Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
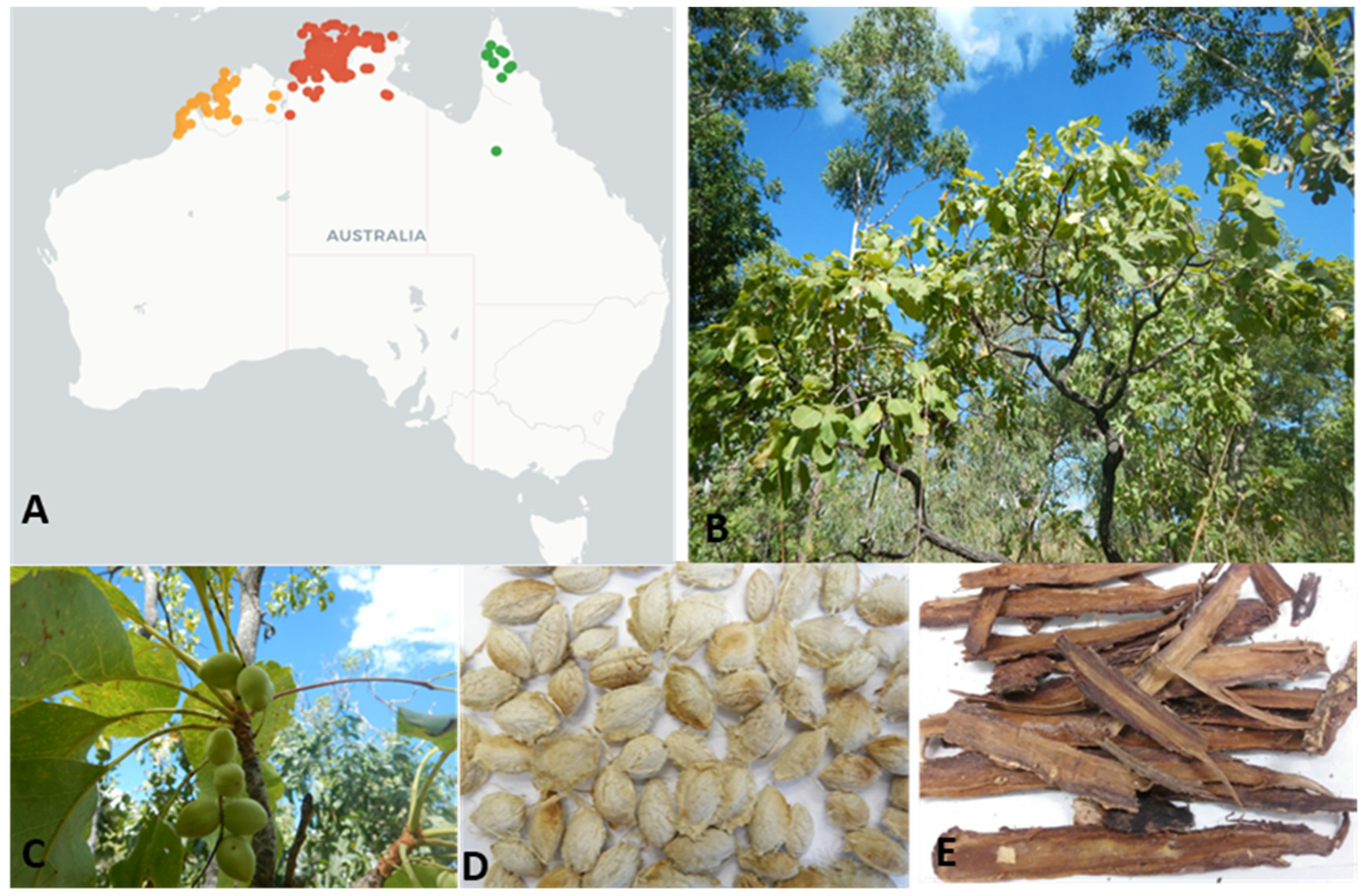
2.2. Sample Collection and Processing
2.3. Preparation of Kakadu Plum Extracts
2.4. Antioxidant Capacity
2.4.1. Total Phenolic Content
2.4.2. DPPH Radical Scavenging Activity
2.5. Determination of Total Saponin Content
2.6. Determination of Condensed Tannin Content
2.7. Determination of Hydrolysable Tannin
2.8. Antimicrobial Activity
2.8.1. Foodborne Microorganisms
2.8.2. Disc Diffusion Assay
2.8.3. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
2.8.4. Scanning Electron Microscopy
2.9. Statistical Analysis
3. Results and Discussion
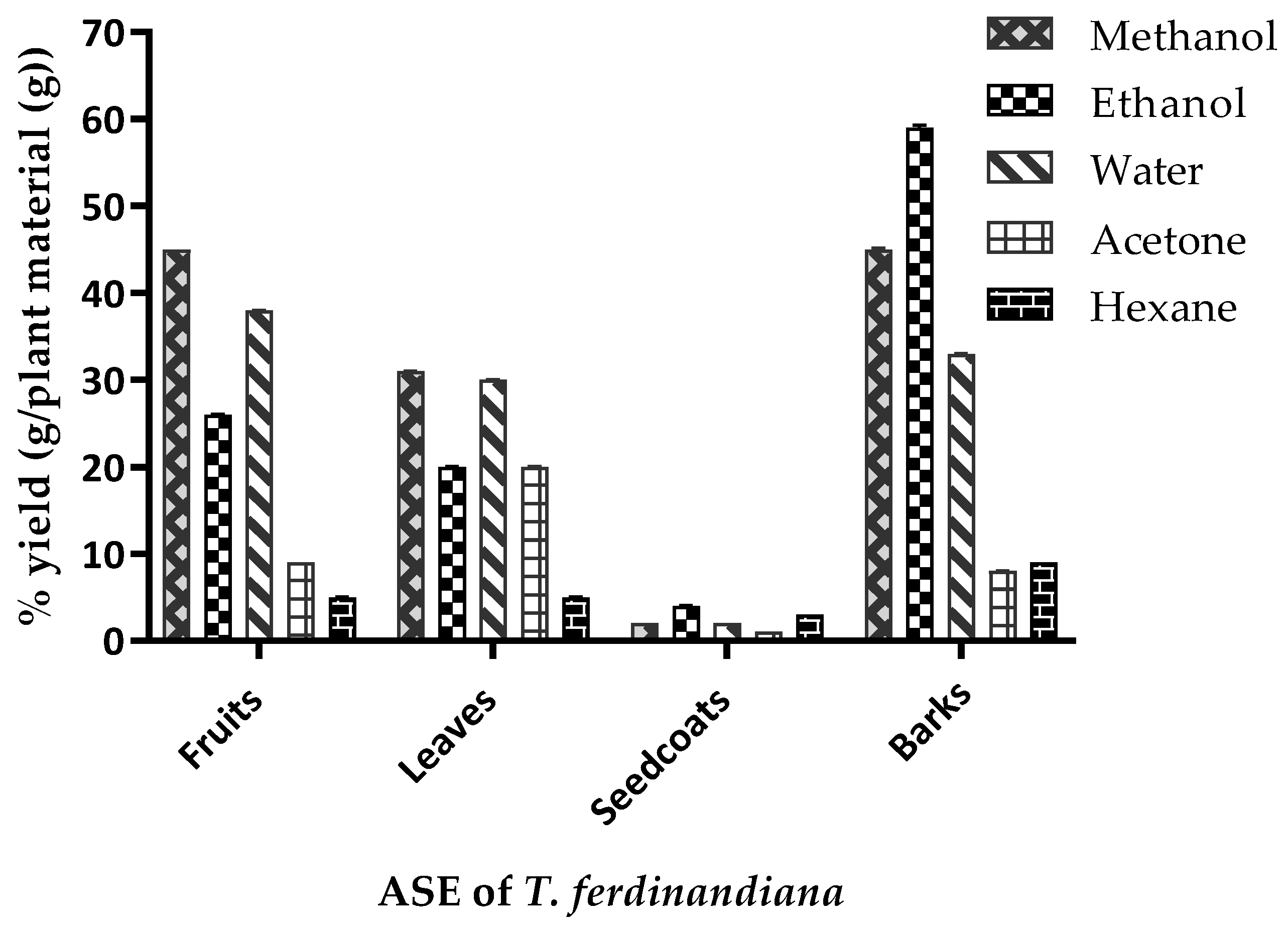
3.1. Extraction Yields
3.2. Antioxidant Capacity
3.2.1. Total Phenolic Content
3.2.2. DPPH radical Scavenging Capacity
3.3. Determination of Total Saponins
3.4. Determination of Condensed and Hydrolysable Tannins
3.5. Antimicrobial Activity
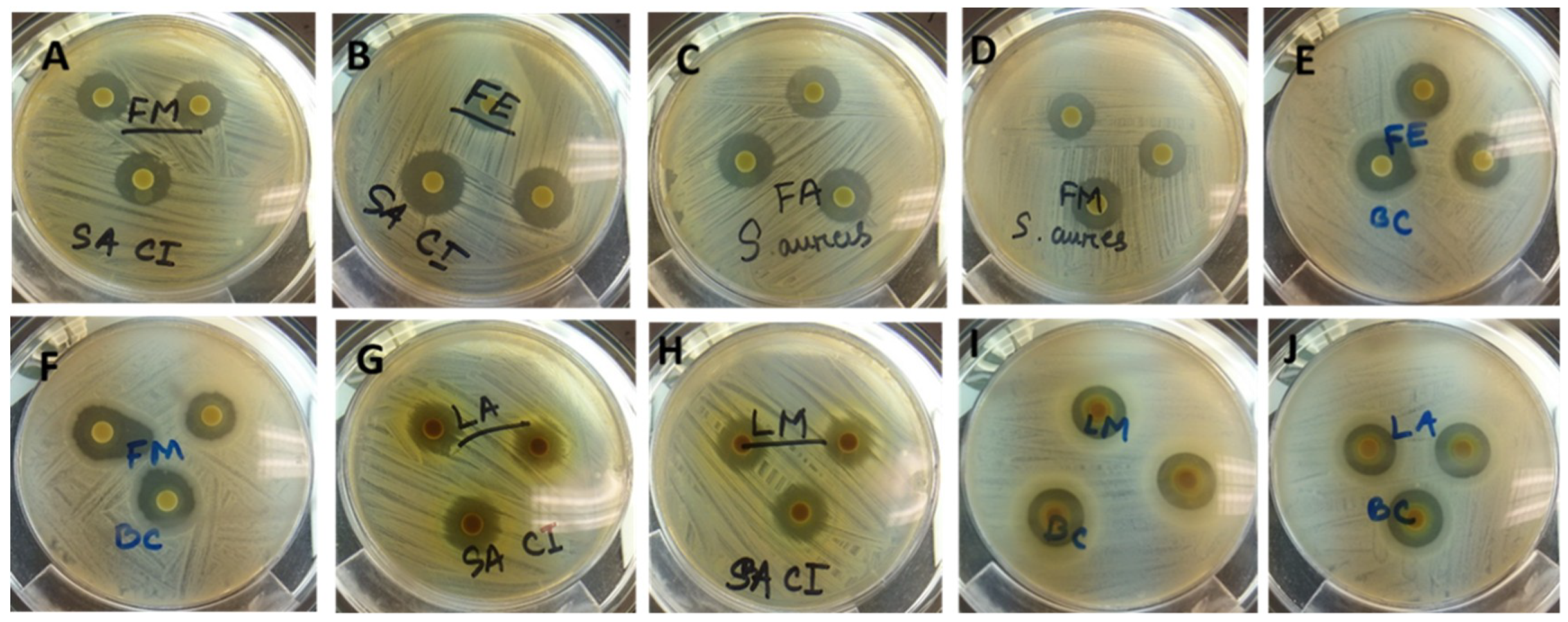
3.5.1. Disc Diffusion Assay
3.5.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
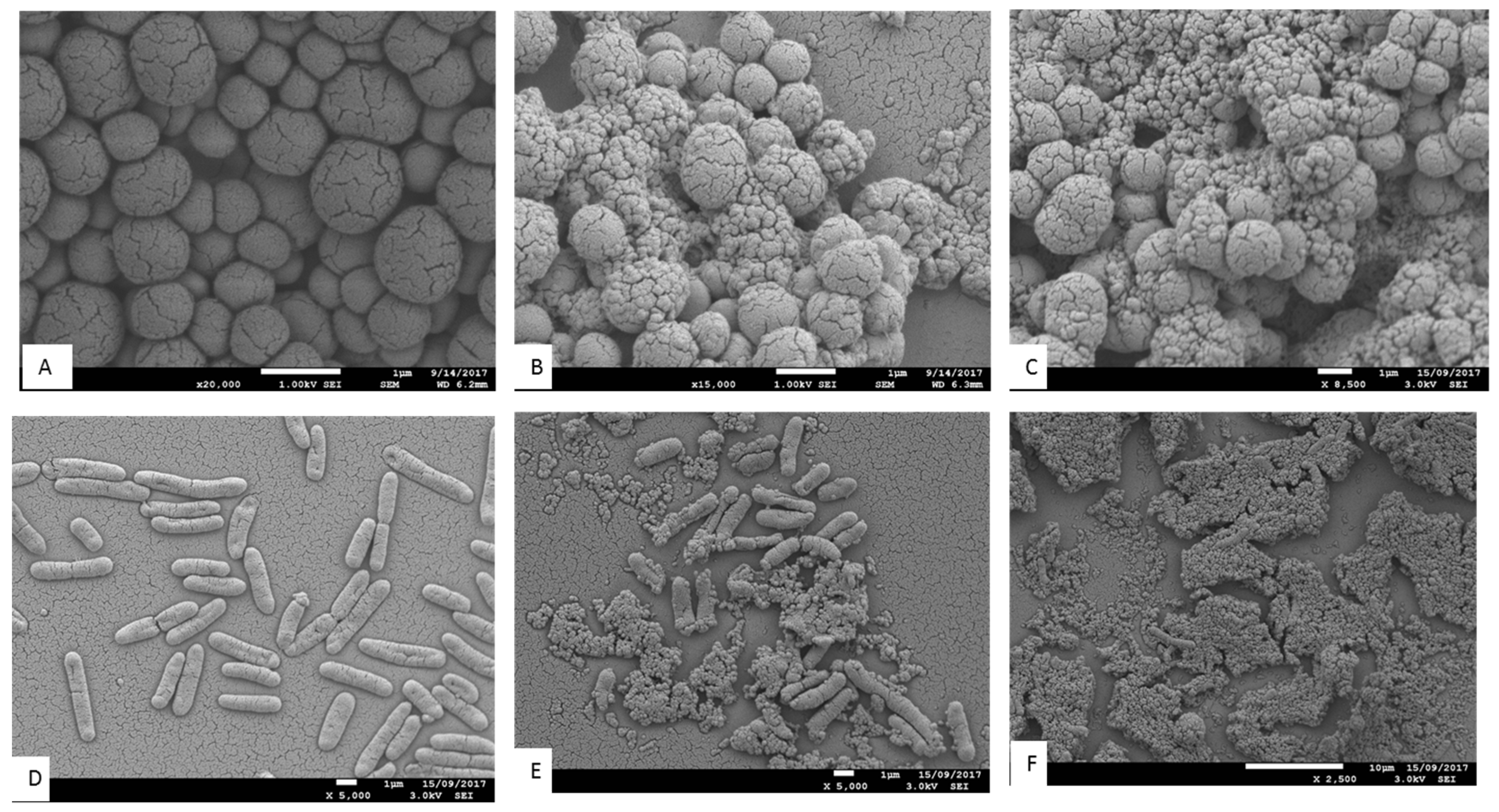
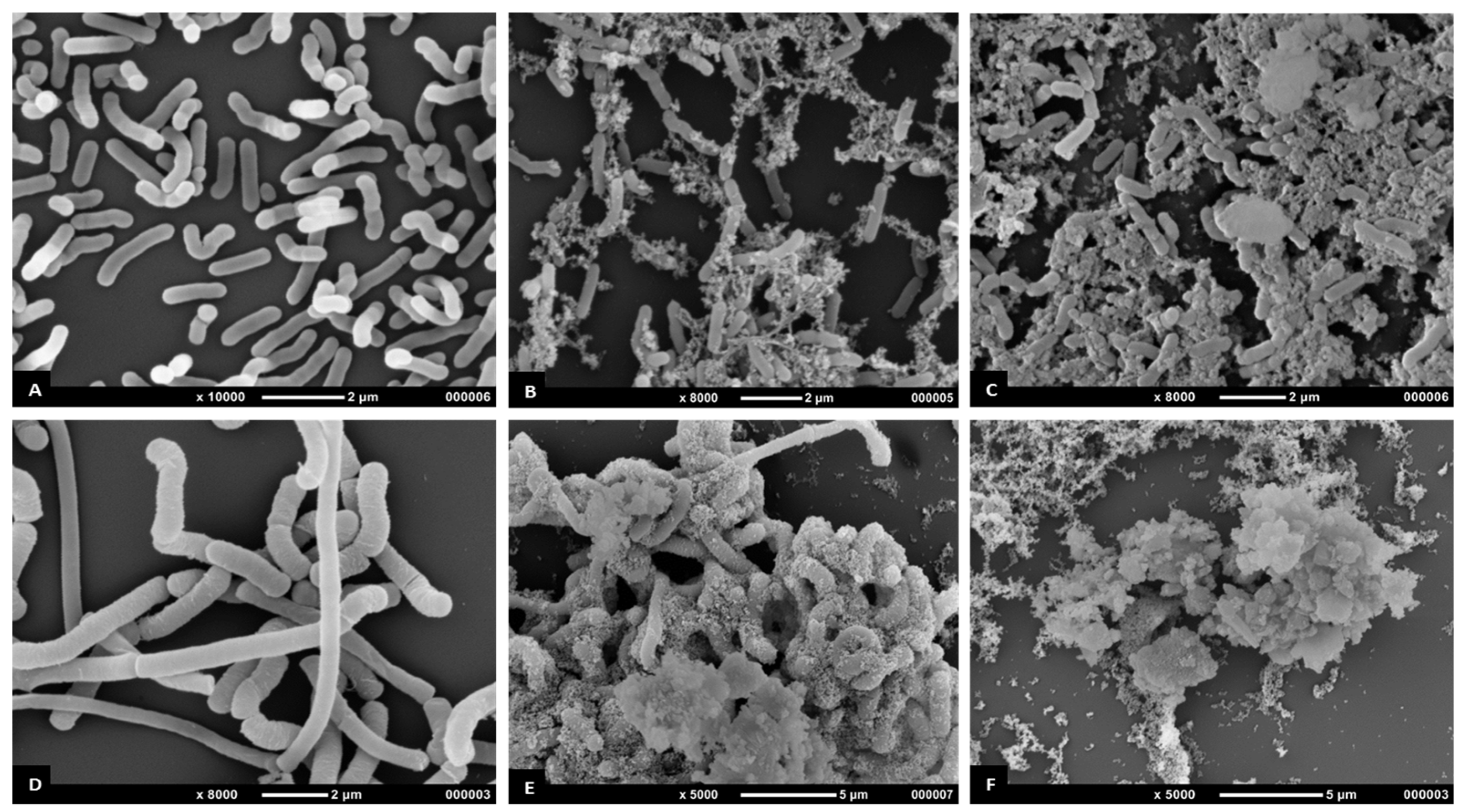
3.5.3. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayouni, E.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Yanishlieva, N.; Marinova, E. Stabilisation of edible oils with natural antioxidants. Eur. J. Lipid Sci. Technol. 2001, 103, 752–767. [Google Scholar] [CrossRef]
- Pietta, P. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.; Montville, T.; Nes, I.; Chikindas, M. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Negi, P. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.; Ibrahim, S.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Cunningham, A.; Garnett, S.; Gorman, J.; Courtenay, K.; Boehme, D. Eco-enterprises and Terminalia ferdinandiana: “Best laid plans” and Australian policy lessons. Econ. Bot. 2009, 63, 16–28. [Google Scholar] [CrossRef]
- Gorman, J.; Griffiths, A.; Whitehead, P. An analysis of the use of plant products for commerce in remote aboriginal communities of northern Australia. Econ. Bot. 2006, 60, 362–373. [Google Scholar] [CrossRef]
