Influence of Salting Method on the Chemical and Texture Characteristics of Ovine Halloumi Cheese
Abstract
1. Introduction
2. Materials and Methods
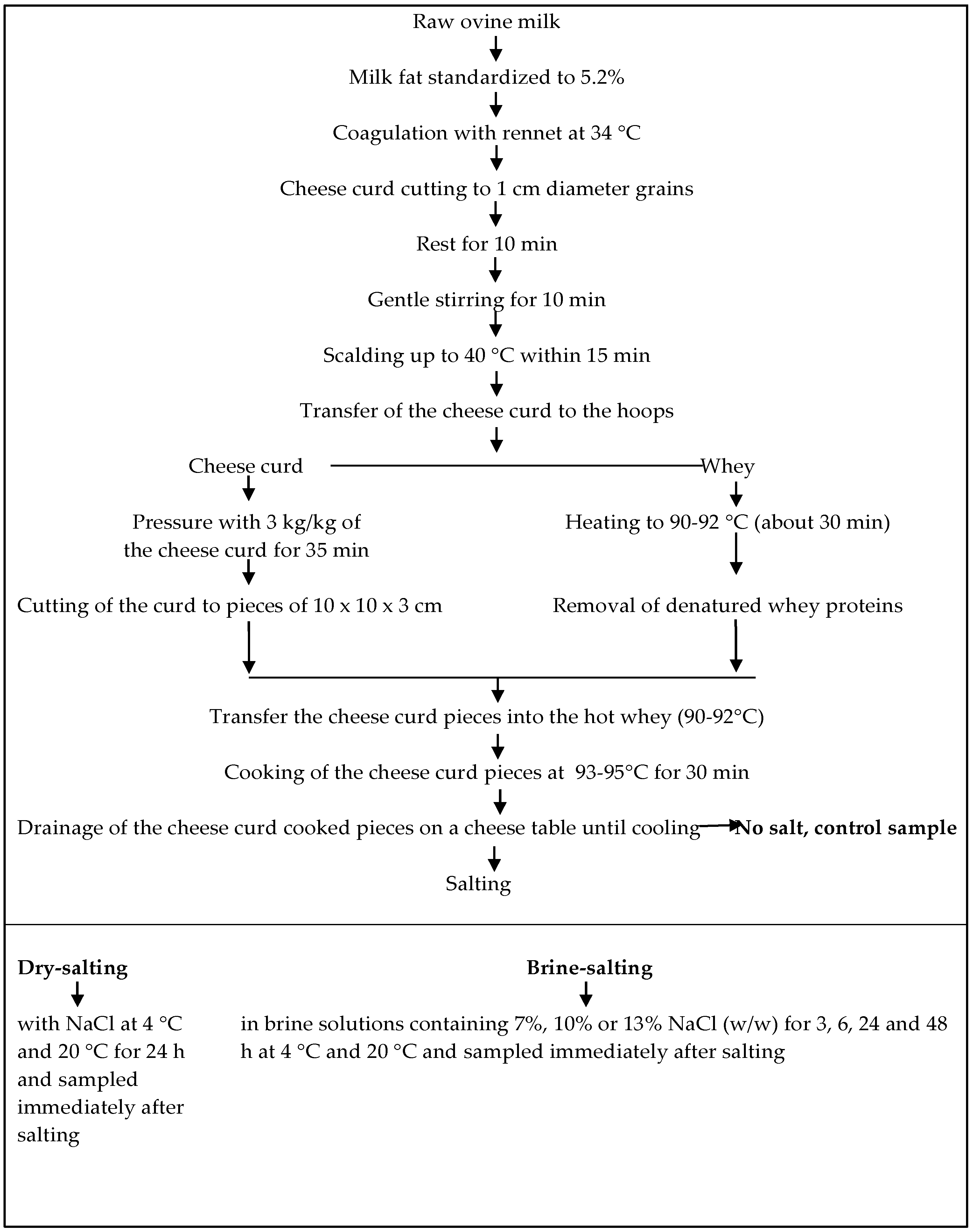
2.1. Cheesemaking
2.2. Chemical Analyses
2.3. Assessment of Textural Properties
2.4. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Inorganic Elements
3.3. Texture Profile
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- CYS 94. Cyprus Standards, for Halloumi Cheese; CYS/ TS10. CY594: Parts 1 and 2; Cyprus Ministry of Commerce and Industry: Nicosia, Cyprus, 1985. [Google Scholar]
- Papademas, P. Halloumi Cheese. In Brine Cheeses; Tamime, A.Y., Ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 117–138. [Google Scholar]
- Kaminarides, S.E.; Stamou, P.; Massouras, T. Changes of organic acids, volatile aroma compounds and sensory characteristics of Halloumi cheese kept in brine. Food Chem. 2007, 100, 219–225. [Google Scholar] [CrossRef]
- Guinee, T.P.; Fox, P.F. Salt in cheese: Physical chemical and biological aspects. In Cheese: Chemistry, Physics and Microbiology, 2nd ed.; Fox, P.F., Ed.; Chapman & Hall: London, UK, 1993; pp. 257–302. [Google Scholar]
- Guinee, T.P. Salting and the role of salt in cheese. Int. J. Dairy Technol. 2004, 57, 99–108. [Google Scholar] [CrossRef]
- Atasever, M.; Keleş, A.; Uçar, G.; Guner, A. Use of different salting techniques in Halloumi cheese: Effect on sensory, microbiological and chemical properties. Acta Aliment. 2003, 32, 7–14. [Google Scholar] [CrossRef]
- Ayyash, M.; Shah, N. Effect of partial substitution of NaCl with KCl on Halloumi cheese during storage: Chemical composition, lactic bacterial count, and organic acids production. J. Food Sci. 2010, 75, C525–C529. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Shah, N. Effect of Partial Substitution of NaCl with KCl on Proteolysis of Halloumi Cheese. J. Food Sci. 2011, 76, C31–C37. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Sherkat, M.; Francis, P.; Williams, R.; Shah, N. The effect of sodium chloride substitution with potassium chloride on texture profile and microstructure of Halloumi cheese. J. Dairy Sci. 2011, 94, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kamleh, R.; Olabi, A.; Toufeili, I.; Najm, N.; Younis, T.; Ajib, R. The effect of substitution of sodium chloride with potassium chloride on the physicochemical, microbiological, and sensory properties of Halloumi cheese. J. Dairy Sci. 2012, 95, 1140–1151. [Google Scholar] [CrossRef]
