Use of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy in Combination with Multivariate Methods for the Rapid Determination of the Adulteration of Grape, Carob and Mulberry Pekmez
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Synthetically Adulterated Pekmez Samples
2.3. ATR-FTIR Analyses of Pekmez Samples
2.4. Chemometric Analysis
3. Results and Discussion
3.1. Brix Values of the Pekmez Samples
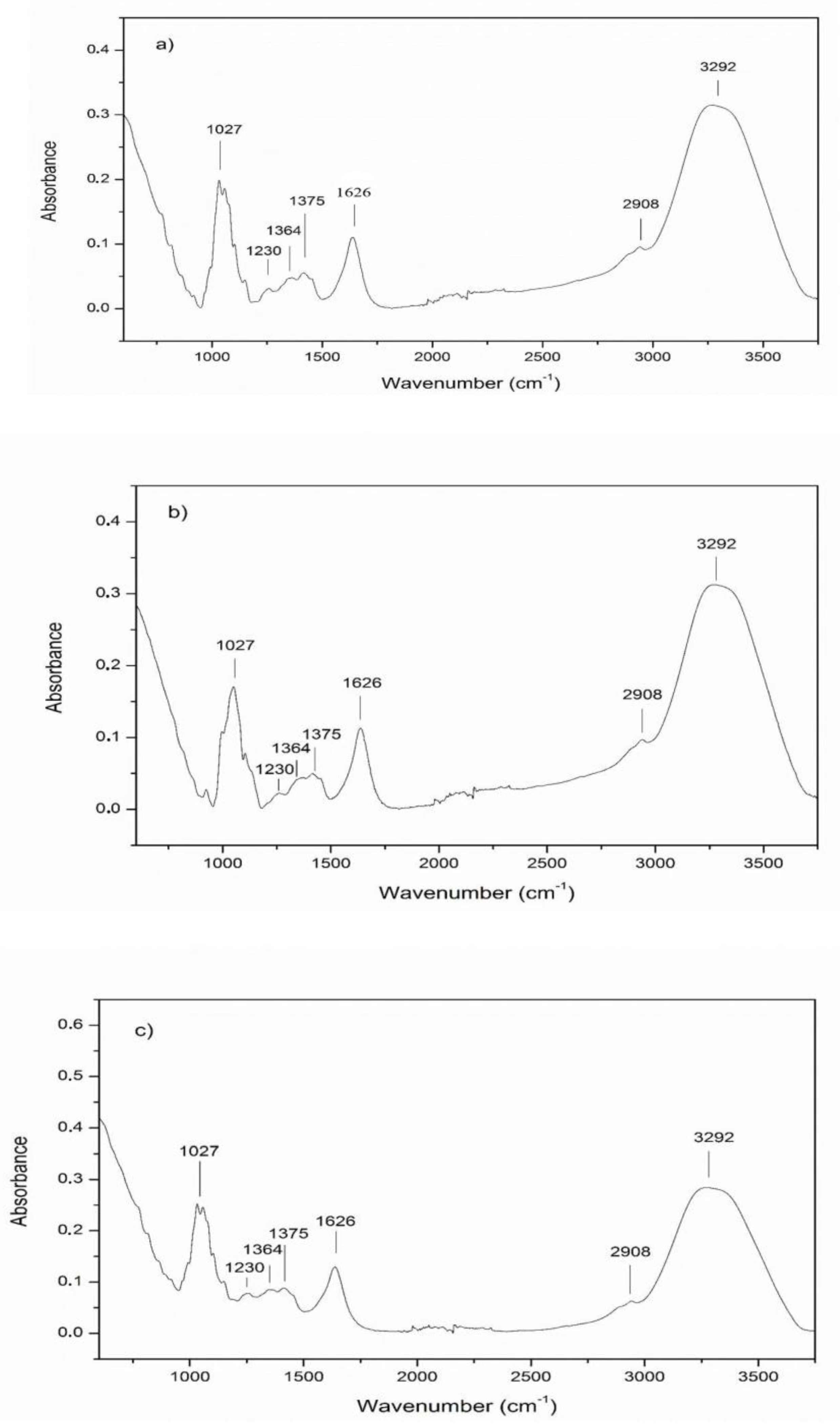
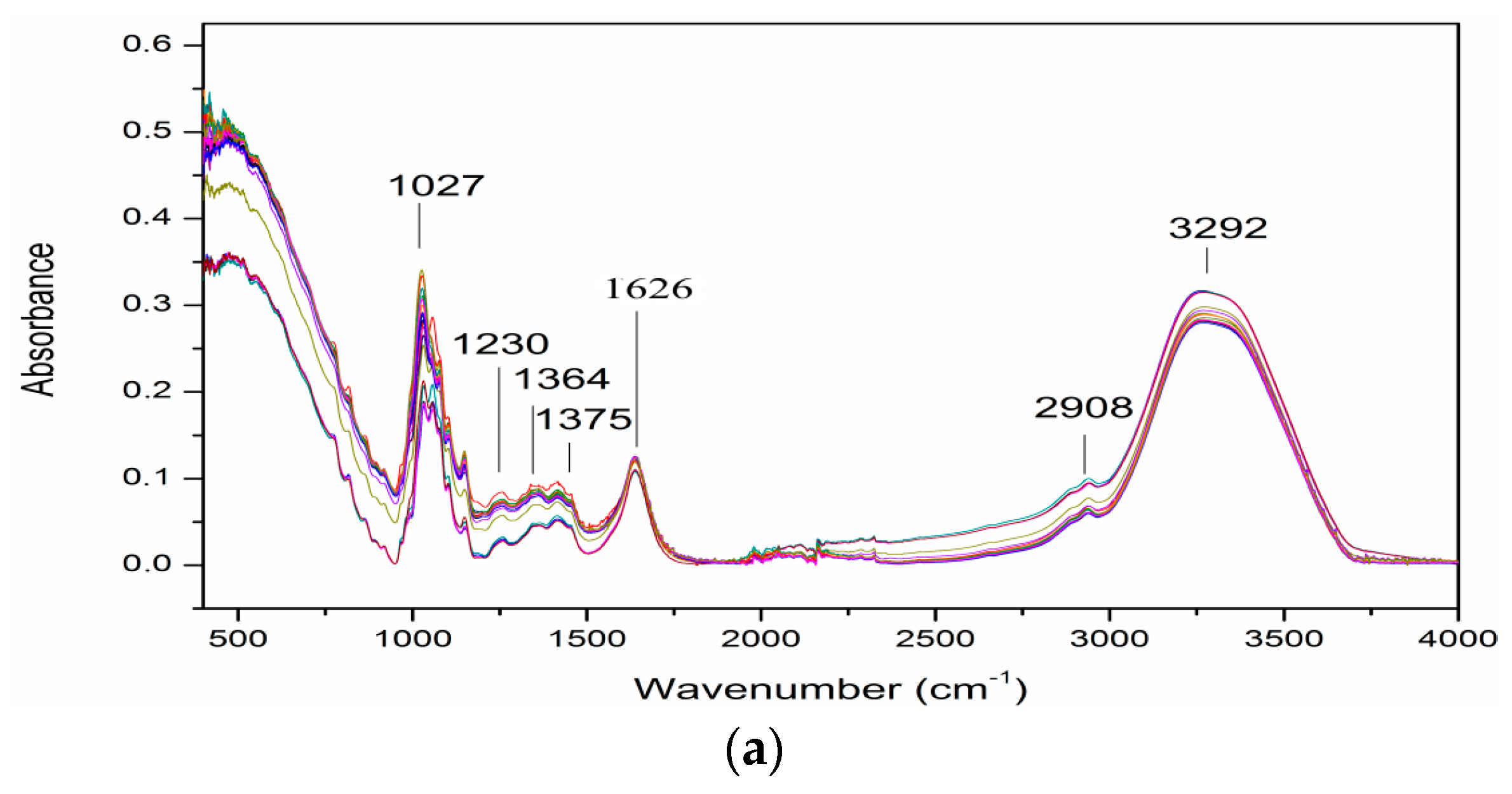
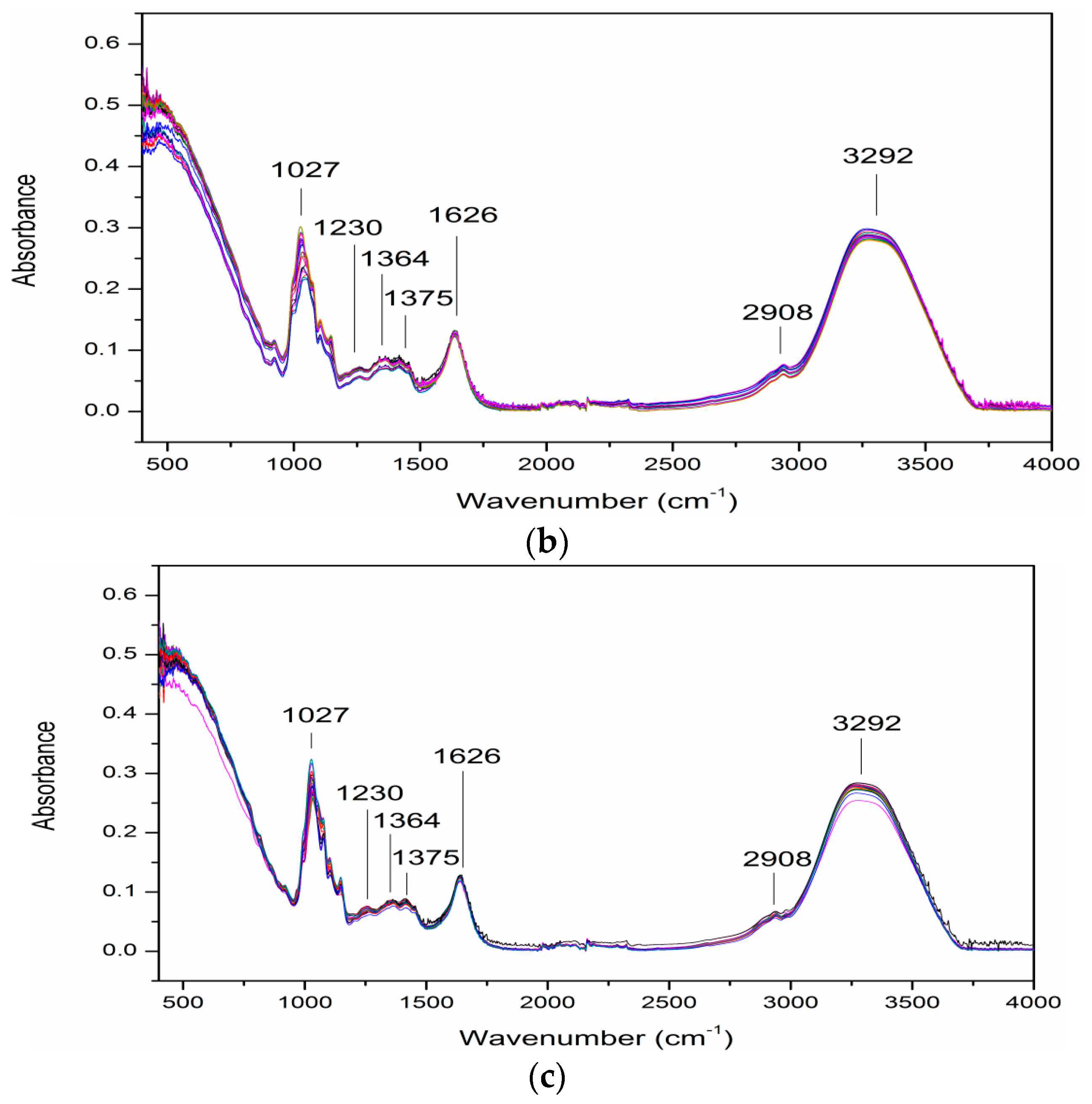
3.2. ATR-FTIR Spectra of Samples
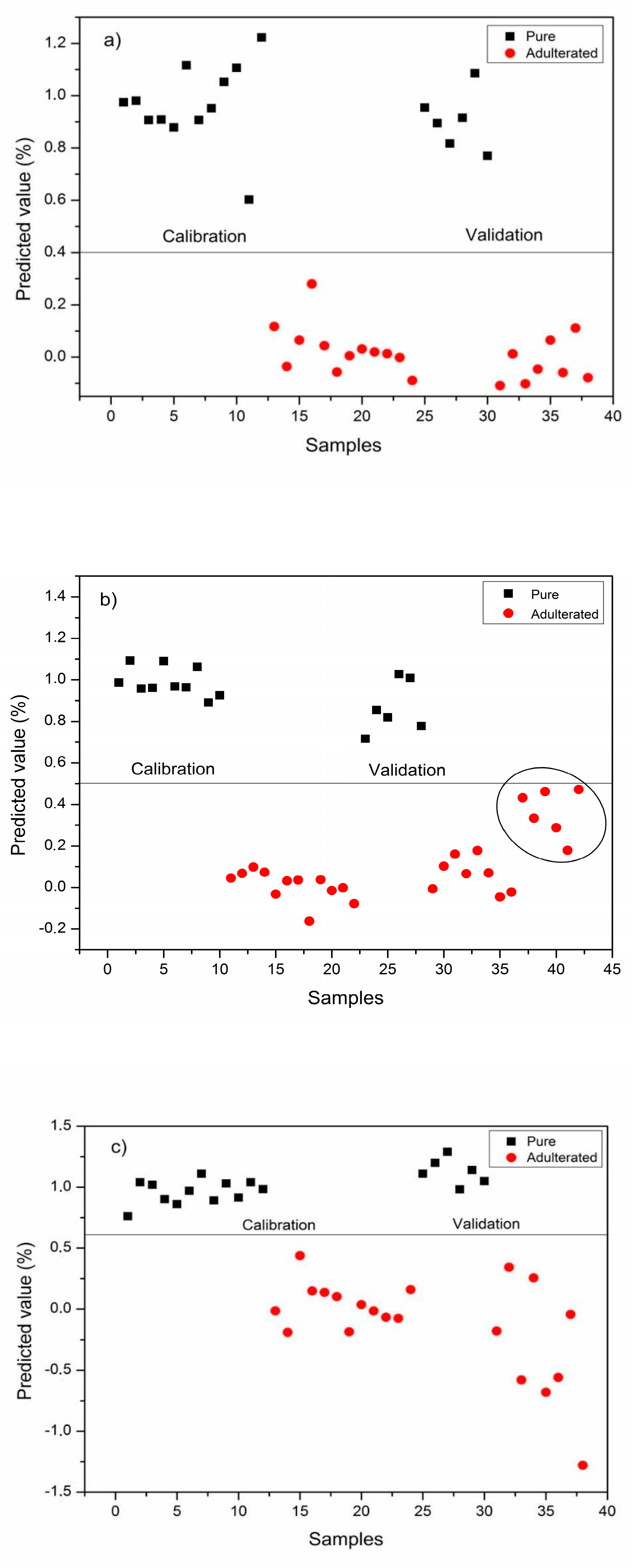
3.3. Discrimination of Pekmez Samples Using PLS-DA
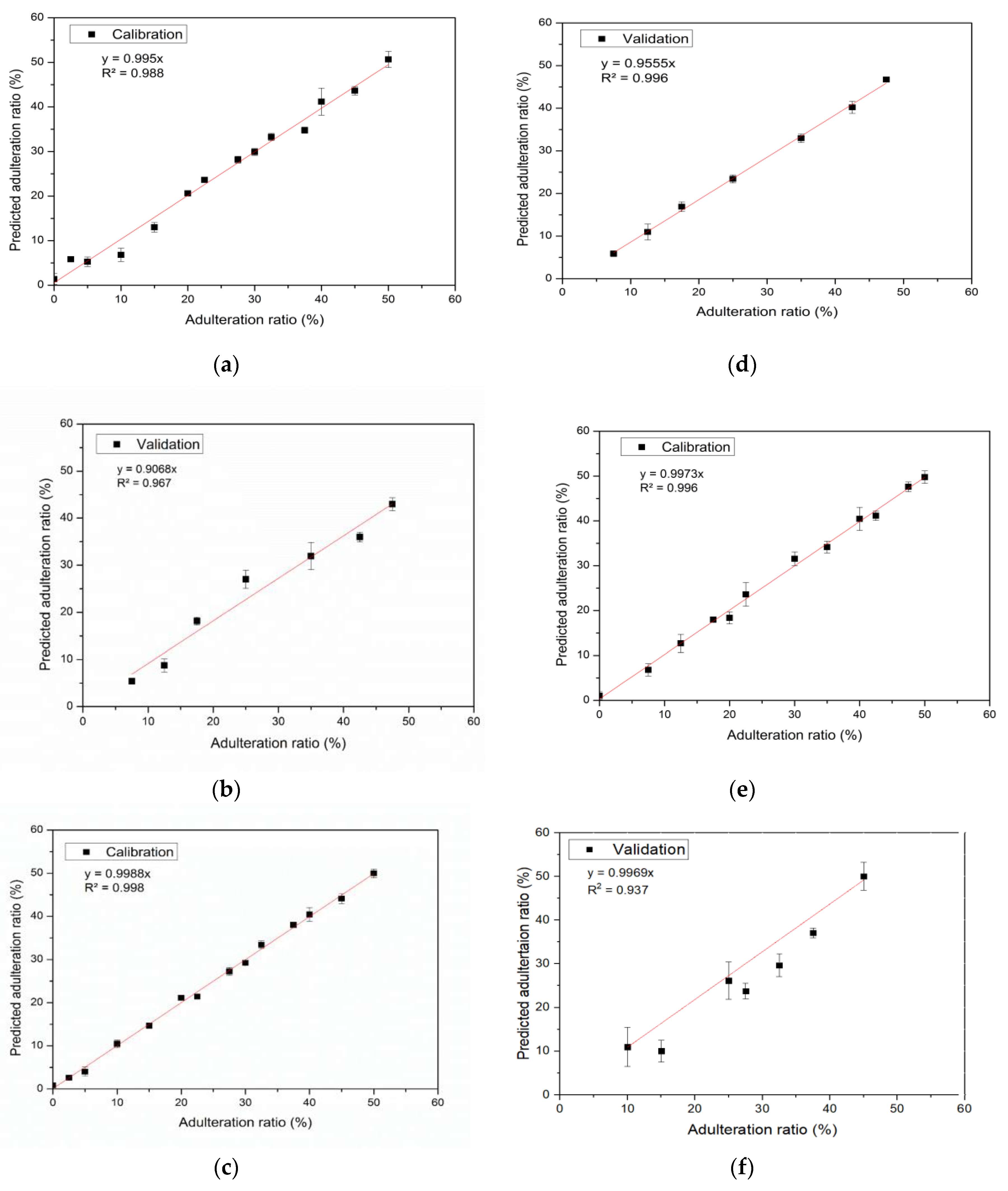
3.4. Quantification of the Adulteration of Pekmez Samples with Glucose Syrup Using PLS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Şimşek, A.; Artık, N.; Baspinar, E. Detection of raisin concentrate (Pekmez) adulteration by regression analysis method. J. Food Compos. Anal. 2004, 17, 155–163. [Google Scholar] [CrossRef]
- Akbulut, M.; Özcan, M.M. Comparison of mineral contents of mulberry (Morus spp.) fruits and their pekmez (boiled mulberry juice) samples. Int. J. Food Sci. Nutr. 2009, 60, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Türkben, C.; Suna, S.; İzli, G.; Uylaşer, V.; Demir, C. Physical and chemical properties of pekmez (Molasses) produced with different grape cultivars. Tarım Bilimleri Derg. 2016, 22, 339–348. [Google Scholar]
