Aeration, Agitation and Cell Immobilization on Corncobs and Oak Wood Chips Effects on Balsamic-Styled Vinegar Production
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Fermentation Medium
2.2. Inoculum Preparation
2.3. Sterilization of Corncobs and Oak Wood Chips
2.4. Cell Immobilization on Corncobs and Oak Wood Chips
2.5. Quantification of Cells Adsorbed on Corncobs and Oak Wood Chips Prior- and Post-Fermentation
2.6. Inoculation
2.6.1. Phase 1: Static vs. Agitated Fermentations
2.6.2. Phase 2: Effect of Aeration
2.7. Data Handling
3. Results and Discussions
3.1. Phase 1: Non-Aerated Fermentations
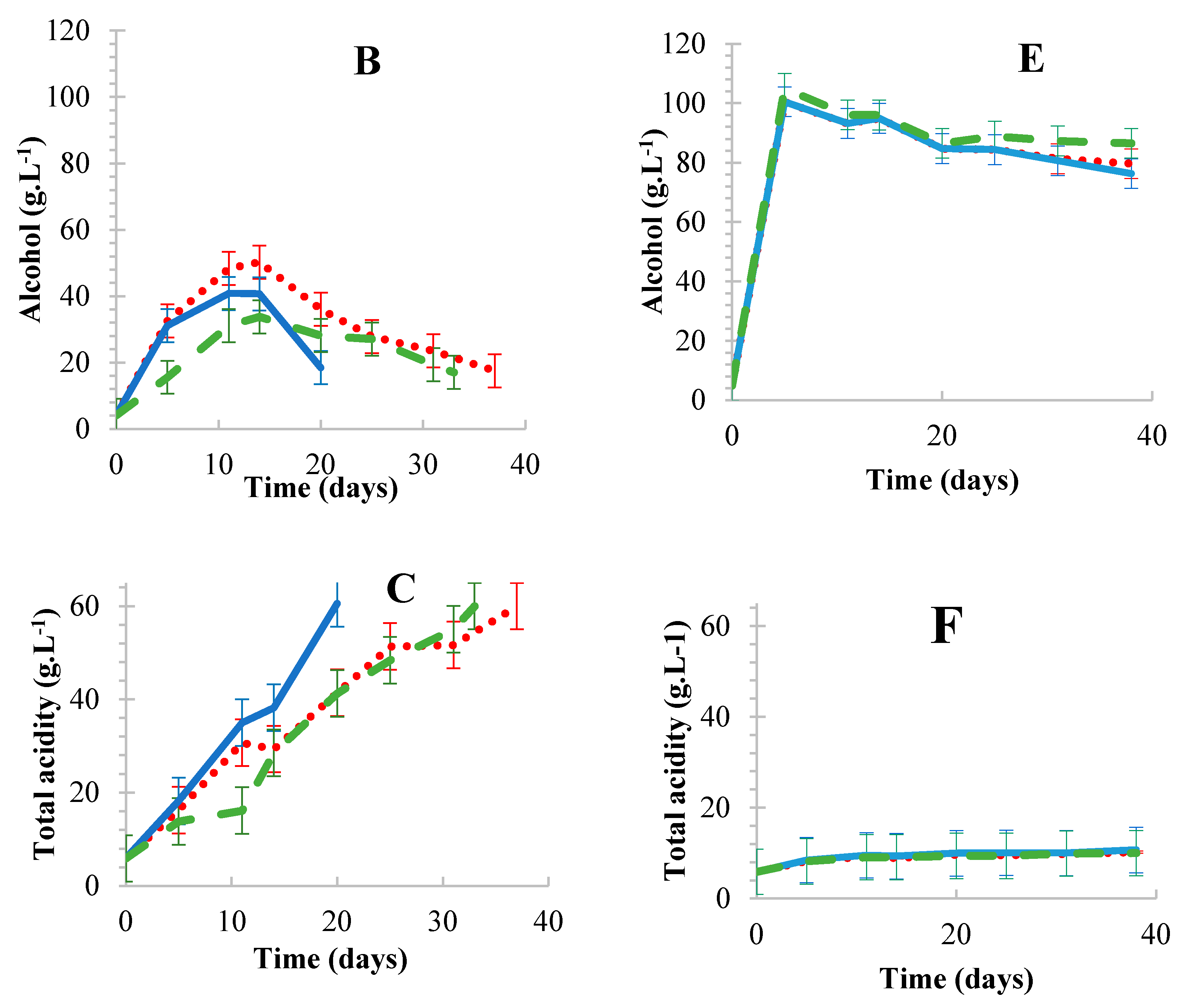
3.1.1. Static vs. Agitated Fermentations
3.1.2. Effect of the Adsorbents Used
3.2. Phase 2: Aerated Fermentations
3.2.1. Effect of Aeration Rates
3.2.2. Performance of Adsorbents Used Under Aerated Conditions
3.3. Variations in Cell Adsorption Capabilities among the Yeast/Bacterial Species Used
3.3.1. Individual Yeast Adsorption on Corncobs and Oak Wood Chips
3.3.2. Individual Bacteria Adsorption on Corncobs and Oak Wood Chips
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solieri, L.; Giudici, P. Vinegars of the World. In Vinegars of the World; Springer: Berlin, Germany, 2009; pp. 1–16. [Google Scholar]
- Giudici, P.; Gullo, M.; Solieri, L.; Falcone, P.M. Chapter 4 Technological and Microbiological Aspects of Traditional Balsamic Vinegar and Their Influence on Quality and Sensorial Properties. Adv. Food Nutr. Res. 2009, 58, 137–182. [Google Scholar] [PubMed]
- Truta, D.M.; Tofana, M.; Cheleman, R. Preliminary studies regarding the physicochemical characteristics of some balsamic vinegars. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2009, 66, 506–511. [Google Scholar]
- SAWIS. SA WINE INDUSTRY 2017 STATISTICS NR 42. 2017. Available online: http://www.sawis.co.za/info/download/Book_2017_statistics_year_english_final.pdf (accessed on 2 December 2018).
- De Ory, I.; Romero, L.E.; Cantero, D. Optimization of immobilization conditions for vinegar production. Siran, wood chips and polyurethane foam as carriers for Acetobacter aceti. Process. Biochem. 2004, 39, 547–555. [Google Scholar] [CrossRef]
- Kumar, G.; Mudhoo, A.; Sivagurunathan, P.; Nagarajan, D.; Ghimire, A.; Lay, C.-H.; Lin, C.-Y.; Lee, D.-J.; Chang, J.-S. Recent insights into the cell immobilization technology applied for dark fermentative hydrogen production. Bioresour. Technol. 2016, 219, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Kocher, G.; Kalra, K.; Phutela, R. Comparative Production of Sugarcane Vinegar by Different Immobilization Techniques. J. Inst. Brew. 2006, 112, 264–266. [Google Scholar] [CrossRef]