- Αnifantakis, E.M.; Kaminarides, S.E. Contribution to the study of Halloumi cheese made from sheep’s milk. Aust. J. Dairy Technol. 1983, 58, 29–31. [Google Scholar]
- IDF Standard 4:2004. Cheese and Processed Cheese—Determination of the Total Solids Content; International Dairy Federation: Brussels, Belgium, 2004. [Google Scholar]
- AOAC. Official Methods of Analysis, 12th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1975; p. 254. [Google Scholar]
- IDF Standard 88:2006. Cheese and Processed Cheese Products—Determination of Chloride Content—Potentiometric Titration Method; International Dairy Federation: Brussels, Belgium, 2006. [Google Scholar]
- IDF Standard 119:2007. Milk and Milk Products—Determination of Calcium, Sodium, Potassium and Magnesium Content—Atomic Absorption Spectrometric Method; International Dairy Federation: Brussels, Belgium, 2007. [Google Scholar]
- IDF Standard 42:2006. Milk—Determination of Total Phosphorous Content—Method Using Molecular Absorption Spectrometry; International Dairy Federation: Brussels, Belgium, 2006. [Google Scholar]
- Kaminarides, S.; Stachtiaris, S. Production of processed cheese using kasseri cheese and processed cheese analogues incorporating whey protein concentrate and soybean oil. Int. J. Dairy Technol. 2000, 53, 69–74. [Google Scholar] [CrossRef]
- Geurts, T.J.; Walstra, P.; Mulder, H. Transport of salt and water during salting of cheese. 1. Analysis of the processes involved. Neth. Milk Dairy J. 1974, 28, 102–129. [Google Scholar]
- Guinee, T.P.; Fox, P.F. Transport of sodium chloride and water in Romano-type cheese slices during brining. Food Chem. 1986, 19, 49–64. [Google Scholar] [CrossRef]
- Kindstedt, P.S.; Kiely, L.J.; Gilmore, J.A. Variation in composition and functional properties within brine salted Mozzarella cheese. J. Dairy Sci. 1992, 75, 2913–2921. [Google Scholar] [CrossRef]
- Pappas, C.P.; Kondyli, E.; Voutsinas, L.P.; Mallatou, H. Effects of salting method and storage time on composition and quality of Feta cheese. J. Soc. Dairy Technol. 1996, 49, 113–118. [Google Scholar] [CrossRef]