- Tosun, M. Detection of adulteration in mulberry pekmez samples added various sugar syrups with 13C/12C isotope ratio analysis method. Food Chem. 2014, 165, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Naderi-Boldaji, M.; Mishra, P.; Ahmadpour-Samani, M.; Ghasemi-Varnamkhasti, M.; Ghanbarian, D.; Izadi, Z. Potential of two dielectric spectroscopy techniques and chemometric analyses for detection of adulteration in grape syrup. Measurement 2018, 127, 518–524. [Google Scholar] [CrossRef]
- Grape Pekmez Notification. Türk Gıda Kodeksi Üzüm Pekmezi Tebliği. Available online: http://www.resmigazete.gov.tr/eskiler/2017/06/20170930-24.htm (accessed on 15 May 2019). (In Turkish)
- Yılmaz, M.; Afşar, H. An approach to determination of geographical origin and authenticity of grape and some grape products using SNIF-NMR®. Sigma 2012, 30, 102–112. [Google Scholar]
- Durmaz, G.; İzci, Y.; Yilmaztekin, M.; Cam, M. Adulteration in pekmez and detection methods. In Proceedings of the 4th International Symposium on Traditional Foods from Adriatic to Caucasus, Cyprus, Turkey, 19–21 April 2018; p. 4. [Google Scholar]
