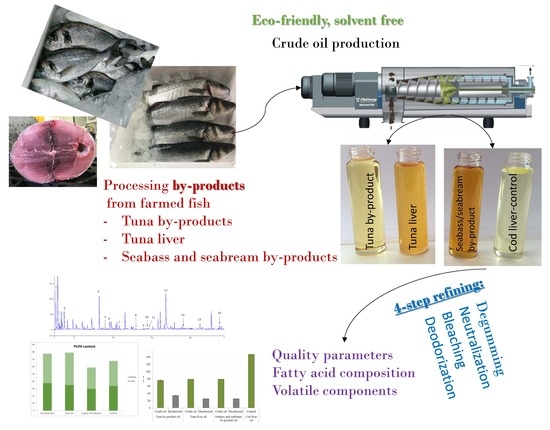
Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Raw Materials and Fish Oil Extraction
2.3. Oil Refinement Process
2.4. Analytical Methodologies
2.5. Tocopherol Content
2.6. Fatty Acid Profile
2.7. Analysis of Volatiles
2.8. Statistical Analysis
3. Results and Discussion
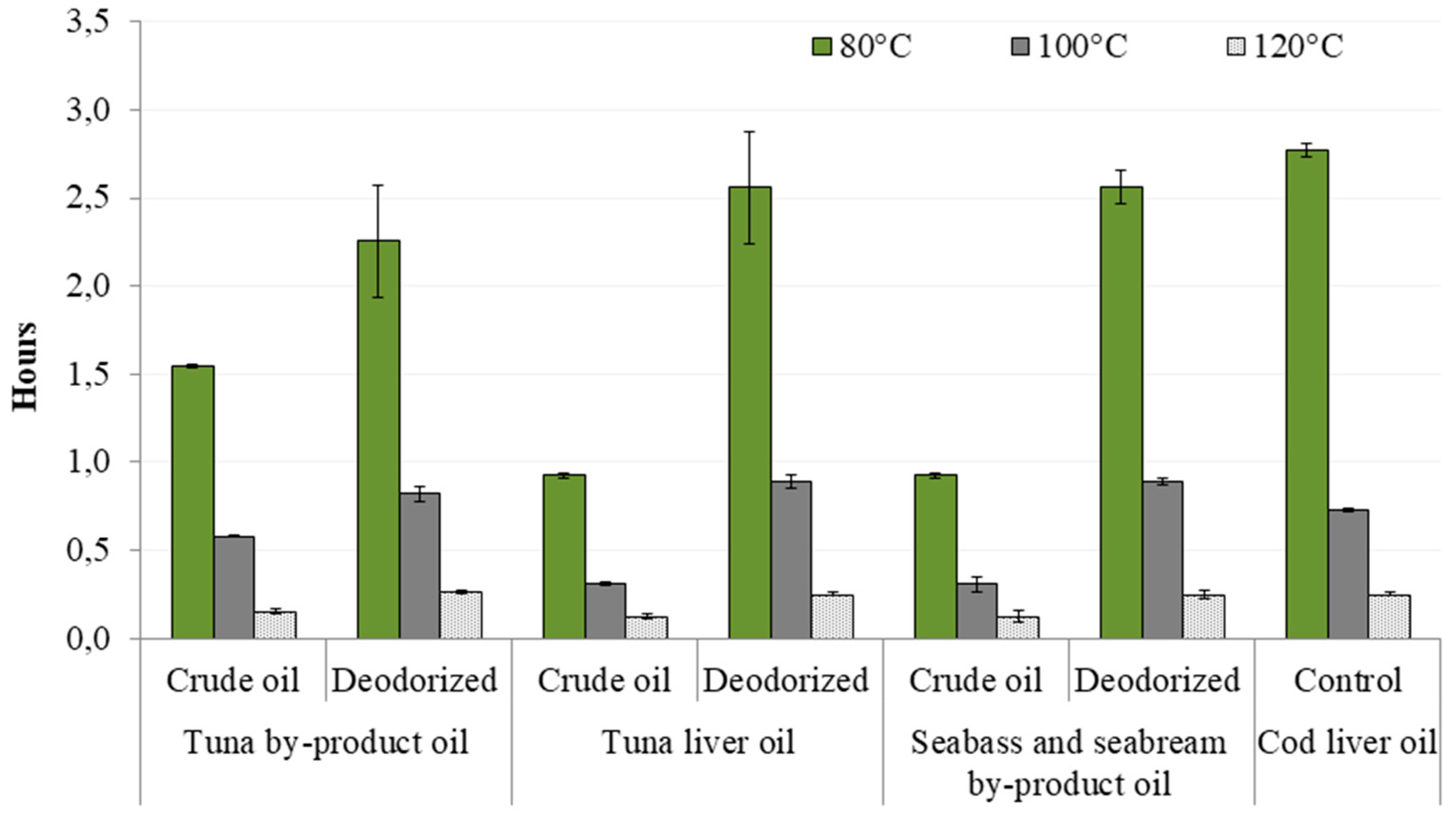
3.1. Chemical Characterization and Oxidative Stability of Fish Oils
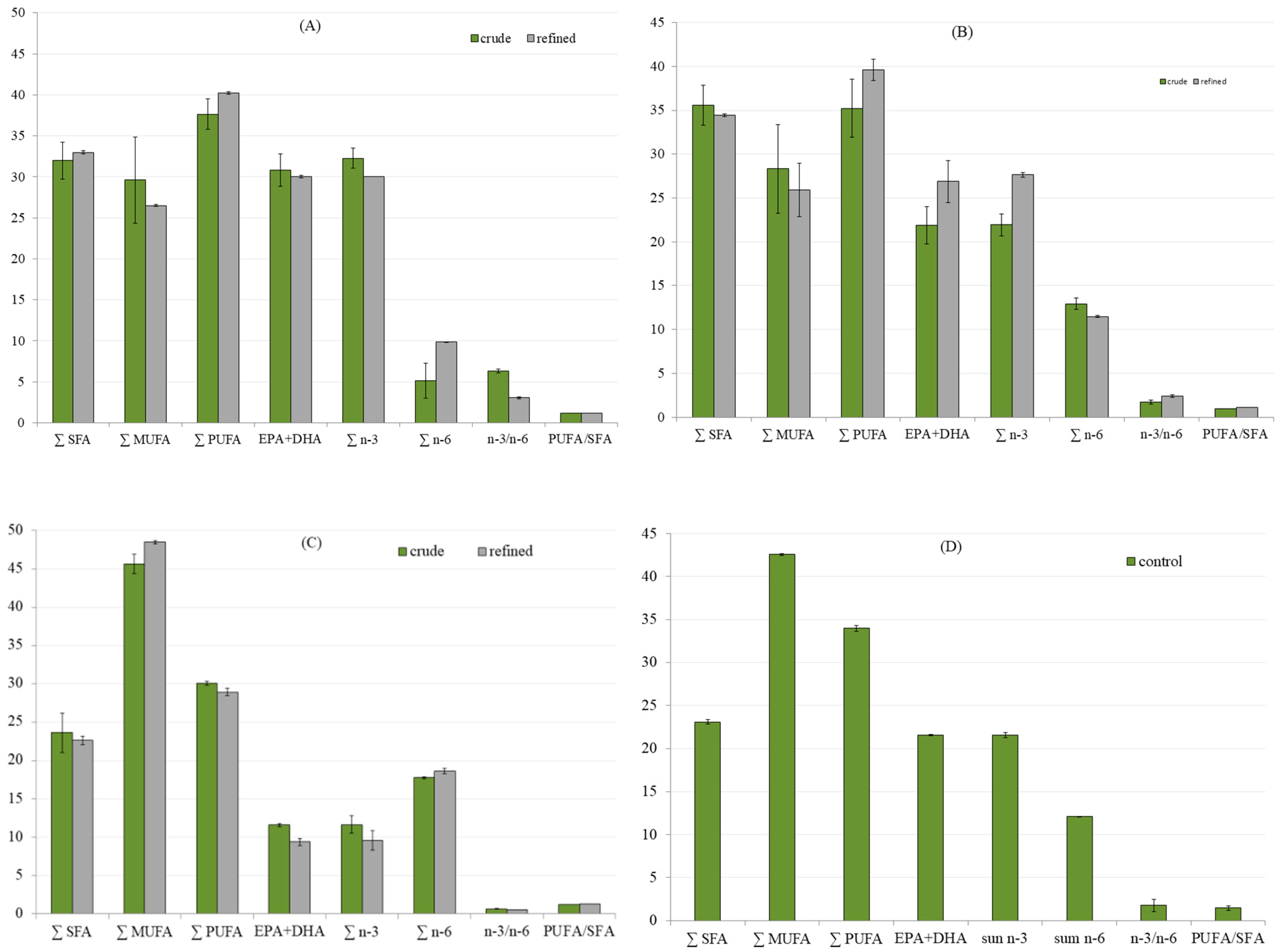
3.2. Fatty Acid Profile of Oils
3.3. Volatile Profile of Oils
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; p. 227. [Google Scholar]
- Constantinos, C.M.; De La Gándara, F.; Corriero, A.; Belmonte Ríos, A. Atlantic bluefin tuna (Thunnus Thynnus) farming and fattening in the Mediterranean Sea. Rev. Fish. Sci. 2010, 18, 266–280. [Google Scholar] [CrossRef]
- Rustad, T. Utilization of marine by-product. EJEAFChe 2003, 2, 458–463. [Google Scholar]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Arason, S.; Karlsdottir, M.; Valsdottir, T.; Slizyte, R.; Rustad, T.; Falch, E.; Eysturskard, J.; Jakobsen, G. Maximum Resource Utilisation—Value Added Fish By-Products; Nordic Innovation Centre: Oslo, Norway, 2009; p. 108. Available online: http://nordicinnovation.org/Global/_Publications/Reports/2010/Maximum%20resource%20utilisation%20-%20Value%20added%20fish%20by-products.pdf (accessed on 24 January 2019).