- Melilli, C.; Barbano, D.M.; Licitra, G.; Tumino, G.; Farina, G.; Carpino, S. Influence of presalting and brine concentration on salt uptake by Ragusano cheese. J. Dairy Sci. 2003, 86, 1083–1100. [Google Scholar] [CrossRef]
- Turhan, M.; Kaletunc, G. Modelling of salt diffusion in white cheese during long-term brining. J. Food Sci. 1992, 57, 1082–1085. [Google Scholar] [CrossRef]
- Payne, M.R.; Morison, K.R. A multicomponent approach to salt diffusion in cheese. Int. Dairy J. 1999, 9, 887–894. [Google Scholar] [CrossRef]
- Pierre, A.; Brulé, G. Mineral and protein equilibria between the colloidal and soluble phases of milk at low temperature. J. Dairy Res. 1981, 48, 417–428. [Google Scholar] [CrossRef]
- Pierre, A.; Brule, G.; Fauquant, J. Etude de la mobilite du calcium dans le lait h l’aide du calcium. Le Lait 1983, 63, 473–489. [Google Scholar] [CrossRef]
- Van Hooydonk, A.C.M.; Hagedoom, H.G.; Boenigter, J. pH-Induced physicochemical changes of casein micelles in milk and their effect on renneting. Effects of acidification on physicochemical properties. Neth. Milk Dairy J. 1986, 40, 281–296. [Google Scholar]
- Zoidou, E.; Plakas, N.; Giannopoulou, D.; Kotoula, M.; Moatsou, G. Effect of supplementation of brine with calcium on the Feta cheese ripening. Int. J. Dairy Technol. 2015, 68, 420–426. [Google Scholar] [CrossRef]
- Lucas, A.; Rock, E.; Chamba, J.; Verdier-Metz, I.; Brachet, P.; Coulon, J. Respective effects of milk composition and the cheese-making process on cheese compositional variability in components of nutritional interest. Le Lait 2006, 86, 21–41. [Google Scholar] [CrossRef]
- Kindstedt, P.S.; Kosikowski, F.V. Calcium, phosphorus and sodium concentrations in Cheddar cheese. J. Dairy Sci. 1988, 71, 285–289. [Google Scholar] [CrossRef]
- Gaucheron, F.; Le Graet, Y.; Briad, V. Effect of NaCl addition on the mineral equilibrium of concentrated and acidified casein micelles. Milchwissenschaft 2000, 55, 82–86. [Google Scholar]
- Fernandez del Pozo, B.; Gaya, P.; Medina, M.; Rodriguez-Marin, A.; Nuňez, M. Changes in chemical and rheological characteristics of La Serena ewes’ milk cheese during ripening. J. Dairy Res. 1988, 55, 457–464. [Google Scholar] [CrossRef]
- Kaminarides, S.; Litos, I.; Massouras, T.; Georgala, A. The effect of cooking time on curd composition and textural properties of sheep Halloumi cheese. Small Ruminant Res. 2015, 125, 106–114. [Google Scholar] [CrossRef]