- Boyaci, I.H.; Temiz, H.T.; Geniş, H.E.; Soykut, E.A.; Yazgan, N.N.; Güven, B.; Uysal, R.S.; Bozkurt, A.G.; İlaslan, K.; Torun, Ö.; et al. Dispersive and FT-Raman spectroscopic methods in food analysis. RSC Adv. 2015, 5, 56606–56624. [Google Scholar] [CrossRef]
- Li, S.; Shan, Y.; Zhu, X.; Zhang, X.; Ling, G. Detection of honey adulteration by high fructose corn syrup and maltose syrup using Raman spectroscopy. J. Food Compos. Anal. 2012, 28, 69–74. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Paduret, S. Honey adulteration detection using Raman spectroscopy. Food Anal. Methods 2018, 11, 959–968. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chem. 2017, 218, 231–236. [Google Scholar] [CrossRef]
- Kelly, J.D.; Petisco, C.; Downey, G. Application of Fourier transform midinfrared spectroscopy to the discrimination between Irish artisanal honey and such honey adulterated with various sugar syrups. J. Agric. Food Chem. 2006, 54, 6166–6171. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Koca, N.; Harper, W.J.; Alvarez, V.B. Rapid determination of Swiss cheese composition by Fourier transform infrared/attenuated total reflectance spectroscopy. J. Dairy Sci. 2006, 89, 1407–1412. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of adulterated neem and flaxseed oil compositions by FTIR spectroscopy and multivariate regression analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- Basar, B.; Ozdemir, D. Determination of honey adulteration with beet sugar and corn syrup using infrared spectroscopy and genetic-algorithm-based multivariate calibration. J. Sci. Food Agric. 2018, 98, 5616–5624. [Google Scholar] [CrossRef]