- Solieri, L.; Giudici, P. Yeasts associated to Traditional Balsamic Vinegar: Ecological and technological features. Int. J. Food Microbiol. 2008, 125, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Gullo, M.; Giudici, P. Acetic acid bacteria in traditional balsamic vinegar: Phenotypic traits relevant for starter cultures selection. Int. J. Food Microbiol. 2008, 125, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Chaves-López, C.; Di Fabio, F.; Schirone, M.; Felis, G.E.; Torriani, S.; Paparella, A.; Suzzi, G. Molecular identification and osmotolerant profile of wine yeasts that ferment a high sugar grape must. Int. J. Food Microbiol. 2009, 130, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.J.; Ludovico, P.; Rodrigues, F.; Leão, C.; Côrte-Real, M. Stress and cell death in yeast induced by acetic acid. In Cell Metabolism-Cell Homeostasis and Stress Response; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Giudici, P.; Lemmetti, F.; Mazza, S. Balsamic Vinegars. Tradition, Technology, Trade; Springer: Cham, Germany, 2015. [Google Scholar]
- Oliver, J.D. The public health significance of viable but nonculturable bacteria. In Nonculturable Microorganisms in the Environment; Springer: Berlin, Germany, 2000; pp. 277–300. [Google Scholar]
- Mamlouk, D.; Gullo, M. Acetic Acid Bacteria: Physiology and Carbon Sources Oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.; Marchant, R.; Koutinas, A.; Kourkoutas, I.; Banat, I. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Hutchinson, U.F.; Ntwampe, S.K.O.; Ngongang, M.M.; Du Plessis, H.W.; Chidi, B.S.; Saulse, C.; Jolly, N.P. Cell immobilization by Gel Entrapment in Ca-alginate Beads for Balsamic-styled Vinegar Production. In Proceedings of the 10th International Conference on Advances in Science, Engineering, Technology & Healthcare (ASETH-18), Cape Town, South Africa, 19–20 November 2018. [Google Scholar]
- Inglett, G.E. Corn: Culture, Processing, Products; Major Fred and Food Crops in Agriculture and Food Series; The Avi Publishing: St. Petersburg, FL, USA, 1970. [Google Scholar]
- Vaughan, T.; Seo, C.W.; E Marshall, W. Removal of selected metal ions from aqueous solution using modified corncobs. Bioresour. Technol. 2001, 78, 133–139. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Ruengruglikit, C.; Hang, Y.D. L (+)-lactic acid production from corncobs by Rhizopus oryzae NRRL-395. LWT-Food Sci. Technol. 2003, 36, 573–575. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Ma, T.; Bao, X.; Du, F.; Zhuang, G.; Qu, Y. Simultaneous saccharification and fermentation of acid-pretreated corncobs with a recombinant Saccharomyces cerevisiae expressing β-glucosidase. Bioresour. Technol. 2008, 99, 5099–5103. [Google Scholar] [CrossRef]
- Afonso, V.G. Sensory Descriptive Analysis Between White Wines Fermented with Oak Chips and in Barrels. J. Food Sci. 2002, 67, 2415–2419. [Google Scholar] [CrossRef]