- Prentice, J.H.; Langley, K.R.; Aarshall, R.J. Cheese rheology. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., Ed.; Chapman and Hall: London, UK, 1993; pp. 303–340. [Google Scholar]
| Chemical Characteristics | No Salt | Salting Method | Temperature of Cheese Salting (°C) | Pairs of Means That Significantly Differed at (p ≤ 0.05) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 20 | ||||||||||
| Salting Time (h) | |||||||||||
| 3 | 6 | 24 | 48 | 3 | 6 | 24 | 48 | ||||
| Moisture (%) | 47.10 ± 0.36 | Dry salting | - | - | 46.2 ± 0.12 | - | - | - | 42.62 ± 0.55 | - | (4–20 °C) |
| Brine 7% | 51.80 ± 0.39 | 52.01 ± 1.18 | 52.67 ± 0.60 | 53.55 ± 0.60 | 50.29 ± 0.18 | 50.31 ± 0.60 | 50.94 ± 0.10 | 52.53 ± 0.12 | (7–10%) (7–13%) | ||
| Brine 10% | 50.15 ± 0.07 | 50.85 ± 1.20 | 49.95 ± 0.13 | 50.66 ± 0.28 | 49.32 ± 0.30 | 50.20 ± 1.06 | 49.26 ± 0.68 | 48.95 ± 0.1 | (10–13%) | ||
| Brine 13% | 48.89 ± 0.75 | 49.10 ± 0.64 | 48.41 ± 0.18 | 48.02 ± 0.38 | 48.56 ± 0.55 | 47.93 ± 0.10 | 47.98 ± 0.84 | 45.53 ± 0.17 | (4–20 °C) | ||
| Ash (%) | 2.64 ± 0.11 | Dry salting | - | - | 4.13±0.11 | - | - | - | 4.15±0.22 | - | NS |
| Brine 7% | 4.25 ± 0.01 | 4.44 ± 0.14 | 4.75 ± 0.06 | 4.87 ± 0.03 | 4.37 ± 0.02 | 4.47 ± 0.14 | 4.79 ± 0.02 | 5.09 ± 0.10 | (7–10%) (7–13%) | ||
| Brine 10% | 5.12 ± 0.03 | 5.65 ± 0.07 | 5.91 ±0.06 | 6.03 ± 0.10 | 5.03 ± 0.03 | 5.62 ± 0.05 | 6.05 ± 0.03 | 6.34 ± 0.12 | (10–13%) | ||
| Brine 13% | 6.01 ± 0.02 | 6.18 ± 0.02 | 6.89 ± 0.11 | 7.06 ± 0.10 | 5.80 ± 0.04 | 6.19 ± 0.03 | 6.58 ± 0.16 | 6.99 ± 0.03 | (3–6 h) (3–24 h) (3–48 h) (6–24 h) (6–48 h) (24–48 h) | ||
| Salt (%) | 0.10 ± 0.02 | Dry salting | - | - | 1.51 ± 0.13 | - | - | - | 1.61 ± 0.22 | - | NS |
| Brine 7% | 2.17 ± 0.01 | 2.39 ± 0.11 | 2.77 ± 0.03 | 2.98 ± 0.04 | 2.15 ± 0.02 | 2.16 ± 0.10 | 2.79 ± 0.02 | 3.09 ± 0.07 | (7–10%) (7–13%) | ||
| Brine 10% | 2.92 ± 0.15 | 3.72 ± 0.09 | 3.98 ± 0.07 | 4.08 ± 0.07 | 2.72 ± 0.09 | 3.63 ± 0.18 | 4.05 ± 0.25 | 4.21 ± 0.14 | (10–13%) | ||
| Brine 13% | 3.68 ± 0.20 | 4.19 ± 0.07 | 4.89 ± 0.07 | 4.97 ± 0.10 | 3.86 ± 0.02 | 3.95 ± 0.08 | 4.69 ± 0.06 | 4.82 ± 0.11 | (3–6 h) (3–24 h) (3–48 h) (6–24 h) (6–48 h) (24–48 h) | ||
| Acidity as lactic acid (%) | 0.50 ± 0.02 | Dry salting | - | - | 0.52 ± 0.02 | - | - | - | 0.62 ± 0.06 | (4–20 °C) | |