- Cascant, M.; Kuligowski, J.; Garrigues, S.; Guardia, M. Determination of sugars in depilatory formulations: A green analytical method employing infrared detection and partial least squares regression. Talanta 2011, 85, 1721–1729. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; Seeram, N.P.; Lopez, M.G. Comparative analysis of maple syrups and natural sweeteners: Carbohydrates composition and classification (differentiation) by HPAEC-PAD and FTIR spectroscopy-chrmometrics. J. Food Compos. Anal. 2016, 52, 1–8. [Google Scholar] [CrossRef]
- Özbalci, B.; Boyaci, I.H.; Topcu, A.; Kadılar, C.; Tamer, U. Rapid analysis of sugars in honey by processing Raman spectrum using chemometric methods and artificial neural networks. Food Chem. 2013, 136, 1444–1452. [Google Scholar] [CrossRef]
- Anjos, O.; Campos, M.G.; Ruiz, P.C.; Antunes, P. Application of FTIR-ATR spectroscopy to the quantification of sugar in honey. Food Chem. 2015, 169, 218–223. [Google Scholar] [CrossRef]
- Gok, S.; Severcan, M.; Goormaghtigh, E.; Kandemir, I.; Severcan, F. Differentiation of Anatolian honey samples from different botanical origins by ATR-FTIR spectroscopy using multivariate analysis. Food Chem. 2015, 170, 234–240. [Google Scholar] [CrossRef]