- Hegarty, M.; Hegarty, E. Food safety of Australian plant bushfoods. Rural Industr. Res. Develop. Corporat. 2001, 01/28, AGP-1A. [Google Scholar]
- Mohanty, S.; Cock, I. The chemotherapeutic potential of Terminalia ferdinandiana: Phytochemistry and bioactivity. Pharmacogn Rev. 2012, 6, 29–36. [Google Scholar] [PubMed]
- Konczak, I.; Maillot, F.; Dalar, A. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (kakadu plum). Food Chem. 2014, 151, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Sultanbawa, Y.; Williams, D.; Smyth, H. Changes in Quality and Bioactivity of Native Food During Storage; Rural Industries Research and Development Corporation (RIRDC): Canberra, Australia, 2015. [Google Scholar]
- Courtney, R.; Sirdaarta, J.; Matthews, B.; Cock, I. Tannin components and inhibitory activity of kakadu plum leaf extracts against microbial triggers of autoimmune inflammatory diseases. Pharmacog. J. 2015, 7, 18–31. [Google Scholar] [CrossRef]
- Williams, D.; Edwards, D.; Pun, S.; Chaliha, M.; Burren, B.; Tinggi, U.; Sultanbawa, Y. Organic acids in kakadu plum (Terminalia ferdinandiana): The good (ellagic), the bad (oxalic) and the uncertain (ascorbic). Food Res. Int. 2016, 89, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Akter, S.; Netzel, M.E.; Fletcher, M.T.; Tinggi, U.; Sultanbawa, Y. Chemical and nutritional composition of Terminalia ferdinandiana (kakadu plum) kernels: A novel nutrition source. Foods 2018, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Stanley, R.; Cusack, A.; Yasmina, S. Combinations of plant-derived compounds against campylobacter in vitro. J. Appl. Poul. Res. 2015, 24, 352–363. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Nirmal, N.; Chaliha, M.; Williams, D.; Mereddy, R.; Shelat, K.; Sultanbawa, Y. Physico-chemical characteristics and fungal profile of four saudi fresh date (Phoenix dactylifera l.) cultivars. Food Chem. 2017, 221, 644–649. [Google Scholar] [CrossRef]
- Musa, K.; Abdullah, A.; Kuswandi, B.; Hidayat, M. A novel high throughput method based on the dpph dry reagent array for determination of antioxidant activity. Food Chem. 2013, 141, 4102–4106. [Google Scholar] [CrossRef]