| Brine 7% | 0.45 ± 0.03 | 0.40 ± 0.08 | 0.37 ± 0.05 | 0.32 ± 0.02 | 0.55 ± 0.06 | 0.48 ± 0.06 | 0.43 ± 0.05 | 0.35 ± 0.03 | (4–20 °C) | ||
| Brine 10% | 0.47 ± 0.02 | 0.42 ± 0.05 | 0.42 ± 0.03 | 0.35 ± 0.03 | 0.53 ± 0.08 | 0.52 ± 0.06 | 0.47 ± 0.03 | 0.40 ± 0.03 | (3–6 h) (3–24 h) | ||
| Brine 13% | 0.50 ± 0.06 | 0.40 ± 0.06 | 0.37 ± 0.05 | 0.37 ± 0.03 | 0.55 ± 0.06 | 0.45 ± 0.06 | 0.43 ± 0.05 | 0.42 ± 0.05 | (3–48 h) (6–48 h) | ||
| Inorganic Elements | No Salt | Salting Method | Temperature of Cheese Salting (°C) | Pairs of Means That Significantly Differed at (p ≤ 0.05) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 20 | ||||||||||
| Salting time (h) | |||||||||||
| 3 | 6 | 24 | 48 | 3 | 6 | 24 | 48 | ||||
| Na | 25 ± 2 | Dry salting | - | - | 558 ± 133 | - | - | - | 568 ± 84 | - | (4–20 °C) |
| Brine 7% | 741 ± 200 | 579 ± 48 | 1097 ± 154 | 1042 ± 80 | 694 ± 141 | 806 ± 241 | 1023 ± 204 | 1107 ± 144 | 20 °C→(3–6 h) (3–24 h) (3–48 h) (6–48 h) 4 °C→(3–24 h) (3–48 h) (6–24 h) | ||
| Brine 10% | 1141 ± 113 | 1436 ± 68 | 1430 ± 95 | 1539 ± 190 | 1042 ± 101 | 1360 ± 193 | 1489 ± 127 | 1625 ± 186 | |||
| Brine 13% | 1347 ± 384 | 1477 ± 358 | 1742 ± 362 | 1641 ± 218 | 1233 ± 330 | 1447 ± 300 | 1527 ± 210 | 1647 ± 355 | |||
| K | 64 ± 6 | Dry salting | - | - | 71 ± 6 | - | - | - | 71 ± 10 | - | (4–20 °C) |
| Brine 7% | 30 ± 6 | 26 ± 17 | 19 ± 6 | 18 ± 10 | 34 ±17 | 29 ± 12 | 17 ± 7 | 19 ± 5 | (3–6 h) (3–24 h) (3–48 h) (6–24 h)(6–48 h) (DS,−7%)(DS, −10%)(DS, −13%) | ||
| Brine 10% | 38 ± 6 | 30 ± 11 | 19 ±8 | 23 ± 6 | 39 ± 10 | 27 ± 7 | 14 ± 7 | 23 ± 14 | |||
| Brine 13% | 32 ± 13 | 27 ± 8 | 18 ±9 | 16 ± 9 | 26 ± 10 | 28 ± 16 | 17 ± 14 | 15 ± 11 | |||
| Ca | 970 ± 90 | Dry salting | - | - | 1064 ± 80 | - | - | - | 1093 ± 124 | - | (4–20 °C) |
| Brine 7% | 841 ± 111 | 992 ± 134 | 845 ± 114 | 780 ± 70 | 875 ± 24 | 924 ± 54 | 854 ± 67 | 882 ± 134 | (6–24 h)(6–48 h) (DS, −7%)(DS, −10%)(DS, −13%) | ||
| Brine 10% | 926 ± 82 | 821 ± 101 | 898 ± 86 | 834 ± 76 | 1026 ± 115 | 1009 ± 137 | 913 ± 37 | 922 ± 113 | |||
| Brine 13% | 912 ± 39 | 847 ± 75 | 751 ± 82 | 857 ± 30 | 871 ± 109 | 1048 ± 281 | 869 ± 30 | 855 ± 177 | |||
| Mg | 42 ± 3 | Dry salting | - | - | 47 ± 2 | - | - | - | 48 ± 4 | - | NS |
| Brine 7% | 37 ± 5 | 36 ± 4 | 34 ± 6 | 33 ± 2 | 37 ± 3 | 37 ± 4 | 36 ± 5 | 34 ± 2 | (3–6 h) (3–24 h) (3–48 h) (6–24 h)(6–48 h) (DS, −7%)(DS, −10%)(DS, −13%) | ||