- Se, K.W.; Ghoshal, S.K.; Wahab, R.A.; Ibrahim, R.K.I.; Lani, M.N. A simple approach for rapid detection and quantification of adulterants in stingless bees (Heterotrigona itama) honey. Food Res. Int. 2018, 105, 453–460. [Google Scholar] [CrossRef]
- Wang, J.; Kliks, M.M.; Jun, S.; Jackson, M.; Li, Q.X. Rapid analysis of glucose, fructose, sucrose, and maltose in honeys from different geographic regions using fourier transform infrared spectroscopy and multivariate analysis. J. Food Sci. 2010, 75, 208–214. [Google Scholar] [CrossRef]
- Trullols, E.; Ruisanchez, I.; Rius, F.X. Validation of qualitative analytical methods. TrAC Trends Anal. Chem. 2004, 23, 137–145. [Google Scholar] [CrossRef]
- Hubert, P.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of strategies for the validation of quantitative analytical procedures: A SFSTP proposal–part II. J. Pharm. Biomed. Anal. 2007, 45, 70–81. [Google Scholar] [CrossRef]
- Hineno, M. Infrared spectra and normal vibrations of β D-glucopyranose. Carbohydr. Res. 1977, 56, 219–227. [Google Scholar] [CrossRef]
- Tewari, J.; Irudayaraj, J. Quantification of saccharides in multiple floral honeys using fourier transform infrared microattenuated total reflectance spectroscopy. J. Agric. Food Chem. 2004, 52, 3237–3243. [Google Scholar] [CrossRef]