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef]
- Kara, K.; Ouanji, F.; El Lotfia, M.; El Mahia, M.; Kacimi, M.; Ziyad, M. Biodiesel production from waste fish oil with high free fatty acid content from Moroccan fish-processing industries. Egypt. J. Pet. 2017, 27, 249–255. [Google Scholar] [CrossRef]
- Rustad, T.; Storro, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Menegazzo, M.L.; Petenuci, M.E.; Fonseca, G.G. Production and characterization of crude and refined oils obtained from the co-products of Nile tilapia and hybrid sorubim processing. Food Chem. 2014, 157, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sathivel, S. Purifying salmon oil using adsorption, neutralization, and a combined neutralization and adsorption process. J. Food Eng. 2010, 96, 51–58. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, Z.I.; Norulaini, N.; Oliviera, A.; Yunus, K.; Choedury, A.J.; Arkanda, J.; Omar, M. Quality of tuna fish oils extracted from processing the by- products of three species of neritic tuna using supercritical carbon dioxide. J. Food Process Preserv. 2015, 39, 432–441. [Google Scholar] [CrossRef]
- Kuo, C.H.; Liao, H.Z.; Wang, Y.H.; Wang, H.M.D.; Shieh, C.J.; Tseng, C.Y. Highly efficient extraction of EPA/DHA-enriched oil from cobia liver using homogenization plus sonication. Eur. J. Lipid Sci. Technol. 2017, 119, 1600466. [Google Scholar] [CrossRef]
- Nazir, N.; Diana, A.; Sayuti, K. Physicochemical and fatty acid profile of fish oil from head of tuna (Thunnus albacares) extracted from various extraction method. IJASEIT 2017, 7, 709–715. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D. Production and characterization of refined oils obtained from Indian oil sardine (Sardinella longiceps). J. Agric. Food Chem. 2015, 63, 998–1009. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Generalić Mekinić, I.; Čagalj, M.; Hamed, I.; Skroza, D. The quality of crude marine oils from seafood processing by-products: Production and characterization. Food Biosci. 2019, in press. [Google Scholar]
- AOCS. The Official Methods and Recommended Practices of the American Oil Chemists’ Society; The American Oil Chemists’ Society: Champaign, IL, USA, 1994. [Google Scholar]
- Ke, P.; Woyewoda, A. Microdetermination of thiobarbituric acid values in marine lipids by a direct spectrophotometric method with a monophasic reaction system. Anal. Chim. Acta 1979, 106, 279–284. [Google Scholar] [CrossRef]
- Šimat, V.; Ficović, M.; Čagalj, M.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Preventive effect of herb extracts on lipid oxidation in fish oil. Croat. J. Food Technol. Biotechnol. Nutr. 2017, 12, 30–36. [Google Scholar]