- Xi, M.; Hai, C.; Tang, H.; Chen, M.; Fang, K.; Liang, X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother. Res. 2008, 22, 228–237. [Google Scholar] [CrossRef]
- Ahmed, A.; McGaw, L.; Moodley, N.; Naidoo, V.; Eloff, J. Cytotoxic, antimicrobial, antioxidant, antilipoxygenase activities and phenolic composition of Ozoroa and Searsia species (Anacardiaceae) used in south African traditional medicine for treating diarrhoea. South Afr. J. Bot. 2014, 95, 9–18. [Google Scholar] [CrossRef]
- Hoang, V.; Pierson, J.; Curry, M.; Shaw, P.; Dietzgen, R.; Gidley, M.; Thomson, S.; Monteith, G. Polyphenolic contents and the effects of methanol extracts from mango varieties on breast cancer cells. Food Sci. Biotechnol. 2015, 24, 265–271. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.; Lacroix, M. In vitro evaluation of antimicrobial activities of various commercial essential oils, oleoresin and pure compounds against food pathogens and application in ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultanbawa, Y.; Cusack, A.; Currie, M.; Davis, C. An innovative microplate assay to facilitate the detection of antimicrobial activity in plant extracts. J. Rapid Methods Autom. Microbiol. 2009, 17, 519–534. [Google Scholar] [CrossRef]
- Shami, A.; Philip, K.; Muniandy, S. Synergy of antibacterial and antioxidant activities from crude extracts and peptides of selected plant mixture. BMC Complement. Alter. Med. 2013, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Alderees, F.; Mereddy, R.; Webber, D.; Nirmal, N.; Sultanbawa, Y. Mechanism of action against food spoilage yeasts and bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum plant solvent extracts. Foods 2018, 7, 179. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Ahmed, A.; McGaw, L.; Eloff, J. Evaluation of pharmacological activities, cytotoxicity and phenolic composition of four maytenus species used in southern african traditional medicine to treat intestinal infections and diarrhoeal diseases. BMC Complement. Altern. Med. 2013, 13, 100. [Google Scholar] [CrossRef]
