Regulation (EU) No 589/2014. Off. J. Eur. Union 2017, 92, 9–34.
- Commission Regulation (EU) 2017/771 of 3 May 2017 amending Regulation (EC) No 152/2009 as regards the methods for the determination of the levels of dioxins and polychlorinated biphenyls. Off. J. Eur. Communities 2017, 115, 22–42.
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef]
- Focant, J.F.; Eppe, G.; Massart, A.C.; Scholl, G.; Pirard, C.; De Pauw, E. High-throughput biomonitoring of dioxins and polychlorinated biphenyls at the sub-picogram level in human serum. J. Chromatogr. A 2006, 1130, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Eppe, G.; Focant, J.F.; Pirard, C.; Pauw, E.D. PTV-LV-GC/MS/MS as screening and complementary method to HRMS for the monitoring of dioxin levels in food and feed. Talanta 2004, 63, 1135–1146. [Google Scholar] [CrossRef]
| Native Congeners | Retention Time (min) | Lowest Cali. Point (pg/μL) | Lowest Cali. Point RSD (%) | Highest Cali. Point (pg/μL) | Determination Coef. (R2) | Average RRF | Difference (%) RRF (Lowest Point)-RRF (All) | iLOQ (pg/μL) |
|---|---|---|---|---|---|---|---|---|
| PCDDs | ||||||||
| 2,3,7,8-TCDD | 29.41 | 0.05 | 1.9 | 10 | 0.99998 | 1.35 | 8.7 | 0.028 |
| 1,2,3,7,8-PeCDD | 34.61 | 0.05 | 15 | 10 | 0.9999 | 1.07 | 4.5 | 0.046 |
| 1,2,3,4,7,8-HxCDD | 41.11 | 0.1 | 11.6 | 20 | 0.99996 | 1.16 | 22.7 | 0.168 |
| 1,2,3,6,7,8-HxCDD | 41.3 | 0.1 | 15 | 20 | 0.9993 | 1.18 | −3.6 | 0.094 |
| 1,2,3,7,8,9-HxCDD | 41.76 | 0.1 | 8.3 | 20 | 0.99999 | 1.19 | 6.3 | 0.103 |
| 1,2,3,4,6,7,8-HpCDD | 45.5 | 0.1 | 15 | 20 | 0.99998 | 1.38 | −25.5 | 0.06 |
| OCDD | 49.97 | 0.25 | 12.3 | 50 | 0.9999 | 1.04 | 8.3 | 0.164 |
| PCDFs | ||||||||
| 2,3,7,8-TCDF | 28.81 | 0.05 | 11.4 | 10 | 0.9998 | 1.24 | −12.7 | 0.032 |
| 1,2,3,7,8-PeCDF | 32.99 | 0.05 | 10.9 | 10 | 0.99999 | 1.09 | 1.8 | 0.051 |
| 2,3,4,7,8-PeCDF | 34.24 | 0.05 | 10.2 | 10 | 0.99997 | 1.1 | 6.4 | 0.033 |
| 1,2,3,4,7,8-HxCDF | 39.33 | 0.1 | 9.9 | 20 | 0.99999 | 1.03 | −5.2 | 0.09 |
| 1,2,3,6,7,8-HxCDF | 39.61 | 0.1 | 10.2 | 20 | 0.99998 | 1.07 | −3 | 0.053 |
| 2,3,4,6,7,8-HxCDF | 40.84 | 0.1 | 6 | 20 | 0.99999 | 1.23 | 7.6 | 0.066 |
| 1,2,3,7,8,9-HxCDF | 42.33 | 0.1 | 8 | 20 | 0.999 | 1.03 | −8.5 | 0.11 |
| 1,2,3,4,6,7,8-HpCDF | 44.1 | 0.1 | 11.9 | 20 | 0.9999 | 1.29 | 3.9 | 0.075 |