- Kyraleou, M.; Kallithraka, S.; Chira, K.; Tzanakouli, E.; Ligas, I.; Kotseridis, Y. Differentiation of Wines Treated with Wood Chips Based on Their Phenolic Content, Volatile Composition, and Sensory Parameters. J. Food Sci. 2015, 80, C2701–C2710. [Google Scholar] [CrossRef] [PubMed]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Obreque-Slier, E. Wines in contact with oak wood: The impact of the variety (Carménère and Cabernet Sauvignon), format (barrels, chips and staves), and aging time on the phenolic composition. J. Sci. Food Agric. 2018, 99, 436–448. [Google Scholar] [CrossRef]
- Stone, K.M.; Roche, F.W.; Thornhill, N.F. Dry weight measurement of microbial biomass and measurement variability analysis. Biotechnol. Tech. 1992, 6, 207–212. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Ton, N.M.N.; Le, V.V.M. Optimization of Saccharomyces cerevisiae immobilization in bacterial cellulose by ‘adsorption-incubation’method. Int. Food Res. J. 2009, 16, 59–64. [Google Scholar]
- Hutchinson, U.F.; Ntwampe, S.K.O.; Ngongang, M.M.; Chidi, B.S.; Hoff, J.W.; Jolly, N.P. Product and Microbial Population Kinetics During Balsamic-Styled Vinegar Production. J. Food Sci. 2019, 84, 572–579. [Google Scholar] [CrossRef]
- Saltini, R.; Akkerman, R. Testing improvements in the chocolate traceability system: Impact on product recalls and production efficiency. Food Control. 2012, 23, 221–226. [Google Scholar] [CrossRef]
- González-Sáiz, J.-M.; Garrido-Vidal, D.; Pizarro, C. Modelling the industrial production of vinegar in aerated-stirred fermentors in terms of process variables. J. Food Eng. 2009, 91, 183–196. [Google Scholar] [CrossRef]
- Talabardon, M.; Schwitzguébel, J.-P.; Peringer, P.; Yang, S.-T.; Schwitzguébel, J. Acetic Acid Production from Lactose by an Anaerobic Thermophilic Coculture Immobilized in a Fibrous-Bed Bioreactor. Biotechnol. Prog. 2000, 16, 1008–1017. [Google Scholar] [CrossRef]
- Horiuchi, J.-I.; Kanno, T.; Kobayashi, M. Effective onion vinegar production by a two-step fermentation system. J. Biosci. Bioeng. 2000, 90, 289–293. [Google Scholar] [CrossRef]
- Park, Y.S.; Ohtake, H.; Fukaya, M.; Okumura, H.; Kawamura, Y.; Toda, K. Effects of dissolved oxygen and acetic acid concentrations on acetic acid production in continuous culture of Acetobacter aceti. J. Ferment. Bioeng. 1989, 68, 96–101. [Google Scholar] [CrossRef]
- Pochat-Bohatier, C.; Bohatier, C.; Ghommidh, C. Modelling the Kinetics of Growth of Acetic Acid Bacteria to Increase vinegar Production: Analogy with Mechanical Modelling. Available online: https://www.math.ucsd.edu/~helton/MTNSHISTORY/CONTENTS/2000PERPIGNAN/CDROM/articles/B57.pdf (accessed on 8 December 2018).