- Tewari, J.C.; Irudayaraj, J.M.K. Floral classification of honey using mid-infrared spectroscopy and surface acoustic wave based z-nose sensor. J. Agric. Food Chem. 2005, 53, 6955–6966. [Google Scholar] [CrossRef]
- Gallardo-Velázquez, T.; Osorio-Revilla, G.; Zuñiga-de Loa, M.; Rivera-Espinoza, Y. Application of FTIR-HATR spectroscopy and multivariate analysis to the quantification of adulterants in Mexican honeys. Food Res. Int. 2009, 42, 313–318. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhao, Q.; Ouyang, J.; Wub, Y. Rapid detection of authenticity and adulteration of walnut oil by FTIR and fluorescence spectroscopy: A comparative study. Food Chem. 2015, 181, 25–30. [Google Scholar] [CrossRef]
| Parameters | Grape | Carob | Mulberry |
|---|---|---|---|
| RMSEC | 0.128 | 0.068 | 0.141 |
| RMSECV | 0.381 | 0.327 | 0.300 |
| STR (%) | 100 a,b | 100 a,b | 100 a,b |
| SPR (%) | 100 a,b | 100 a,b | 100 a,b |
| FPR (%) | 0 a,b | 0 a,b | 0 a,b |
| FNR (%) | 0 a,b | 0 a,b | 0 a,b |
| EFR (%) | 100 a,b | 100 a,b | 100 a,b |
| Pekmez Type | R2 | |
|---|---|---|
| Calibration | Validation | |
| Grape | 0.988 | 0.967 |
| Carob | 0.998 | 0.996 |
| Mulberry | 0.996 | 0.937 |
| Pekmez Type | LOD (%) | LOQ (%) | RMSEC | RMSECV | RMSEP |
|---|---|---|---|---|---|
| Grape | 1.33 | 3.98 | 2.017 | 2.721 | 3.908 |