| 1,2,3,4,7,8,9-HpCDF | 46.3 | 0.1 | 9.5 | 20 | 0.99996 | 1.2 | 7.1 | 0.1 |
| OCDF | 50.33 | 0.25 | 8.2 | 50 | 0.9988 | 1.13 | 1.6 | 0.273 |
| NO-PCBs | ||||||||
| PCB 77 | 24.76 | 0.5 | 4.8 | 20 | 0.9997 | 1.1 | −5.2 | 0.118 |
| PCB 81 | 25.33 | 0.5 | 6.5 | 20 | 0.9997 | 1.17 | −7.5 | 0.057 |
| PCB 126 | 29.72 | 0.5 | 4.3 | 20 | 0.9999 | 1.37 | −0.3 | 0.253 |
| PCB 169 | 34.41 | 0.5 | 8 | 20 | 0.9999 | 1.12 | −3.2 | 0.397 |
| MO-PCBs | ||||||||
| PCB 123 | 26.37 | 0.4 | 3.7 | 140 | 0.9913 | 1.2 | 14.1 | 0.774 |
| PCB 118 | 26.57 | 0.4 | 2.5 | 140 | 0.9908 | 1.26 | 15.8 | 0.233 |
| PCB114 | 27.16 | 0.4 | 6.3 | 140 | 0.995 | 1.19 | 6.6 | 0.756 |
| PCB 105 | 27.98 | 0.4 | 6.8 | 140 | 0.9976 | 1.25 | 14.6 | 0.226 |
| PCB 167 | 30.68 | 0.4 | 5.1 | 140 | 0.9926 | 1.15 | 15.1 | 0.121 |
| PCB 156 | 32.01 | 0.4 | 9.2 | 140 | 0.9928 | 1.13 | 15.5 | 0.255 |
| PCB 157 | 32.31 | 0.4 | 13.7 | 140 | 0.9998 | 1.21 | 11.7 | 0.494 |
| PCB 189 | 37.16 | 0.4 | 10.1 | 140 | 0.9998 | 1.14 | 14.1 | 0.119 |
| NDL-PCBs | ||||||||
| PCB 28 | 17.95 | 0.4 | 7.5 | 500 | 0.9963 | 0.78 | 17 | 0.21 |
| PCB 52 | 19.23 | 0.4 | 8 | 500 | 0.9914 | 1.21 | 14.3 | 0.176 |
| PCB 101 | 23.29 | 0.4 | 7.8 | 500 | 0.9961 | 1.31 | 19.8 | 0.13 |
| PCB 153 | 27.64 | 0.4 | 14.9 | 500 | 0.9906 | 1.05 | −4.9 | 0.434 |
| PCB 138 | 29.13 | 0.4 | 4.5 | 500 | 0.9908 | 0.98 | 2.5 | 0.235 |
| PCB 180 | 32.92 | 0.4 | 12 | 500 | 0.9927 | 0.99 | −12.5 | 0.249 |
| plant origins ng WHO2005TEQ/kg | |||||||
| PCDD/Fs | Regulation | Average | SD | RSD % | Target | Bias % | MSE |
| ML | 0.75 | 0.573 | 0.018 | 3.21 | 0.584 | −1.88 | 0.00035 |
| ML/2 | 0.38 | 0.126 | 0.003 | 2.41 | 0.117 | 7.69 | 0.00009 |
| 2ML | 1.5 | 1.166 | 0.012 | 1.06 | 1.168 | −0.15 | 0.00011 |
| animal origins (fat) ng WHO2005TEQ/kg | |||||||
| PCDD/Fs | Regulation | Average | SD | RSD % | Target | Bias % | MSE |
| ML | 1.5 | 1.72 | 0.055 | 3.21 | 1.75 | −1.95 | 0.00308 |
| ML/2 | 0.75 | 0.378 | 0.009 | 2.41 | 0.35 | 7.87 | 0.00084 |
| 2ML | 3 | 3.52 | 0.037 | 1.06 | 3.5 | 0.34 | 0.00115 |
| plant origins ng WHO2005TEQ/kg | |||||||
| DL-PCBs and PCDD/Fs | Regulation | Average | SD | RSD % | Target | Bias % | MSE |
| ML | 1.25 | 1.375 | 0.014 | 1.02 | 1.372 | 0.28 | 0.00014 |