- Schlepütz, T.; Gerhards, J.P.; Büchs, J. Ensuring constant oxygen supply during inoculation is essential to obtain reproducible results with obligatory aerobic acetic acid bacteria in vinegar production. Process. Biochem. 2013, 48, 398–405. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Humphreys, J.D.; Barker, S.A.; Greenshields, R.N. Application of living immobilized cells to the acceleration of the continuous conversions of ethanol (wort) to acetic acid (vinegar)—Hydrous titanium(IV) oxide-immobilized Acetobacter species. Enzym. Microb. Technol. 1980, 2, 209–216. [Google Scholar] [CrossRef]
- Ghommidh, C.; Navarro, J.M.; Messing, R.A. A study of acetic acid production by immobilizedAcetobacter cells: Product inhibition effects. Biotechnol. Bioeng. 1982, 24, 1991–1999. [Google Scholar] [CrossRef]
- Kaur, P.; Kocher, G.S.; Phutela, R.P. Production of tea vinegar by batch and semicontinuous fermentation. J. Food Sci. Technol. 2011, 48, 755–758. [Google Scholar] [CrossRef][Green Version]
- Thiripurasundari, G.; Usharani, G. Comparative production of vinegar using cashew apple juice by different immobilization techniques. Curr. Bot. 2011, 2, 31–33. [Google Scholar]
- Kocher, G.S.; Dhillon, H.K. Fermentative production of sugarcane vinegar by immobilized cells of Acetobacter aceti under packed bed conditions. Sugar Tech 2013, 15, 71–76. [Google Scholar] [CrossRef]
- Krusong, W.; Vichitraka, A. An air-lift acetifier with mash recycling system for corn vinegar production by adsorbed cells of Acetobacter aceti WK on surface of loofa sponge. In Proceedings of the 2nd International Conference on Biotechnology and Food Science IPCBEE, Bali Island, Indonesia, 1–3 April 2011; Volume 7, pp. 86–90. [Google Scholar]
- Krusong, W.; Tantratian, S. Acetification of rice wine by Acetobacter aceti using loofa sponge in a low-cost reciprocating shaker. J. Appl. Microbiol. 2014, 117, 1348–1357. [Google Scholar] [CrossRef]