| Brine 10% | 39 ± 1 | 36 ± 6 | 38 ± 3 | 35 ± 4 | 43 ± 4 | 40 ± 4 | 30 ± 12 | 38 ± 6 | |||
| Brine 13% | 36 ± 9 | 36 ± 7 | 32 ± 4 | 33 ± 5 | 38 ± 8 | 40 ± 8 | 34 ± 5 | 34 ± 7 | |||
| P | 470 ± 30 | Dry salting | - | - | 519 ± 41 | - | - | - | 519 ± 29 | - | (4–20 °C) |
| Brine 7% | 442 ± 21 | 403 ± 13 | 391 ± 21 | 402 ± 22 | 488 ± 40 | 442 ± 26 | 436 ± 8 | 445 ± 22 | 3–6 h) (3–24 h) (3–48 h) 4°C→(7–13%) (DS, −7%)(DS, −10%)(DS, −13%) | ||
| Brine 10% | 435 ± 39 | 420 ± 30 | 436 ± 39 | 400 ± 7 | 469 ± 30 | 448 ± 26 | 426 ± 39 | 419 ± 3 | |||
| Brine 13% | 447 ± 24 | 422 ± 17 | 423 ± 11 | 457 ± 18 | 458 ± 10 | 440 ± 36 | 437 ± 16 | 457 ± 14 | |||
| Textural characteristics | No Salt | Salting Method | Temperature of Cheese Salting (°C) | Pairs of Means That Significantly Differed at (p ≤ 0.05) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 20 | ||||||||||
| Salting Time (h) | |||||||||||
| 3 | 6 | 24 | 48 | 3 | 24 | 48 | |||||
| Hardness (Ν) | 7.88 ± 0.39 | Dry salting | - | - | 10.52 ± 0.07 | - | - | - | 14.01 ± 1.38 | - | (4–20°C) |
| Brine 7% | 6.85 ± 0.66 | 7.51 0.72 | 8.67 ± 0.09 | 9.11 ± 0.51 | 7.51 ± 1.04 | 8.25 ± 0.61 | 8.51 ± 1.22 | 9.39 ± 1.31 | (7–10%) (7–13%) (10– 13%) (3–48 h) | ||
| Brine 10% | 9.90 ± 0.53 | 11.04 ± 1.19 | 11.48 ± 0.06 | 11.92 ± 0.94 | 9.93 ± 0.20 | 10.08 ± 0.64 | 11.94 ± 0.86 | 12.43 ± 1.88 | |||
| Brine 13% | 10.16 ± 0.35 | 10.63 ± 0.02 | 12.82 ± 1.18 | 15.46 ± 2.37 | 11.33 ± 0.83 | 11.24 ± 1.06 | 16.02 ± 1.38 | 19.54 ± 2.21 | |||
| Fracturability (N) | 4.80 ± 0.55 | Dry salting | - | - | 8.07 ± 0.53 | - | - | - | 10.27 ± 0.53 | - | (4–20°C) |
| Brine 7% | 4.65 ± 0.32 | 5.50 ± 0.322 | 6.04 ± 0.24 | 6.68 ± 0.64 | 6.63 ± 0.75 | 6.16 ± 0.46 | 7.29 ± 0.21 | 8.43 ± 1.21 | (7–10%) (7–13%) (10–13%) (3–48 h) (6–48 h) | ||
| Brine 10% | 7.15 ± 0.33 | 7.83 ± 0.4 | 9.01 ± 0.17 | 10.30 ± 0.24 | 7.76 ± 0.16 | 8.45 ± 0.38 | 10.51 ± 0.73 | 12.16 ±0.44 | |||
| Brine 13% | 8.41 ± 0.09 | 9.65 ± 0.15 | 11.10 ±0.41 | 13.91 ± 0.47 | 9.07 ± 0.14 | 10.66 ±0.31 | 13.88 ± 1.11 | 18.72 ±1.65 | |||
| Adhesiveness (Ν.mm) | 6.83 ± 3.07 | Dry salting | - | - | 11.30 ± 2.08 | - | - | - | 7.00 ± 0.66 | - | (4–20°C) |
| Brine 7% | 10.58 ± 3.41 | 4.59 ± 1.03 | 9.63 ±2.23 | 11.93 ± 3.89 | 9.52 ± 0.47 | 5.47 ±0.33 | 7.67 ± 2.17 | 9.42 ± 3.27 | (7–13%) | ||