- Soldo, B.; Šimat, V.; Vlahović, J.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Sardine by-products as an alternative to whole sardines in oil production: Chemical composition and quality parameters during refining process. Eur. J. Lipid Sci. Technol. 2019, in press. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [PubMed]
- Šimat, V.; Vlahović, J. University Department of Marine Studies, University of Split, Split, Croatia. Unpublished work. 2016. [Google Scholar]
- Honold, P.J.; Nouard, M.-L.; Jacobsen, C. Fish oil extracted from fish-fillet by-products is weakly linked to the extraction temperature but strongly linked to the omega-3 content of the raw material. Eur. J. Lipid Sci. Technol. 2016, 118, 874–884. [Google Scholar] [CrossRef]
- Ceriani, R.; Paiva, F.R.; Goncalves, C.B.; Batista, E.A.C.; Meirelles, A.J.A. Densities and viscosities of vegetable oils of nutritional value. Chem. Eng. Data 2008, 53, 1846–1853. [Google Scholar]
- Bimbo, A.P. Guidelines for characterization food-grade fish oil. Inform 1998, 9, 473–483. [Google Scholar]
- Özyurt, G.; Şimşek, A.; Etyemez, M.; Polat, A. Fatty acid composition and oxidative stability of fish oil products in Turkish retail market. J. Aquat. Food Prod. Technol. 2013, 22, 322–329. [Google Scholar] [CrossRef]
- Vaisali, C.; Charanyaa, S.; Belur, P.D.; Regupathi, I. Refining of edible oils: A critical appraisal of current and potential technologies. Int. J. Food Sci. Technol. 2015, 50, 13–23. [Google Scholar] [CrossRef]
- Bako, T.; Victor, U.I.; Awulu, O. Criteria for the extraction of fish oil. Agric. Eng. Int. CIGR J. 2017, 19, 120–132. [Google Scholar]
- FAO/WHO. Proposed draft standard for fish oils. In Proceedings of the 23rd Session Codex Committee on Fats and Oils, Langkawi, Malaysia, 25 February–1 March 2013. [Google Scholar]
- Codex Alimentarius Commision. Standards for Fish Oils; CXS 329-2017 1–6; Codex Alimentarius Commision: Rome, Italy, 2017. [Google Scholar]
- Crexi, V.T.; Monte, M.L.; Soares, L.A.S.; Pinto, L.A.A. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chem. 2010, 119, 945–950. [Google Scholar] [CrossRef]
- Deepika, D.; Vegneshwaran, V.R.; Julia, P.; Sukhinder, K.C.; Sheila, T.; Heather, M.; Wade, M. Investigation on oil extraction methods and its influence on omega-3 content from cultured salmon. J. Food Process Technol. 2014, 5, 12. [Google Scholar] [CrossRef]
- Sabliov, C.M.; Fronczek, C.; Astete, C.E.; Khachaturyan, M.; Khachatryan, L.; Leonardi, C. Effects of temperature and UV light on degradation of α-tocopherol in free and dissolved form. J. Am. Oil Chem. Soc. 2009, 86, 895–902. [Google Scholar] [CrossRef]
- Song, G.; Dai, Z.; Shen, Q.; Peng, X.; Zhang, M. Analysis of the changes in volatile compound and fatty acid profiles of fish oil in chemical refining process. Eur. J. Lipid Sci. Technol. 2017, 120, 1700219. [Google Scholar] [CrossRef]
- FAO. Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation; FAO: Rome, Italy, 2010; p. 166. [Google Scholar]