- Ahmed, A.; Elgorashi, E.; Moodley, N.; McGaw, L.; Naidoo, V.; Eloff, J. The antimicrobial, antioxidative, anti-inflammatory activity and cytotoxicity of different fractions of four south African Bauhinia species used traditionally to treat diarrhoea. J. Ethnopharmacol. 2012, 143, 826–839. [Google Scholar] [CrossRef]
- Ye, C.; Dai, D.; Hu, W. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 2013, 30, 48–53. [Google Scholar] [CrossRef]
- Harborne, J.; Baxter, H.; Moss, G.P. Phytochemical Dictionary: Handbook of Bioactive Compounds from Plants, 2nd ed.; Taylor & Francis: London, UK, 1999. [Google Scholar]
- Nickel, J.; Spanier, L.; Botelho, F.; Gularte, M.; Helbig, M. Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem. 2017, 234, 285–294. [Google Scholar]
- Ruiz, K.; Khakimov, B.; Engelsen, S.; Bak, S.; Biondi, S.; Jacobsen, S. Quinoa seed coats as an expanding and sustainable source of bioactive compounds: An investigation of genotypic diversity in saponin profiles. Indust. Crops Prod. 2017, 104, 156–163. [Google Scholar] [CrossRef]
- Price, K.; Johnson, I.; Fenwick, G.; Malinow, M. The chemistry and biological significance of saponins in foods and feedingstuffs. C R C Crit. Rev. Food Sci. Nutr. 1987, 26, 27–135. [Google Scholar] [CrossRef]
- Lásztity, R.; Hidvégi, M.; Bata, Á. Saponins in food. Food Rev. Int. 1998, 14, 371–390. [Google Scholar] [CrossRef]
- Fenwick, D.; Oakenfull, D. Saponin content of food plants and some prepared foods. J. Sci. Food Agric. 1983, 34, 186–191. [Google Scholar] [CrossRef]
- Hartzfeld, P.; Forkner, R.; Hunter, M.; Hagerman, A. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Food Chem. 2002, 50, 1785–1790. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Balogun, A.; Fetuga, B. Tannin, phytin and oxalate contents of some wild under-utilized crop-seeds in Nigeria. Food Chem. 1988, 30, 37–43. [Google Scholar] [CrossRef]
- Humeera, N.; Kamili, A.; Bandh, S.; Amin, S.; Lone, B.; Gousia, N. Antimicrobial and antioxidant activities of alcoholic extracts of Rumex dentatus L. Microb. Pathog. 2013, 57, 17–20. [Google Scholar] [CrossRef]