| Brine 10% | 11.95 ± 3.14 | 8.09 ± 1.54 | 10.43 ± 3.07 | 12.04 ±1.93 | 11.73 ±1.3 | 11.10 ± 2.74 | 7.67 ± 1.34 | 9.59 ± 4.68 | |||
| Brine 13% | 13.15 ± 4.16 | 10.76 ± 2.19 | 12.76 ± 3.6 | 17.24 ± 1.56 | 12.25 ± 1.73 | 11.74 ± 5.73 | 10.38 ± 3.1 | 12.11 ± 5.65 | |||
| Elasticity (mm) | 0.95 ± 0.01 | Dry salting | - | - | 0.92 ± 0.02 | - | - | - | 0.91 ± 0.03 | - | NS |
| Brine 7% | 0.93 ± 0.86 | 0.93 ± 0.01 | 0.94 ± 0.9 | 0.95 ± 0.01 | 0.93 ±0.86 | 0.94 ± 0.02 | 0.94 ± 0.01 | 0.95 ± 0.01 | NS | ||
| Brine 10% | 0.93 ± 0.02 | 0.94 ± 0.02 | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.93 ± 0.01 | 0.94 ± 0.86 | 0.94 ± 0.02 | 0.94 ± 0.02 | NS | ||
| Brine 13% | 0.94 ± 0.02 | 0.94 ± 0.01 | 0.93 ± 0.02 | 0.93 ±0.02 | 0.92 ± 0.01 | 0.92 ± 0.01 | 0.93 ± 0.02 | 0.92 ± 0.01 | NS | ||
| Cohesiveness | 0.47 ± 0.01 | Dry salting | - | - | 0.48 ± 0.01 | - | - | - | 0.48 ± 0.01 | - | NS |
| Brine 7% | 0.52 ± 0.01 | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.50 ± 0.01 | 0.48 ± 0.01 | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.48 ± 0.01 | NS | ||
| Brine 10% | 0.51 ± 0.01 | 0.49 ± 0.01 | 0.49 ± 0.01 | 0.49 ± 0.01 | 0.49 0.01 | 0.49 ± 0.01 | 0.48 ± 0.01 | 0.48 ± 0.01 | NS | ||
| Brine 13% | 0.49 ± 0.01 | 0.50 ± 0.01 | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.48 ± 0.01 | 0.49 ± 0.01 | 0.48 ± 0.01 | 0.47 ± 0.01 | NS | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminarides, S.; Moschopoulou, E.; Karali, F. Influence of Salting Method on the Chemical and Texture Characteristics of Ovine Halloumi Cheese. Foods 2019, 8, 232. https://doi.org/10.3390/foods8070232
Kaminarides S, Moschopoulou E, Karali F. Influence of Salting Method on the Chemical and Texture Characteristics of Ovine Halloumi Cheese. Foods. 2019; 8(7):232. https://doi.org/10.3390/foods8070232
Chicago/Turabian StyleKaminarides, Stelios, Ekaterini Moschopoulou, and Fotini Karali. 2019. "Influence of Salting Method on the Chemical and Texture Characteristics of Ovine Halloumi Cheese" Foods 8, no. 7: 232. https://doi.org/10.3390/foods8070232
APA StyleKaminarides, S., Moschopoulou, E., & Karali, F. (2019). Influence of Salting Method on the Chemical and Texture Characteristics of Ovine Halloumi Cheese. Foods, 8(7), 232. https://doi.org/10.3390/foods8070232