- Šimat, V.; Bogdanović, T.; Poljak, V.; Petričević, S. Changes in fatty acid composition, atherogenic and thrombogenic health lipid indices and lipid stability of bogue (Boops boops Linnaeus, 1758) during storage on ice: Effect of fish farming activities. J. Food Comp. Anal. 2015, 40, 120–125. [Google Scholar] [CrossRef]
- Cameron-Smith, D.; Albert, B.B.; Cutfield, W.S. Fishing for answers: Is oxidation of fish oil supplements a problem? J. Nutr. Sci. 2015, 4, e36. [Google Scholar] [CrossRef]
- Chung, H.; Choi, A.; Cho, I.H.; Kim, Y.-S. Changes in fatty acids and volatile components in mackerel by broiling. Eur. J. Lipid Sci. Technol. 2011, 113, 1481–1490. [Google Scholar] [CrossRef]
- Jónsdóttir, R.; Bragadóttir, M.; Arnarson, G.Ö. Oxidatively derived volatile compounds in microencapsulated fish oil monitored by solid-phase microextraction (SPME). J. Food Sci. 2005, 70, 433–440. [Google Scholar] [CrossRef]
- Roh, H.S.; Park, J.Y.; Park, S.Y.; Chun, B.S. Isolation of off-flavours from tuna fish oil using supercritical carbon dioxide. Biotechnol. Bioprocess Eng. 2006, 11, 496–502. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques: Review. Am. J Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Giogios, I.; Grigorakis, K.; Nengas, I.; Papasolomontos, S.; Papaioannou, N.; Alexis, M.N. Fatty acid composition and volatile compounds of selected marine oils and meals. J Sci. Food Agric. 2008, 89, 88–100. [Google Scholar] [CrossRef]
- De Oliveira, D.; Minozzo, M.G.; Licodiedoff, S.; Waszczynskyj, N. Physicochemical and sensory characterization of refined and deodorized tuna (Thunnus albacares) by-product oil obtained by enzymatic hydrolysis. Food Chem. 2016, 207, 187–194. [Google Scholar] [CrossRef]

| Oil Samples | Refining Phase | Measured Parameter | ||||
|---|---|---|---|---|---|---|
| FFA (% Oleic Acid) | PV (meq O2/kg) | p-AV | TOTOX | TBARS (μM/g) | ||
| Tuna by-product oil | Crude oil | 2.94 ± 0.12 a | 2.31 ± 0.73 a | 25.2 ± 0.08 a | 29.7 ± 0.20 a | 0.94 ± 0.33 a |
| Degummed | 3.51 ± 0.03 b | 3.93 ± 0.18 b | 19.2 ± 1.25 b | 27.1 ± 0.79 b | 1.02 ± 0.14 a | |
| Neutralized | 3.13 ± 0.10 b | 3.46 ± 0.39 b | 16.9 ± 1.23 c | 23.8 ± 1.06 c | 2.02 ± 0.45 b | |
| Bleached | 2.93 ± 0.04 a | 2.88 ± 0.11 ab | 14.4 ± 0.90 d | 20.2 ± 1.34 d | 1.88 ± 0.37 b | |
| Deodorized | 2.83 ± 0.01a | 3.78 ± 0.22 b* | 19.5 ± 0.94 b | 27.1 ± 2.15 b | 1.14 ± 0.11 a | |
| Tuna liver oil | Crude oil | 3.13 ± 0.00 a | 2.68 ± 0.86 a | 20.9 ± 1.34 a | 26.0 ± 0.14 a | 2.88 ± 0.73 a |
| Degummed | 2.52 ± 0.05 b | 3.01 ± 0.05 ab | 19.1 ± 2.47 b | 25.2 ± 1.17 a | 1.35 ± 0.02 b | |
| Neutralized | 2.65 ± 0.11 c | 2.89 ± 0.22 ab | 17.0 ± 4.78 c | 22.7 ±1.22 b | 1.76 ± 0.03 b | |
| Bleached | 2.17 ± 0.03 b | 2.85 ± 0.53 ab | 14.0 ± 0.65 d | 19.7 ± 0.43 c | 1.92 ± 0.35 b | |