| ML/2 | 0.625 | 0.654 | 0.019 | 2.85 | 0.665 | −1.77 | 0.00024 |
| 2ML | 2.5 | 6.152 | 0.103 | 1.67 | 6.247 | −1.52 | 0.00714 |
| animal origins (fat) ng WHO2005TEQ/kg | |||||||
| DL-PCBs and PCDD/Fs | Regulation | Average | SD | RSD % | Target | Bias % | MSE |
| ML | 2 | 1.961 | 0.056 | 2.846 | 1.996 | −1.77 | 0.00407 |
| ML/2 | 1 | 0.493 | 0.01 | 2.016 | 0.47 | 4.82 | 0.00048 |
| 2ML | 4 | 4.126 | 0.042 | 1.017 | 4.115 | 0.28 | 0.00129 |
| Analytes | Measured Values | SD | RSD (%) | Certified Values * | Accuracy (%) |
|---|---|---|---|---|---|
| 2,3,7,8-TCDD | 0.26 | 0.025 | 10 | 0.25(0.03) | 105 |
| 1,2,3,7,8-PeCDD | 0.85 | 0.057 | 7 | 0.79(0.04) | 107 |
| 1,2,3,4,7,8-HxCDD | 0.34 | 0.018 | 5 | 0.42(0.07) | 81 |
| 1,2,3,6,7,8-HxCDD | 0.65 | 0.071 | 11 | 0.98(0.11) | 66 |
| 1,2,3,7,8,9-HxCDD | 0.28 | 0.024 | 8 | 0.34(0.05) | 83 |
| 2,3,7,8-TCDF | 0.04 | 0.004 | 10 | 0.05(0.03) | 82 |
| 1,2,3,7,8-PeCDF | 0.06 | 0.005 | 8 | 0.054(0.013) | 114 |
| 2,3,4,7,8-PeCDF | 1.49 | 0.152 | 10 | 1.81(0.13) | 82 |
| 1,2,3,4,7,8-HxCDF | 0.91 | 0.073 | 8 | 0.94(0.04) | 97 |
| 1,2,3,6,7,8-HxCDF | 1.06 | 0.074 | 7 | 1.01(0.09) | 105 |
| 2,3,4,6,7,8-HxCDF | 0.97 | 0.051 | 5 | 1.07(0.05) | 90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchina, F.A.; Lazzari, E.; Scholl, G.; Focant, J.-F. Assessment of a New GC-MS/MS System for the Confirmatory Measurement of PCDD/Fs and (N)DL-PCBs in Food under EU Regulation. Foods 2019, 8, 302. https://doi.org/10.3390/foods8080302
Franchina FA, Lazzari E, Scholl G, Focant J-F. Assessment of a New GC-MS/MS System for the Confirmatory Measurement of PCDD/Fs and (N)DL-PCBs in Food under EU Regulation. Foods. 2019; 8(8):302. https://doi.org/10.3390/foods8080302
Chicago/Turabian StyleFranchina, Flavio Antonio, Eliane Lazzari, George Scholl, and Jean-François Focant. 2019. "Assessment of a New GC-MS/MS System for the Confirmatory Measurement of PCDD/Fs and (N)DL-PCBs in Food under EU Regulation" Foods 8, no. 8: 302. https://doi.org/10.3390/foods8080302
APA StyleFranchina, F. A., Lazzari, E., Scholl, G., & Focant, J.-F. (2019). Assessment of a New GC-MS/MS System for the Confirmatory Measurement of PCDD/Fs and (N)DL-PCBs in Food under EU Regulation. Foods, 8(8), 302. https://doi.org/10.3390/foods8080302