| Carob | 2.01 | 6.04 | 0.983 | 1.184 | 1.856 |
| Mulberry | 2.99 | 9.06 | 1.618 | 10.852 | 10.282 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaman, N.; Durakli Velioglu, S. Use of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy in Combination with Multivariate Methods for the Rapid Determination of the Adulteration of Grape, Carob and Mulberry Pekmez. Foods 2019, 8, 231. https://doi.org/10.3390/foods8070231
Yaman N, Durakli Velioglu S. Use of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy in Combination with Multivariate Methods for the Rapid Determination of the Adulteration of Grape, Carob and Mulberry Pekmez. Foods. 2019; 8(7):231. https://doi.org/10.3390/foods8070231
Chicago/Turabian StyleYaman, Nihal, and Serap Durakli Velioglu. 2019. "Use of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy in Combination with Multivariate Methods for the Rapid Determination of the Adulteration of Grape, Carob and Mulberry Pekmez" Foods 8, no. 7: 231. https://doi.org/10.3390/foods8070231
APA StyleYaman, N., & Durakli Velioglu, S. (2019). Use of Attenuated Total Reflectance—Fourier Transform Infrared (ATR-FTIR) Spectroscopy in Combination with Multivariate Methods for the Rapid Determination of the Adulteration of Grape, Carob and Mulberry Pekmez. Foods, 8(7), 231. https://doi.org/10.3390/foods8070231





