Effect of the Plastein Reaction in Presence of Extrinsic Amino Acids on the Protective Activity of Casein Hydrolysate against Ethanol-Induced Damage in HHL-5 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation and Modification of Casein Hydrolysate
2.3. Assays of Protein and Free Amino Contents
2.4. Antioxidant Activity Assays
2.4.1. Ferrous Reducing Power Activity Assay
2.4.2. DPPH Radical-Scavenging Activity Assay
2.5. Cell Culture Conditions and Ethanol-Induced HHL-5 Damage Assay
2.6. Cell Viability Assay
2.7. Cell Cycle Assay
2.8. Cell Apoptosis Assay
2.9. Statistical Analysis
3. Results
3.1. The Free Amino Group Content
3.2. Antioxidant Activity
3.3. Proliferative Effect
3.4. The Effect on the Cell Cycle
3.5. Anti-Apoptosis Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | Casein hydrolysate |
| CH-P | Plastein reaction-modified CH in the absence of extrinsic Phe or Trp |
| CH-P-Phe | Plastein reaction-modified CH in the presence of extrinsic Phe |
| CH-P+Phe | Mixture of CH and extrinsic Phe |
| CH-P-Trp | Plastein reaction-modified CH in the presence of extrinsic Trp |
| CH-P+Trp | Mixture of CH and extrinsic Trp |
| CCK-8 | Cell counting kit-8 |
| EDTA | Ethylenediamine tetra-acetic acid |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
| Trp | Tryptophan |
| Phe | Phenylalanine |
| Ethanol | ETOH |
References
- World Health Organization. The global status report on alcohol and health 2011. Geneva, 2011. Available online: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ (accessed on 14 April 2014).
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Hennig, M.; Yip-Schneider, M.T.; Klein, P.; Matos, J.M.; Doyle, C.; Choi, J.; Wu, H.; O’Mara, A.; Menze, A.; Noble, S.; et al. Ethanol-TGF-a-MEK signal promotes growth of human hepatocellular carcinoma. Food Res. Int. 2009, 154, 187–195. [Google Scholar]
- Minana, J.B.; Gomez-Cambronero, L.; Lloret, A.; Pallardo, F.V.; Del Olmo, J.; Escudero, A.; Rodrigo, J.M.; Pellin, A.; Vina, J.R.; Sastre, J. Mitochondrial oxidative stress and CD95 ligand: A dual mechanism for hepatocyte apoptosis in chronic alcoholism. Hepatology 2002, 35, 1205–1214. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- García, M.C.; Puchalska, P.; Esteve, C.; Marine, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Decker, E.A. Antioxidant mechanisms of caseinophosphorpeptides and casein hydrolysates and their application in ground beef. J. Agr. Food Chem. 2004, 52, 8208–8213. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, J.L.; Ma, H.L.; Yagoub, A.E.A.; Yu, X.J.; Owusu, J.; Ma, H.Y.; Qin, X.P. Antioxidant peptides from corn gluten meal: Orthogonal design evaluation. Food Chem. 2015, 187, 270–278. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras, M.D.M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Hernández-Jabalera, A.; Cortés-Giraldo, I.; Dávila-Ortíz, G.; Vioque, J.; Alaiz, M.; Girón-Calle, J.; Megías, C.; Jiménez-Martínez, C. Influence of peptides–phenolics interaction on the antioxidant profile of protein hydrolysates from Brassica napus. Food Chem. 2015, 178, 346–357. [Google Scholar]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell B. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Zhao, X.H.; Li, Y.Y. An approach to improve ACE-inhibitory activity of casein hydrolysates with plastein reaction catalyzed by Alcalase. Eur. Food Res. Technol. 2009, 229, 795–805. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, X.H. Angiotensin I converting enzyme inhibition and enzymatic resistance in vitro of casein hydrolysate treated by plastein reaction and fractionated with ethanol/water or methanol/water. Int. Dairy J. 2012, 24, 27–32. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Rajendran, S.R.C.K. Old products, new applications? Considering the multiple bioactivities of plastein in peptide-based functional food design. Curr. Opin. Food Sci. 2016, 8, 8–13. [Google Scholar] [CrossRef]
- Zhao, X.H.; Fu, Y.; Yue, N. In vitro cytoprotection of modified casein hydrolysates by plastein reaction on rat hepatocyte cells. J. Food 2014, 12, 40–47. [Google Scholar]
- Xu, J.L.; Pang, J.N.; Chen, F.F.; Li, T.J.; Zhao, X.H. Antihypertensive activities of the plasteins derived from casein hydrolysates in spontaneously hypertensive rats. CyTA-J. Food 2017, 15, 105–109. [Google Scholar] [CrossRef]