- Fregapane, G.; Nieto, J.; Salvador, M.D.; Rubio-Fernández, H. Wine vinegar production using a noncommercial 100-litre bubble column reactor equipped with a novel type of dynamic sparger. Biotechnol. Bioeng. 1999, 63, 141–146. [Google Scholar] [CrossRef]
- Fregapane, G.; Rubio-Fernández, H.; Salvador, M.D. Influence of fermentation oxygen partial pressure on semicontinuous acetification for wine vinegar production. Eur. Food Res. Technol. 2004, 219, 393–397. [Google Scholar]
- Ouyang, P.; Wang, H.; Hajnal, I.; Wu, Q.; Guo, Y.; Chen, G.-Q. Increasing oxygen availability for improving poly(3-hydroxybutyrate) production by Halomonas. Metab. Eng. 2018, 45, 20–31. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer: An Ancient Yet Modern Biotechnology. Chem. Educ. 2000, 5, 102–112. [Google Scholar] [CrossRef]
- Hiralal, L.; Olaniran, A.O.; Pillay, B. Aroma-active ester profile of ale beer produced under different fermentation and nutritional conditions. J. Biosci. Bioeng. 2014, 117, 57–64. [Google Scholar] [CrossRef]
- Watson, K.; Arthur, H.; Morton, H. Thermal adaptation in yeast: Obligate psychrophiles are obligate aerobes, and obligate thermophiles are facultative anaerobes. J. Bacteriol. 1978, 136, 815–817. [Google Scholar]
- Bekatorou, A.; Psarianos, C.; Koutinas, A.A. Production of food grade yeasts. Food Technol. Biotechnol. 2006, 44, 407–415. [Google Scholar]
- Chatre, L.; Ricchetti, M. Are mitochondria the Achilles’ heel of the Kingdom Fungi? Curr. Opin. Microbiol. 2014, 20, 49–54. [Google Scholar] [CrossRef]
- Boss, M.J.; Day, D.W. Biological Risk Engineering Handbook: Infection Control and Decontamination; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Qi, Z.; Yang, H.; Xia, X.; Xin, Y.; Zhang, L.; Wang, W.; Yu, X. A protocol for optimization vinegar fermentation according to the ratio of oxygen consumption versus acid yield. J. Food Eng. 2013, 116, 304–309. [Google Scholar] [CrossRef]