| Deodorized | 2.12 ± 0.16 b | 4.25 ± 0.28 c | 19.2 ± 2.22 b | 27.7 ± 2.22 a | 1.97 ± 0.53 b | |
| Seabass and gilthead seabream by-product oil | Crude oil | 2.41 ± 0.47 a | 4.63 ± 1.07 a | 18.1 ± 0.19 ab | 19.3 ± 2.45 a | 0.53 ± 0.05 a |
| Degummed | 1.66 ± 0.05 b | 2.70 ± 0.18 b | 15.7 ± 1.12 c | 20.2 ± 0.12 a | 3.46 ± 2.08 b | |
| Neutralized | 1.43 ± 0.18 b | 3.13 ± 0.31 b | 12.7 ± 0.65 a | 18.9 ± 0.26 a | 2.24 ± 1.05 bc | |
| Bleached | 1.40 ± 0.01 b | 3.44 ± 0.25 ab | 11.4 ± 0.19 b | 18.4 ± 0.52 a | 1.14 ± 0.10 ac | |
| Deodorized | 1.28 ± 0.15 b | 4.20 ± 0.15 ab | 16.5 ± 0.39 c | 24.7 ± 0.32 b | 1.42 ± 0.53 ac* | |
| Cod liver oil | Control | 2.15 ± 0.08 | 3.85 ± 0.44 | 13.3 ± 1.11 | 21.0 ± 0.18 | 1.63 ± 0.07 |
| Fatty Acid | Tuna By-Product Oil | Tuna Liver Oil | Seabass and Seabream By-Product Oil | Cod Liver Oil | |||
|---|---|---|---|---|---|---|---|
| Crude Oil | Refined | Crude Oil | Refined | Crude Oil | Refined | Control | |
| C12:0 | n.d. 1 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.02 |
| C13:0 | n.d. | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.02 | n.d. | 0.02 ± 0.00 | 0.01 ± 0.01 |
| C14:0 | 6.08 ± 0.98 | 6.05 ± 0.00 | 6.60 ± 0.49 | 6.50 ± 0.25 | 2.31 ± 0.02 | 2.57 ± 0.14 | 4.64 ± 0.02 |
| C14:1 | 0.46 ± 0.65 | 0.04 ± 0.06 | 0.91 ± 0.06 | n.d. | 0.36 ± 0.00 | 0.40 ± 0.00 | 0.39 ± 0.03 |
| C15:0 | 0.37 ± 0.52 | 0.82 ± 0.01 | 0.07 ± 0.00 | 0.88 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.00 | 0.12 ± 0.01 |
| C15:1 | n.d. | 0.01 ± 0.01 | 0.01 ± 0.00 | n.d. | n.d. | n.d. | n.d. |
| C16:0 | 18.20 ± 2.12 | 18.09 ± 0.23 | 19.85 ± 1.54 | 18.97 ± 0.82 | 12.70 ± 0.02 | 13.87 ± 0.23 | 12.16 ± 0.18 |
| C16:1 | 6.14 ± 0.74 | 6.09 ± 0.06 | 5.61 ± 0.43 | 5.40 ± 0.22 | 3.55 ± 0.09 | 3.88 ± 0.17 | 8.81 ± 0.01 |
| C17:0 | 0.67 ± 0.07 | 0.65 ± 0.00 | 0.75 ± 0.06 | 0.71 ± 0.03 | 0.32 ± 0.02 | 0.34 ± 0.01 | 0.19 ± 0.02 |
| C17:1 | 0.46 ± 0.10 | 0.44 ± 0.01 | 0.40 ± 0.04 | 0.35 ± 0.02 | 0.27 ± 0.01 | 0.29 ± 0.01 | 0.35 ± 0.02 |
| C18:0 | 4.28 ± 0.36 | 4.23 ± 0.01 | 4.97 ± 0.36 | 4.66 ± 0.08 | 2.63 ± 0.01 | 0.17 ± 0.00 | 3.21 ± 0.34 |
| C18:1n-9t | 2.64 ± 0.41 | 0.16 ± 0.00 | 0.17 ± 0.02 | 0.16 ± 0.01 | 0.12 ± 0.00 | 0.15 ± 0.00 | 0.14 ± 0.00 |
| C18:1n-9c | 13.92 ± 1.14 | 13.73 ± 0.18 | 13.42 ± 1.09 | 13.05 ± 0.09 | 37.97 ± 0.08 | 40.52 ± 0.72 | 18.73 ± 0.01 |
| C18:2n-6t | 0.06 ± 0.08 | 0.05 ± 0.03 | 0.08 ± 0.09 | 0.13 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.08 ± 0.00 |
| C18:2n-6c | 1.04 ± 1.47 | 2.18 ± 0.01 | 2.28 ± 0.18 | 2.13 ± 0.02 | 16.35 ± 0.02 | 17.32 ± 0.26 | 1.89 ± 0.02 |
| C20:0 | 0.42 ± 0.03 | 0.42 ± 0.00 | 0.47 ± 0.04 | 0.43 ± 0.01 | 0.51 ± 0.00 | 0.39 ± 0.01 | 0.29 ± 0.01 |
| C18:3n-6 | 0.39 ± 0.55 | 0.13 ± 0.00 | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.19 ± 0.00 | 0.19 ± 0.00 | 0.15 ± 0.00 |