- Tomita, M.; Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J. Dairy Sci. 1991, 74, 4137–4142. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Yue, N.; Li, T.J.; Zhao, X.H. The impact of extrinsic amino acids and solvent fractionation on the in vitro antioxidant activity of plastein reaction-stressed casein hydrolysates. Food Technol. Biotech. 2013, 51, 224–232. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthadialdehyde on determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Canabady-Rochelle, L.L.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.M. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 138, 129–135. [Google Scholar] [CrossRef]
- Santos, J.; Brizola, V.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.K.; Osawa, T.; Kawakishi, S. Hydroxyl radical scavenging effects of species and scavengers from brown mustard (Brassica nigra). Biosci. Biotech. Bioch. 1997, 61, 118–123. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, T.J.; Zhao, X.H. Preparation of Alcalase-catalyzed casein plasteins in the presence of proline addition and the ACE-inhibitory activity of the plasteins in vitro. Eur. Food Res. Technol. 2010, 231, 197–207. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wu, D.; Li, T.J. Preparation and radical scavenging activity of papain-catalyzed casein plasteins. Dairy Sci. Tech. 2010, 90, 521–535. [Google Scholar] [CrossRef]
- Sun, H.; Li, T.J.; Zhao, X.H. ACE inhibition and enzymatic resistance in vitro of a casein hydrolysate subjected to plastein reaction in the presence of extrinsic proline and ethanol- or methanol-water fractionation. Int. J. Food Prop. 2014, 17, 386–398. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Zhao, X.H. Pepsin-catalyzed plastein reaction with tryptophan increases the in vitro activity of lactoferrin hydrolysates with BGC-823 cells. Food Biosci. 2019, 28, 109–115. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, I.H.; Chori, Y.H.; Nam, T.J. A peptide from Porphyra yezoensis stimulates the proliferation of IEC-6 cells by activating the insulin-like growth factor I receptor signaling pathway. Int. J. Mol. Med. 2015, 35, 533–538. [Google Scholar] [CrossRef]
- Chen, I.H.; Lin, Z.B.; Li, W.D. Ganoderma lucidum poly-saccharidesexate-induced small intestinal damage in mice via induction of epithelial cell proliferation and migration. Acta Pharmacol. Sin. 2011, 32, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Wang, C.; Ao, J.; Li, B. Non-gastrointestinal-hydrolysis enhances bioavailability and antioxidant efficacy of casein as compared with its in vitro gastrointestinal digest. Food Res. Int. 2013, 51, 114–122. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Ling, Y.F.; Sun, Z.; Zhang, L.; Yu, H.X.; Mburu Kamau, S.; Lu, R.R. Protective effect of whey protein hydrolysates against hydrogen peroxide-induced oxidative stress on PC12 cells. Biotechnol. Lett. 2012, 34, 2001–2006. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, B.; Song, S.J.; Ma, M.; Si, S.Y.; Wang, Y.H.; Xu, B.X.; Feng, K.; Wu, J.G.; Guo, Y.C. Bovine collagen peptides compounds promote the proliferation and differentiation of MC3T3-E1 pre-osteoblasts. PLoS ONE 2014, 9, e99920. [Google Scholar] [CrossRef]
- Blais, A.; Fan, C.; Voisin, T.; Aattouri, N.; Dubarry, M.; Blachier, F.; Tome, D. Effects of lactoferrin on intestinal epithelial cell growth and differentiation: An in vivo and in vitro study. Biometals 2014, 27, 857–874. [Google Scholar] [CrossRef]
- Pan, X.W.; Zhao, X.H. In vitro proliferation and anti-apoptosis of the papain-generated casein and soy protein hydrolysates towards osteoblastic cells (hFOB1.19). Int. J. Mol. Sci. 2015, 16, 13908–13920. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, X.H. In vitro responses of hFOB1.19 cells towards chum salmon (Oncorhynchus keta) skin gelatin hydrolysates in cell proliferation, cycle progression and apoptosis. J. Funct. Food 2013, 279–288. [Google Scholar] [CrossRef]
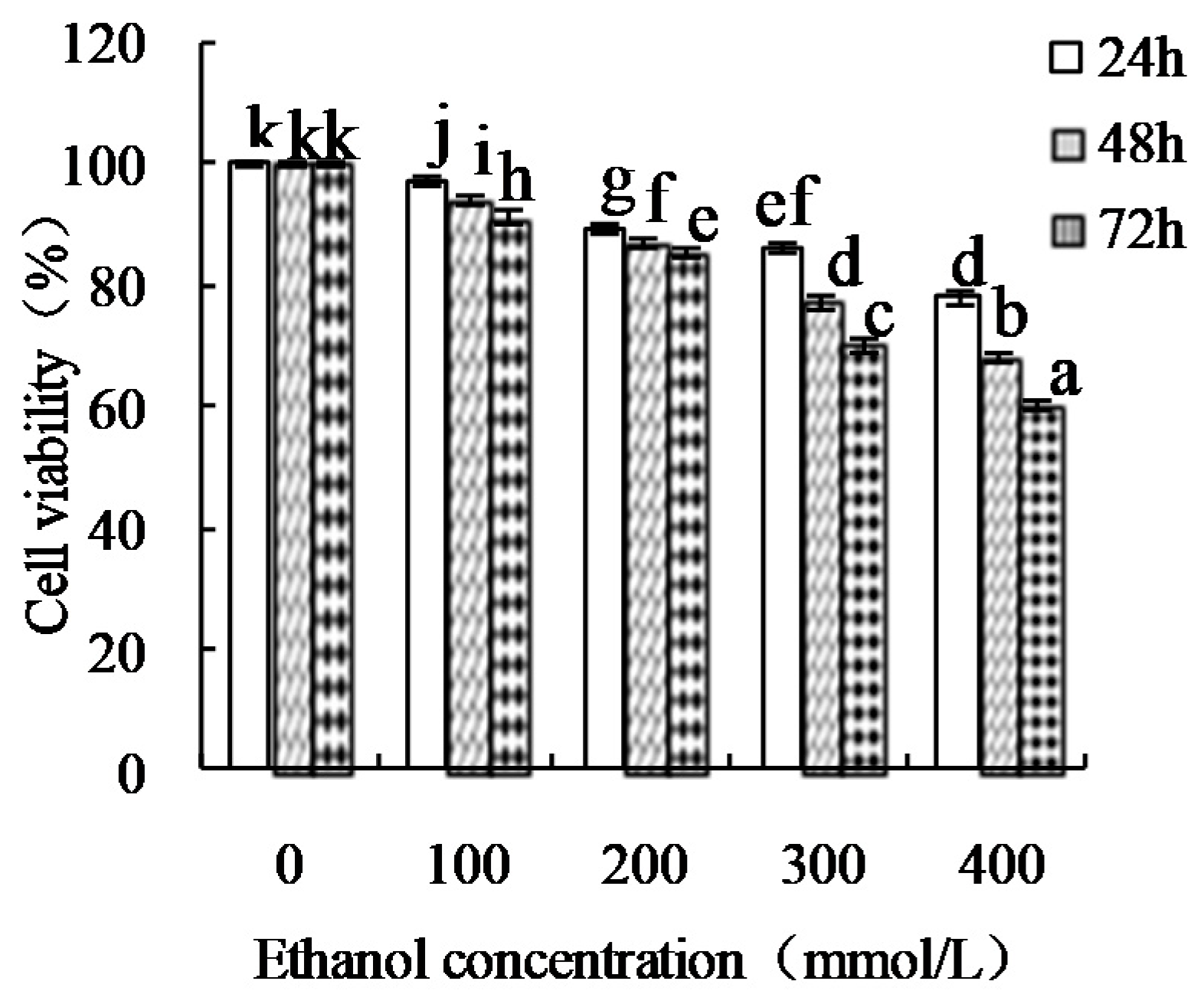

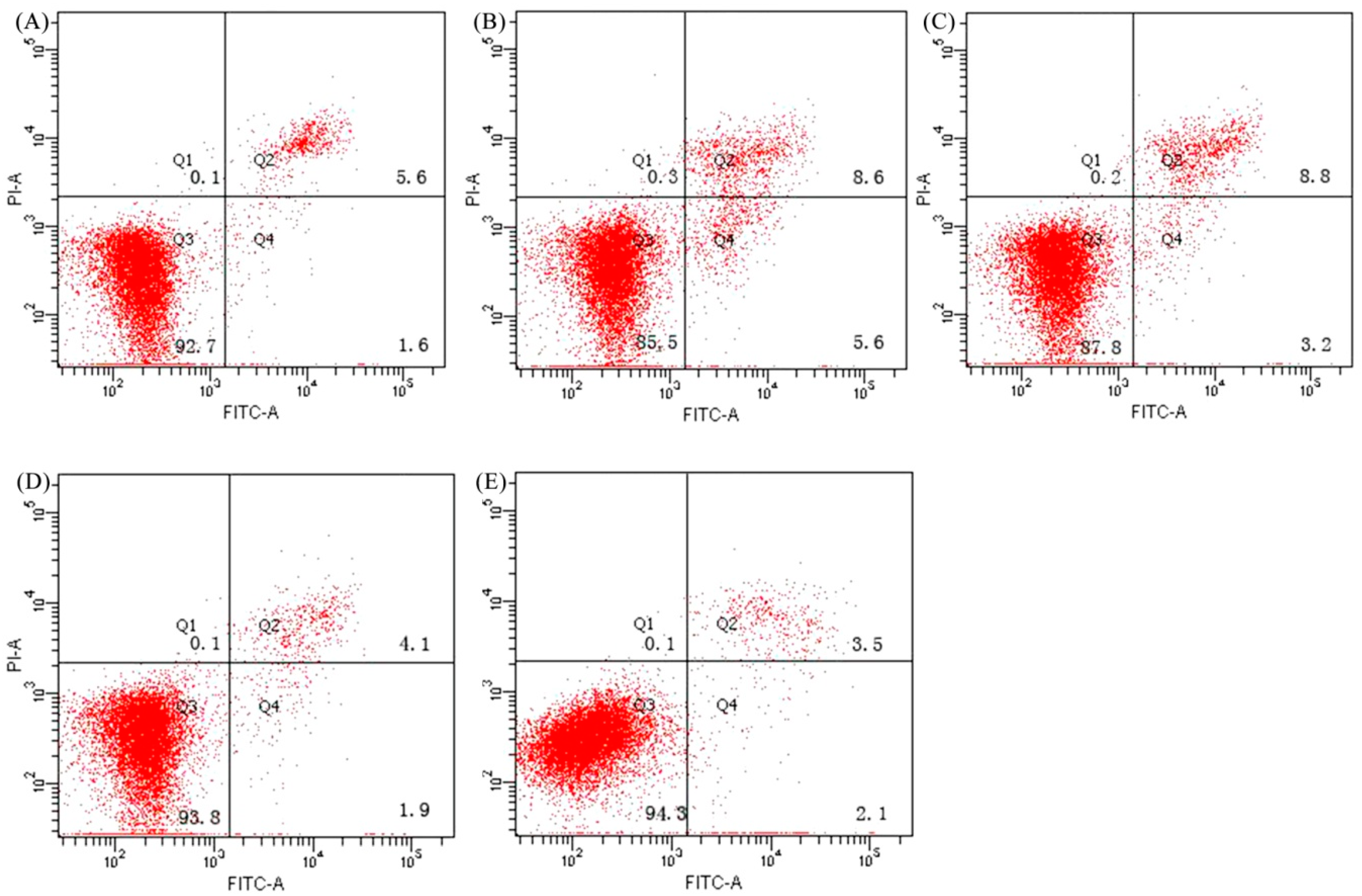
| Sample | Free Amino Group Content (mmol/g) |
|---|---|
| Casein | 0.523 ± 0.002 a |
| CH | 0.941 ± 0.001 c |
| CH-P | 0.734 ± 0.001 b |
| CH-P+Phe | 1.739 ± 0.002 f |
| CH-P-Phe | 1.351 ± 0.003 e |
| CH-P+Trp | 1.742 ± 0.002 g |
| CH-P-Trp | 1.348 ± 0.003 d |
| Samples | Amino Acid Addition Level (mol/mol) | DPPH Scavenging Activity (%) | Reducing Power | Hydroxyl Radicals Scavenging Activity (%) |
|---|---|---|---|---|
| CH | 0 | 35.87 ± 0.075 a | 0.572 ± 0.0093 a | 21.46 ± 0.79 a |
| CH-P | 0 | 36.42 ± 0.071 b | 0.594 ± 0.0084 b | 27.62 ± 0.83 b |
| CH-P+Phe | 0.74 | 39.56 ± 0.085 d | 0.673 ± 0.0087 d | 32.43 ± 0.84 d |
| CH-P-Phe | 0.74 | 38.53 ± 0.082 c | 0.616 ± 0.0092 c | 30.55 ± 0.91 c |
| CH-P+Trp | 0.74 | 48.63 ± 0.059 f | 0.724 ± 0.0095 f | 36.41 ± 0.92 f |
| CH-P-Trp | 0.74 | 46.85 ± 0.065 e | 0.705 ± 0.0082 e | 34.62 ± 0.95 e |