- Cock, I.; Mohanty, S. Evaluation of the antibacterial activity and toxicity of Terminalia ferdinandiana fruit extracts. Pharmacog. J. 2011, 3, 72–79. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Pfundstein, B.; Desouky, S.; Hull, W.; Haubner, R.; Erben, G.; Owen, R. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mitra, M.K.; Chaudhuri, M.G.; Sa, B.; Das, S.; Dey, R. Study of the release mechanism of Terminalia chebula extract from nanoporous silica gel. Appl. Biochem. Biotechnol. 2012, 168, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, T.; Shah, S.; Kumar, S. A validated high-performance liquid chromatography method for determination of tannin-related marker constituents gallic acid, corilagin, chebulagic acid, ellagic acid and chebulinic acid in four Terminalia species from India. J. Chromatogr. Sci. 2015, 53, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, O.; Sood, S.; Osiyemi, O.; Agnihotri, V.; Gulati, A.; Ajaiyeoba, E.; Singh, B. In vitro antimicrobial activity of crude ethanol extracts and fractions of Terminalia catappa and Vitex doniana. Afr. J. Med. Med. Sci. 2015, 44, 21–26. [Google Scholar] [PubMed]
- Sivakumar, V.; Mohan, R.; Rangasamy, T.; Muralidharan, C. Antimicrobial activity of myrobalan (Terminalia chebula retz.) nuts: Application in raw skin preservation for leather making. Ind. J. Nat. Prod. Res. 2016, 7, 65–68. [Google Scholar]
- Mandal, S.; Patra, A.; Samanta, A.; Roy, S.; Mandal, A.; Mahapatra, T.D.; Pradhan, S.; Das, K.; Nandi, D.K. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac. J. Trop. Biomed. 2013, 3, 960–966. [Google Scholar] [CrossRef]
- Macáková, K.; Kolečkář, V.; Cahlíková, L.; Chlebek, J.; Hošt’álková, A.; Kuča, K.; Jun, D.; Opletal, L. Chapter 6—Tannins and their influence on health. In Recent Advances in Medicinal Chemistry; Rahman, A., Choudhary, M., Perry, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 159–208. [Google Scholar]
- Cowan, M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Davidson, P.; Taylor, T. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2007. [Google Scholar]
- Eom, S.; Lee, D.; Jung, Y.; Park, J.; Choi, J.; Yim, M.; Jeon, J.; Kim, H.; Son, K.; Je, J.; et al. The mechanism of antibacterial activity of phlorofucofuroeckol—A against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804. [Google Scholar] [CrossRef]