| C20:1 | 5.07 ± 0.20 | 5.08 ± 0.02 | 6.50 ± 0.56 | 5.84 ± 0.04 | 2.47 ± 0.00 | 2.50 ± 0.05 | 12.67 ± 0.04 |
| C18:3n-3 | 1.41 ± 0.12 | n.d. | n.d. | 0.65 ± 0.06 | 0.02 ± 0.02 | 0.18 ± 0.00 | n.d. |
| C21:0 | n.d. | 1.44 ± 0.04 | 1.48 ± 0.13 | 0.67 ± 0.08 | 4.28 ± 0.01 | 4.46 ± 0.05 | 0.90 ± 0.02 |
| C20:2 | 0.28 ± 0.01 | 0.27 ± 0.01 | 0.31 ± 0.03 | 0.11 ± 0.01 | 0.59 ± 0.01 | 0.58 ± 0.02 | 0.30 ± 0.02 |
| C22:0 | 0.08 ± 0.11 | 0.15 ± 0.01 | 0.18 ± 0.02 | 0.17 ± 0.02 | 0.19 ± 0.02 | 0.17 ± 0.01 | 0.14 ± 0.00 |
| C20:3n-6 | 3.54 ± 5.01 | 7.32 ± 0.02 | 10.25 ± 0.92 | 9.04 ± 0.17 | 1.04 ± 0.00 | 0.97 ± 0.01 | 9.89 ± 0.02 |
| C22:1n-9 | 0.32 ± 0.01 | 0.30 ± 0.00 | 0.39 ± 0.03 | 0.37 ± 0.01 | 0.46 ± 0.03 | 0.43 ± 0.01 | 1.00 ± 0.02 |
| C20:3n-3 | n.d. | 0.02 ± 0.00 | 0.05 ± 0.00 | 0.11 ± 0.11 | 0.03 ± 0.00 | 0.02 ± 0.00 | n.d. |
| C20:4n-6 | 0.19 ± 0.13 | 0.18 ± 0.00 | 0.19 ± 0.01 | 0.09 ± 0.14 | 0.16 ± 0.00 | 0.16 ± 0.00 | 0.16 ± 0.01 |
| C23:0 | 1.02 ± 0.08 | 0.97 ± 0.00 | 1.01 ± 0.07 | 1.92 ± 0.01 | 0.51 ± 0.01 | 0.48 ± 0.01 | 0.58 ± 0.01 |
| C22:2 | n.d. | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.36 ± 0.07 | 0.05 ± 0.01 | 0.05 ± 0.00 | 0.02 ± 0.01 |
| C24:0 | 0.87 ± 0.04 | 0.02 ± 0.00 | 0.07 ± 0.01 | 0.40 ± 0.02 | 0.07 ± 0.00 | 0.05 ± 0.01 | 0.82 ± 0.04 |
| C20:5n-3 | 9.56 ± 0.76 | 9.29 ± 0.04 | 8.81 ± 0.68 | 8.21 ± 0.03 | 3.33 ± 0.03 | 3.04 ± 0.00 | 9.58 ± 0.06 |
| C24:1 | 0.60 ± 0.02 | 0.65 ± 0.00 | 0.90 ± 0.09 | 0.77 ± 0.14 | 0.45 ± 0.01 | 0.34 ± 0.04 | 0.48 ± 0.00 |
| C22:6n-3 | 21.29 ± 1.09 | 20.75 ± 0.19 | 13.07 ± 7.00 | 18.66 ± 1.21 | 8.26 ± 0.24 | 6.37 ± 0.47 | 11.9 ± 0.26 |
| Volatile Compound | Tuna By-Product Oil | Tuna Liver Oil | Seabass and Seabream By-Product Oil | Cod Liver Oil | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Bleached | Deodorized | Crude | Bleached | Deodorized | Crude | Bleached | Deodorized | Control | ||
| Esters | Ethyl acetate | 1.51 ± 0.14 a | 1.45 ± 0.19 a | 1.00 ± 0.02 b* | 0.93 ± 0.28 a | 1.03 ± 0.29 a | 1.19 ± 0.01 a* | 0.50 ± 0.03 a | 1.69 ± 0.25 b | 0.99 ± 0.07 c* | 0.73 ± 0.07 |
| Aldehydes | Pentanal | 1.12 ± 0.14 a | 1.55 ± 0.06 a | 1.94 ± 0.32 a* | 0.95 ± 0.20 ab | 0.81 ± 0.05 ab | 0.54 ± 0.01 bc | 2.09 ± 0.05 a | 1.53 ± 0.11 b | 0.55 ± 0.04 c | 1.22 ± 0.11 |
| E-2-hexenal | 0.08 ± 0.01 a | 0.05 ± 0.01 a | 0.05 ± 0.02 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.02 ± 0.0 a | 0.01 ± 0.0 a | 0.01 ± 0.0 a | 0.02 ± 0.0 a | 0.51 ± 0.04 | |
| Octanal | 0.29 ± 0.25 a | 0.53 ± 0.0 a | 0.39 ± 0.24 a* | 1.15 ± 0.08 a | 0.41 ± 0.20 a | 0.45 ± 0.10 a* | 0.34 ± 0.13 ab | 0.31 ± 0.02 ab | 0.15 ± 0.09 a* | 0.14 ± 0.02 | |
| (E,Z)-2,6-nonadienal | 0.98 ± 0.21 a | 1.07 ± 0.14 a | 0.85 ± 0.13 a | 0.91 ± 0.01 a | 1.20 ± 0.16 a | 1.08 ± 0.08 a | 0.40 ± 0.06 a | 0.62 ± 0.09 b | 0.65 ± 0.03 b | 0.14 ± 0.0 | |