| Samples | Sample Doses and Treating Times of the Cells | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.0 mg/mL | 2.0 mg/mL | 3.0 mg/mL | |||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| CH | 104.2 ± 3.8 a | 110.4 ± 2.5 c | 105.6 ± 3.1 a | 106.5 ± 1.7 ab | 113.0 ± 2.3 b | 111.5 ± 2.1 bc | 105.1 ± 3.1 a | 109.6 ± 5.7 b | 110.8 ± 2.3 b |
| CH-P | 105.3 ± 2.1 a | 109.4 ± 3.3 bc | 107.5 ± 3.4 ab | 110.6 ± 4.4 b | 115.1 ± 3.1 b | 113.4 ± 3.0 cd | 108.7 ± 2.6 ab | 115.2 ± 2.4 bc | 113.2 ± 1.9 bc |
| CH-P+Phe | 103.4 ± 2.3 a | 104.2 ± 1.8 a | 106.4 ± 2.7 ab | 104.6 ± 3.2 a | 105.8 ± 2.4 a | 106.7 ± 2.5 a | 105.3 ± 3.2 a | 106.2 ± 3.4 a | 104.7 ± 2.4 a |
| CH-P-Phe | 107.6 ± 2.3 ab | 112.5 ± 4.2 c | 109.2 ± 2.8 bc | 117.6 ± 3.8 c | 120.2 ± 2.2 c | 117.3 ± 2.4 c | 110.6 ± 3.5 b | 116.7 ± 3.5 bc | 114.6 ± 3.3 bc |
| CH-P+Trp | 104.6 ± 3.1 a | 105.9 ± 3.4 ab | 106.8 ± 3.2 ab | 105.9 ± 2.6 a | 106.8 ± 3.6 a | 107.8 ± 3.4 ab | 105.4 ± 3.2 a | 106.9 ± 2.9 a | 105.2 ± 2.6 a |
| CH-P-Trp | 110.5 ± 4.1 b | 117.4 ± 3.1 d | 112.4 ± 3.3 c | 123.4 ± 3.2 d | 129.1 ± 4.1 c | 123.1 ± 2.6 e | 114.5 ± 3.2 c | 118.2 ± 3.4 c | 115.0 ± 2.3 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, L.-Y.; Pang, J.-N.; Song, C.-L.; Li, T.-J. Effect of the Plastein Reaction in Presence of Extrinsic Amino Acids on the Protective Activity of Casein Hydrolysate against Ethanol-Induced Damage in HHL-5 Cells. Foods 2019, 8, 112. https://doi.org/10.3390/foods8040112
Bo L-Y, Pang J-N, Song C-L, Li T-J. Effect of the Plastein Reaction in Presence of Extrinsic Amino Acids on the Protective Activity of Casein Hydrolysate against Ethanol-Induced Damage in HHL-5 Cells. Foods. 2019; 8(4):112. https://doi.org/10.3390/foods8040112
Chicago/Turabian StyleBo, Li-Ying, Jia-Nan Pang, Chun-Li Song, and Tie-Jing Li. 2019. "Effect of the Plastein Reaction in Presence of Extrinsic Amino Acids on the Protective Activity of Casein Hydrolysate against Ethanol-Induced Damage in HHL-5 Cells" Foods 8, no. 4: 112. https://doi.org/10.3390/foods8040112
APA StyleBo, L.-Y., Pang, J.-N., Song, C.-L., & Li, T.-J. (2019). Effect of the Plastein Reaction in Presence of Extrinsic Amino Acids on the Protective Activity of Casein Hydrolysate against Ethanol-Induced Damage in HHL-5 Cells. Foods, 8(4), 112. https://doi.org/10.3390/foods8040112