- Su, X.; Howell, A.; D’Souza, D. Antibacterial effects of plant-derived extracts on methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2012, 9, 573–578. [Google Scholar] [CrossRef]
- Cox, S.; Mann, C.; Markham, J.; Gustafson, J.; Warmington, J.; Wyllie, G. Determining the antimicrobial actions of tea tree oil. Molecules 2001, 6, 87–91. [Google Scholar] [CrossRef]
- Fahmy, N.; Sayed, E.; Singab, A. Genus Terminalia: A phytochemical and biological review. Med. Arom. Plants 2015, 4, 1–22. [Google Scholar]
- Ekambaram, S.; Perumal, S.; Balakrishnan, A. Scope of hydrolysable tannins as possible antimicrobial agent. Phytother. Res. 2016, 30, 1035–1045. [Google Scholar] [CrossRef]
- Rayan, P.; Matthews, B.; McDonnell, P.; Cock, I. Terminalia ferdinandiana extracts as inhibitors of Giardia duodenalis proliferation: A new treatment for giardiasis. Parasitol. Res. 2015, 114, 2611–2620. [Google Scholar] [CrossRef]
| Total Phenolic Content (GAE g/100 g DW) | ||||
|---|---|---|---|---|
| Fruits | Leaves | Seedcoats | Barks | |
| Methanol | 12.2 ± 2.9 a, w | 11.7 ± 0.5 a, w | 0.2 ± 0.0 x | 18.0 ± 2.0 a, y |
| Ethanol | 11.6 ± 1.0 a, w | 8.8 ± 0.5 b, x | 0.3 ± 0.0 y | 23.5 ± 0.5 b, z |
| Water | 5.2 ± 0.2 b, w | 4.2 ± 0.4 b, c, x | 0.2 ± 0.0 y | 6.7 ± 0.2 c, w |
| Acetone | 8.0 ± 0.2 b, w | 5.2 ± 0.2 c, w | 0.1 ± 0.0 x | 3.5 ± 0.0 d, z |
| Hexane | 0.4 ± 0.0 c | 0.2 ± 0.0 d | ND | 0.04 ± 0.0 e |
| DPPH Radical Scavenging Activity (%) | ||||
|---|---|---|---|---|
| Accelerated solvent extracts (ASE) of T. ferdinandiana | Fruits | Leaves | Seedcoats | Barks |
| Methanol | 93.4 ± 0.3 a, x | 89.4 ± 0.5 a, x | 93.0 ± 0.2 a, x | 84.7 ± 0.1 a, y |
| Ethanol | 94.3 ± 0.1 a,x | 91.8 ± 0.2 a,y | 88.0 ± 0.2 b,y | 85.8 ± 0.2 a,z |
| Water | 93.7 ± 0.2 a, x | 84.0 ± 0.5 b, y | 90.9 ± 0.2 b, x | 79.6 ± 0.2 b, y |
| Acetone | 91.5 ± 0.4 a, w | 79.2 ± 0.3 c, x | 74.2 ± 0.3 c, x | 85.5 ± 0.3 a, y |
| Hexane | 12.9 ± 0.9 b, w | 68.7 ± 0.4 d, x | 2.1 ± 1.3 d, y | 77.5 ± 3.8 b, z |
| T. ferdinandiana Tissues | Saponin Content (QSE g/100 g DW) | Condensed Tannin Content (CaE g/100 g DW) |