 Oak wood chips,
Oak wood chips,  Corncobs,
Corncobs,  Free-floating cells. Results are the average of three biological repeats accounting for standard deviation.
Free-floating cells. Results are the average of three biological repeats accounting for standard deviation.
 Oak wood chips,
Oak wood chips,  Corncobs,
Corncobs,  Free-floating cells. Results are the average of three biological repeats accounting for standard deviation.
Free-floating cells. Results are the average of three biological repeats accounting for standard deviation.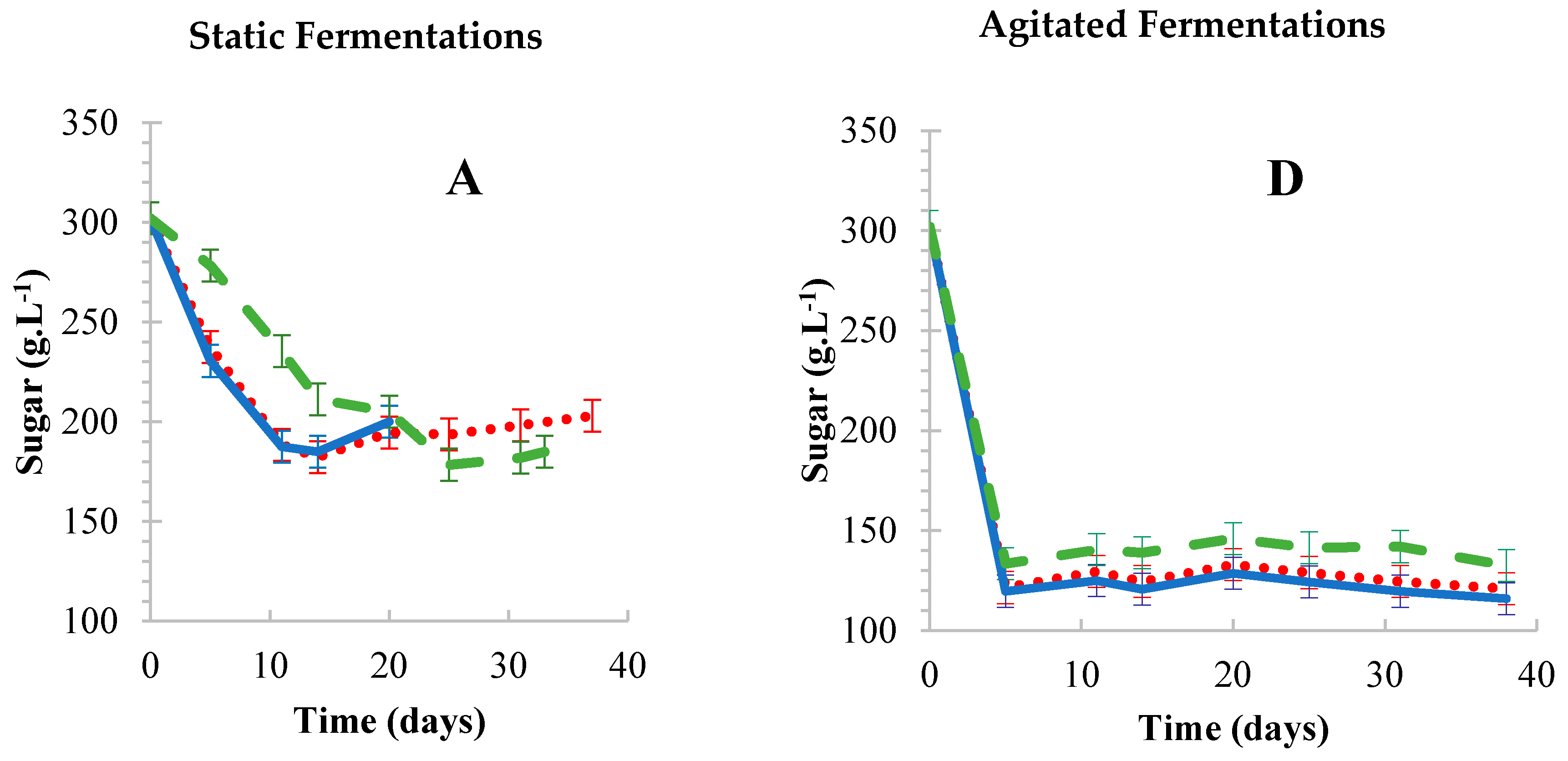
 Oak wood chips,
Oak wood chips,  Corncobs,
Corncobs,  Free-floating cells. Results are the average of three biological repeats ± standard deviation.
Free-floating cells. Results are the average of three biological repeats ± standard deviation.
 Oak wood chips,
Oak wood chips,  Corncobs,
Corncobs,  Free-floating cells. Results are the average of three biological repeats ± standard deviation.
Free-floating cells. Results are the average of three biological repeats ± standard deviation.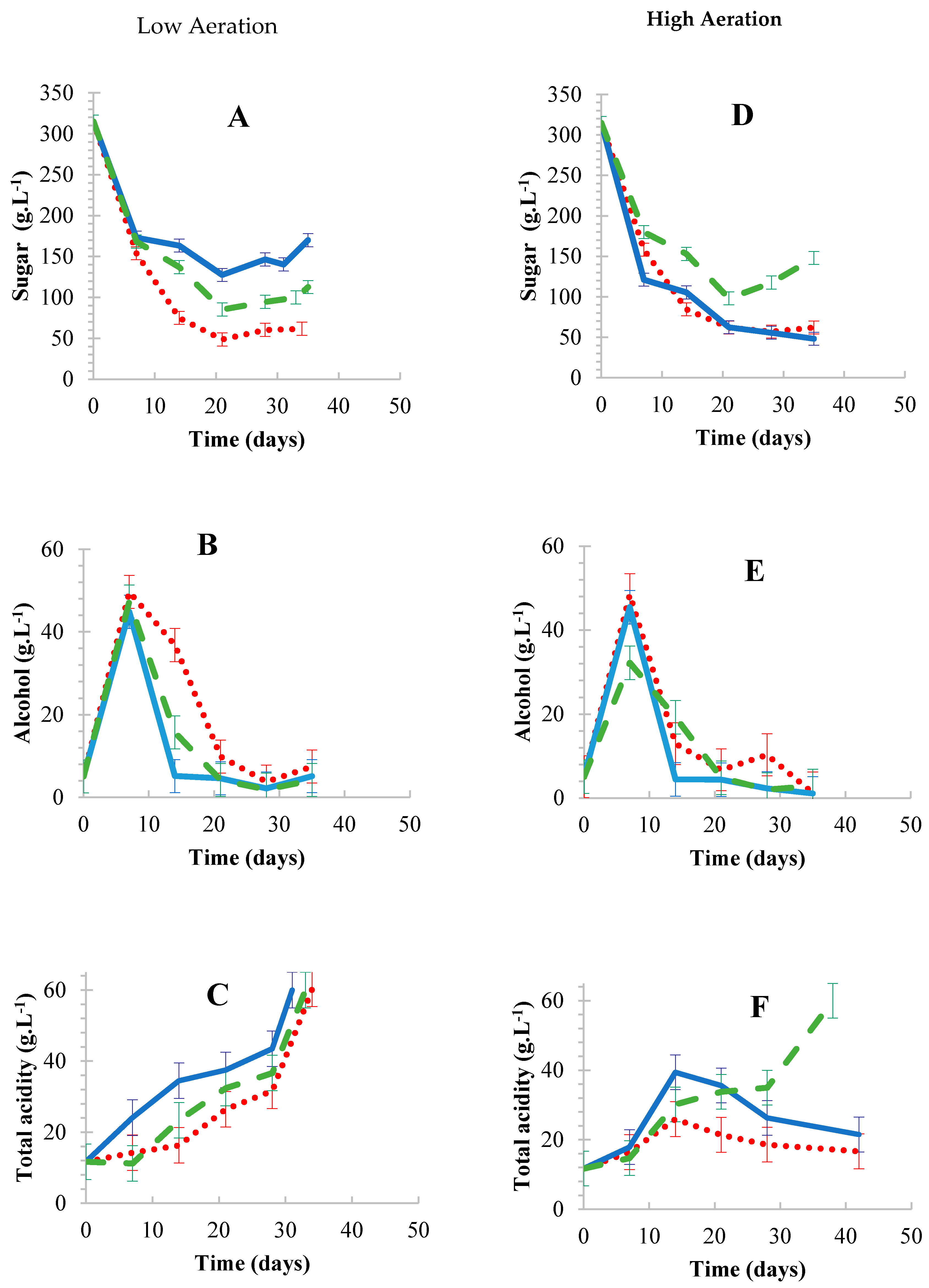
| Non-Saccharomyces Yeast | ||
|---|---|---|
| Identity | ARC Accession Numbers | Origin |
| Candida pulcherrima | Y0839 | Chardonnay grapes |
| Candida zemplinina | Y1020 | Chardonnay grapes |
| Hanseniaspora guilliermondii | Y0558 | Cabernet Sauvignon grapes |
| Kloeckera apiculata | C48V19 | Chardonnay grapes |
| Zygosaccharomyces bailii | C45V69 | Chardonnay grapes |
| Acetic Acid Bacteria | |||
|---|---|---|---|
| Identity | ARC Accession Numbers | NCBI Accession Numbers | Origin |
| Acetobacter pasteurianus | 171/19 | CP 021922.1 | Healthy grapes |
| Acetobacter malorum | 172/36 | KU 686765.1 | Shiraz wine |
| Kozakia baliensis | 179/48 | CP 014681.1 | Grape pomace |
| Gluconobacter cerinus | 126/34 | KX 578017.1 | Shiraz wine |