| (E,E)-2,4-decadienal | 4.59 ± 0.28 a | 6.06 ± 0.57 b | 4.94 ± 0.35 ab | 6.28 ± 1.19 a | 6.25 ± 0.70 a | 6.76 ± 0.42 a* | 11.5 ± 0.46 a | 10.3 ± 0.18 a | 14.1 ± 0.54 b | 6.80 ± 0.21 | |
| 2,4-nonadienal | 0.67 ± 0.04 a | 1.03 ± 0.14 a | 1.02 ± 0.13 a | 2.09 ± 0.16 a | 0.93 ± 0.13 b | 0.96 ± 0.13 b | 6.53 ± 0.58 a | 6.59 ± 0.49 a | 4.77 ± 0.02 b | 0.26 ± 0.05 | |
| Alcohols | 1-penten-3-ol | 0.08 ± 0.0 a | 0.05 ± 0.01 ab | 0.01 ± 0.01 b* | 11.17 ± 0.20 a | 2.29 ± 1.31 b | 1.03 ± 0.0 b | 0.65 ± 0.01 a | 0.37 ± 0.02 b | 0.07 ± 0.0 c | 0.02 ± 0.0 |
| 4-methylpenten-2-ol | 2.66 ± 0.07 a | 1.42 ± 0.17 b | 2.15 ± 0.57 a | 1.14 ± 0.03 a | 1.08 ± 0.57 a | 1.46 ± 0.0 a* | 0.57 ± 0.03 a | 1.55 ± 0.10 b | 1.91 ± 0.10 c | 1.14 ± 0.03 | |
| Hexanol | 0.08 ± 0.01 a | 0.03 ± 0.01 b | 0.05 ± 0.03 b* | 0.06 ± 0.0 a | 0.03 ± 0.01 a | 0.04 ± 0.0 a* | 0.06 ± 0.0 a | 0.02 ± 0.0 b | 0.03 ± 0.0 c | 0.06 ± 0.01 | |
| E-2-hexen-1-ol | 0.08 ± 0.0 a | 0.06 ± 0.04 ab | 0.11 ± 0.03 a | 0.01 ± 0.01 a | 0.13 ± 0.09 a | 0.23 ± 0.02 a | 0.04 ± 0.0 a | 0.12 ± 0.01 b | 0.23 ± 0.02 c | n.d. | |
| Z-3-hexen-1-ol | 0.05 ± 0.01 ab | 0.04 ± 0.01 ab | 0.03 ± 0.0 a | 0.05 ± 0.01 a | 0.20 ± 0.14 a | 0.06 ± 0.0 a* | 0.01 ± 0.01 a | 0.06 ± 0.0 b | 0.02 ± 0.0 a | 0.11 ± 0.01 | |
| Hydrocarbons | Dodecane | 3.85 ± 0.58 a | 4.03 ± 0.11 a | 3.50 ± 0.55 a | 3.22 ± 0.15 a | 4.41 ± 0.59 a | 3.23 ± 0.74 a | 1.17 ± 0.11 a | 1.79 ± 0.59 a | 1.39 ± 0.08 a | 0.76 ± 0.02 |
| Tetradecane | 1.13 ± 0.22 a | 1.19 ± 0.26 a | 0.71 ± 0.41a* | 0.85 ± 0.01 a | 0.60 ± 0.07 b | 1.09 ± 0.10 c | 1.18 ± 0.06 a | 1.00 ± 0.06 a | 1.09 ± 0.15 a | 0.51 ± 0.01 | |
| Tetradecene | 1.22 ± 0.02 a | 1.17 ± 0.21 a | 1.09 ± 0.15 a | 1.32 ± 0.19 a | 1.18 ± 0.08 a | 1.02 ± 0.10 a | 2.66 ± 0.16 a | 2.49 ± 0.37 a | 2.31 ± 0.07 a | 8.62 ± 0.52 | |
| 2,4-heptadienal +pentadecan | 174 ± 22.6 a | 208 ± 27.5 b | 160 ± 19.6 a | 146 ± 11.5 a | 164 ± 10.5 a | 172 ± 9.43 a | 36.0 ± 4.65 a | 51.4±8.07 a | 86.5 ± 0.52 b | 11.0 ± 0.11 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimat, V.; Vlahović, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods 2019, 8, 125. https://doi.org/10.3390/foods8040125
Šimat V, Vlahović J, Soldo B, Skroza D, Ljubenkov I, Generalić Mekinić I. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods. 2019; 8(4):125. https://doi.org/10.3390/foods8040125
Chicago/Turabian StyleŠimat, Vida, Jelena Vlahović, Barbara Soldo, Danijela Skroza, Ivica Ljubenkov, and Ivana Generalić Mekinić. 2019. "Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species" Foods 8, no. 4: 125. https://doi.org/10.3390/foods8040125
APA StyleŠimat, V., Vlahović, J., Soldo, B., Skroza, D., Ljubenkov, I., & Generalić Mekinić, I. (2019). Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods, 8(4), 125. https://doi.org/10.3390/foods8040125