|---|---|---|
| Fruits | 0.4 a ± 0.0 | 0.8 a ± 0.1 |
| Leaves | 0.3 ± 0.1 | 0.02 b ± 0.0 |
| Seedcoats | ND | 0.1 a ± 0.0 |
| Barks | 7.0 b ± 0.2 | 7.0 c ± 0.7 |
| Hydrolysable Tannin Content (TAE mg/100 g DW) | ||||
|---|---|---|---|---|
| ASE | Fruits | Leaves | Seedcoats | Barks |
| Methanol | 55.3 ± 1.6 a,w | 120.8 ± 2.3 a, x | 0.9 ± 0.0 a, y | 16.5 ± 0.2 a, z |
| Ethanol | 33.3 ± 0.8 b, w | 81.4 ± 1.4 b, x | 1.42 ± 0.0 a, y | 20.4 ± 0.2 b, z |
| Water | 7.5 ± 0.4 c, w | 52.0 ± 0.5 c, x | 1.42 ± 0.1 a, y | 13.6 ± 0.0 c, z |
| Acetone | 10.8 ± 0.7 d, w | 66.5 ± 1.1 d, x | 27.1 ± 1.8 b, y | 4.9 ± 0.0 d, z |
| Hexane | 0.1 ± 0.1 e, w | 3.1 ± 0.2 e, x | 2.6 ± 0.2 a, x | 0.2 ± 0.4 e, w |
| ASE Extraction Solvent | T. ferdinandiana Tissues | Zone of Inhibition (in mm) | |||||
|---|---|---|---|---|---|---|---|
| S. aureus | MRSA | Pseudomonas aeruginosa | P. aeruginosa (CI) | B. cereus | Listeria monocytogenes | ||
| Methanol | Fruits | 13.8 ± 0.3 a, w | 16.4 ± 0.0 a, x | -- | 11.2 ± 0.0 a, w | 16.4 ± 0.9 a, x | 20.4 ± 2.0 a, y |
| Leaves | -- | 15.2 ± 0.4 a, w | -- | 14.6 ± 1.5 b, w | 16.0 ± 0.6 a,w | 21.3 ± 0.2 a, x | |
| Seedcoats | -- | 8.8 ± 0.0 b, w | -- | -- | 11.5 ± 0.6 b, x | 11.4 ± 0.8 b, x | |
| Barks | 11.6 ± 0.4 a | 12.0 ± 0.8 c | -- | -- | 12.8 ± 0.3 b | -- | |
| Water | Fruits | -- | -- | -- | 12.9 ± 1.4 a | -- | -- |
| Leaves | -- | 13.3 ± 1.4 c, w | -- | 10.7 ± 0.8 a, x | -- | -- | |
| Seedcoats | -- | -- | -- | -- | -- | -- | |
| Barks | 10.8 ± 1.4 a | 11.6 ± 0.5 c | -- | -- | 11.1 ± 0.4 b | -- | |
| Ethanol | Fruits | -- | 17.1 ± 0.1 a | -- | -- | 17.8 ± 0.6 a | 18.5 ± 0.5 a |
| Leaves | -- | 14.6 ± 0.2 a, w | -- | -- | 16.5 ± 1.0 a, x | 20.0 ± 0.6 a, y | |
| Seedcoats | -- | -- | -- | -- | 9.8 ± 0.8 c | 10.8 ± 0.6 b | |
| Barks | 12.1 ± 0.3 a | 12.7 ± 0.9c | -- | -- | 13.2 ± 1.1 b | -- | |
| Acetone | Fruits | 16.7 ± 0.5 b, w | 16.6 ± 0.3 a, w | -- | 13.3 ± 0.1 b, x | 18.4 ± 1.5 a, y | 20.5 ± 0.4 a, z |
| Leaves | -- | 15.7 ± 0.9 a, w | 8.7 ± 0.8 a, x | 14.1 ± 0.1 b, w | 16.1 ± 0.3 a, w | 21.0 ± 1.3 a, y | |
| Seedcoats | -- | -- | -- | -- | -- | -- | |