| Gluconobacter oxydans | 172/36 | LN 884063.1 | Grape pomace |
| Adsorbents | Length (cm) | Width/Diameter (cm) | Circumference/Perimeter (cm) | Surface Area of One Piece (cm2) | Surface Area of all Adsorbents Used (cm2) |
|---|---|---|---|---|---|
| Corncobs | 6.00 ± 1.05 | 4.00 ± 0.66 | 12.57 | 100.53 | 402 (4 pieces) |
| Oak woodchips | 2.90 ± 0.65 | 1.80 ± 0.53 | 9.60 | 19.64 | 392 (20 chips) |
| Product | Adsorbent | Agitation | Acetification Rate (g·L−1·day−1) | Reference |
|---|---|---|---|---|
| Balsamic-styled vinegar | Corncobs Oak wood chips | 135 rpm | FFC: 0.11 CC: 0.13 OWC: 0.11 | Current study |
| Acetic acid from lactose and milk permeate | Fibrous-Bed/matrix | 100 rpm | FFC (lactose): 1.44 FFC (milk permeate): 1.92 IC (Lactose): 12.96 IC (milk permeate) 7.2 | [31] |
| Rice wine vinegar | Loofa sponge | 1 Hz reciprocating shaking rate = 60 rpm | IC: 1.68–2.4 | [42] |
| Yeast Cells Adsorbed on Corncobs | Yeast Cells Adsorbed on Oak Wood Chips | ||||||
|---|---|---|---|---|---|---|---|
| Identity | Cell Concentration YPD Broth (Cells·mL−1) | Before Fermentation (g·g−1) | After Fermentation (g·g−1) | Relative Difference (%) | Before Fermentation (g·g−1) | After Fermentation (g·g−1) | Relative Difference (%) |
| Candida pulcherrima | 2.34 × 105 | 0.0371 | 1.1580 | 96.80 | 0.0214 | 0.1751 | 87.78 |
| Candida zemplinina | 7.40 × 105 | 0.0006 | 0.0290 | 97.93 | 0.0903 | 0.0566 | −37.32 |
| Hanseniaspora guilliermondii | 7.70 × 105 | 0.0110 | 0.7906 | 98.61 | 0.1001 | 0.1032 | 3.00 |
| Kloeckera apiculata | 1.95 × 106 | 0.0283 | 1.6955 | 98.33 | 0.0926 | 0.1804 | 48.67 |
| Zygosaccharomyces bailii | 3.97 × 105 | 0.0400 | 0.5661 | 92.93 | 0.1785 | 0.1393 | −21.96 |
| Bacteria Cells Adsorbed on Corncobs | Bacteria Cells Adsorbed on Oak Wood Chips | ||||||
|---|---|---|---|---|---|---|---|
| Identity | Cell Concentration GM Broth (Cells·mL−1) | Before Fermentation (g·g−1) | After Fermentation (g·g−1) | Relative Difference (%) | Before Fermentation (g·g−1) | After Fermentation (g·g−1) | Relative Difference (%) |
| Acetobacter pasteurianus | 3.97 × 105 | 0.1461 | 1.1968 | 87.79 | 0.0020 | 0.1772 | 98.87 |
| Acetobacter malorum | 1.25 × 106 | 0.0948 | 1.0882 | 91.29 | 0.1851 | 0.1545 | −16.53 |
| Kozakia baliensis | 3.13 × 105 | 0.0260 | 1.1589 | 97.76 | 0.1224 | 0.1122 | −8.33 |
| Gluconobacter cerinus | 2.22 × 106 | 0.0116 | 1.5618 | 99.26 | 0.1498 | 0.3147 | 52.40 |
| Gluconobacter oxydans | 6.38 × 105 | 0.0426 | 1.0977 | 96.12 | 0.1008 | 0.2308 | 56.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hutchinson, U.F.; Gqozo, S.; Jolly, N.P.; Chidi, B.S.; Du Plessis, H.W.; Mewa-Ngongang, M.; Ntwampe, S.K.O. Aeration, Agitation and Cell Immobilization on Corncobs and Oak Wood Chips Effects on Balsamic-Styled Vinegar Production. Foods 2019, 8, 303. https://doi.org/10.3390/foods8080303
Hutchinson UF, Gqozo S, Jolly NP, Chidi BS, Du Plessis HW, Mewa-Ngongang M, Ntwampe SKO. Aeration, Agitation and Cell Immobilization on Corncobs and Oak Wood Chips Effects on Balsamic-Styled Vinegar Production. Foods. 2019; 8(8):303. https://doi.org/10.3390/foods8080303
Chicago/Turabian StyleHutchinson, Ucrecia F., Sivuyile Gqozo, Neil P. Jolly, Boredi S. Chidi, Heinrich W. Du Plessis, Maxwell Mewa-Ngongang, and Seteno K. O. Ntwampe. 2019. "Aeration, Agitation and Cell Immobilization on Corncobs and Oak Wood Chips Effects on Balsamic-Styled Vinegar Production" Foods 8, no. 8: 303. https://doi.org/10.3390/foods8080303
APA StyleHutchinson, U. F., Gqozo, S., Jolly, N. P., Chidi, B. S., Du Plessis, H. W., Mewa-Ngongang, M., & Ntwampe, S. K. O. (2019). Aeration, Agitation and Cell Immobilization on Corncobs and Oak Wood Chips Effects on Balsamic-Styled Vinegar Production. Foods, 8(8), 303. https://doi.org/10.3390/foods8080303