| Barks | 15.0 ± 1.0 b, w | 11.0 ± 1.5 c, x | -- | -- | 15.7 ± 0.6 a, w | 15.3 ± 1.8 c, w | |
| Oxytetracycline (0.25 mg/mL) | 33.9 ± 0.0 c, w | 29.7 ± 1.9 d, w | 13.8 ± 0.5 b, x | 18.1 ± 0.6 c, y | 17.3 ± 1.5 a, y | 33.3 ± 0.4 d, w | |
| Tested Microorganisms | MIC (mg/mL) | |||||||||||||
| FM | LM | SM | BM | FW | LW | BW | FE | LE | SE | BE | FA | LA | BA | |
| Staphylococcus aureus | 1.5 | 2 | 3 | 1 | 1 | 2 | ||||||||
| MRSA | 3 | 2.5 | 1 | 2.5 | 3 | 3 | 3 | 3 | 1 | |||||
| Pseudomonas aeruginosa | 1 | 1 | 1 | 1 | 1 | |||||||||
| Pseudomonas aeruginosa CI | 1 | 1 | 1 | |||||||||||
| Bacillus cereus | 1.5 | 1 | 1 | 1.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Listeria monocytogenes | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | ||||||
| MBC (mg/mL) | ||||||||||||||
| FM | LM | SM | BM | FW | LW | BW | FE | LE | SE | BE | FA | LA | BA | |
| Staphylococcus aureus | 1.5 | 1 | 2 | 1 | 1 | 2 | ||||||||
| MRSA | 3 | 3 | 1 | 2 | 2 | 3 | 2 | 3 | 1 | |||||
| Pseudomonas aeruginosa | 1 | 1 | 1 | 1 | 1 | |||||||||
| Pseudomonas aeruginosa CI | 1 | 1 | 1 | |||||||||||
| Bacillus cereus | 1.5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Listeria monocytogenes | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, S.; Netzel, M.E.; Tinggi, U.; Osborne, S.A.; Fletcher, M.T.; Sultanbawa, Y. Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria. Foods 2019, 8, 281. https://doi.org/10.3390/foods8080281
Akter S, Netzel ME, Tinggi U, Osborne SA, Fletcher MT, Sultanbawa Y. Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria. Foods. 2019; 8(8):281. https://doi.org/10.3390/foods8080281
Chicago/Turabian StyleAkter, Saleha, Michael E. Netzel, Ujang Tinggi, Simone A. Osborne, Mary T. Fletcher, and Yasmina Sultanbawa. 2019. "Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria" Foods 8, no. 8: 281. https://doi.org/10.3390/foods8080281
APA StyleAkter, S., Netzel, M. E., Tinggi, U., Osborne, S. A., Fletcher, M. T., & Sultanbawa, Y. (2019). Antioxidant Rich Extracts of Terminalia ferdinandiana Inhibit the Growth of Foodborne Bacteria. Foods, 8(8), 281. https://doi.org/10.3390/foods8080281










