Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Basic Characterization of Honey Samples
2.3. Raman and Infrared Spectroscopy
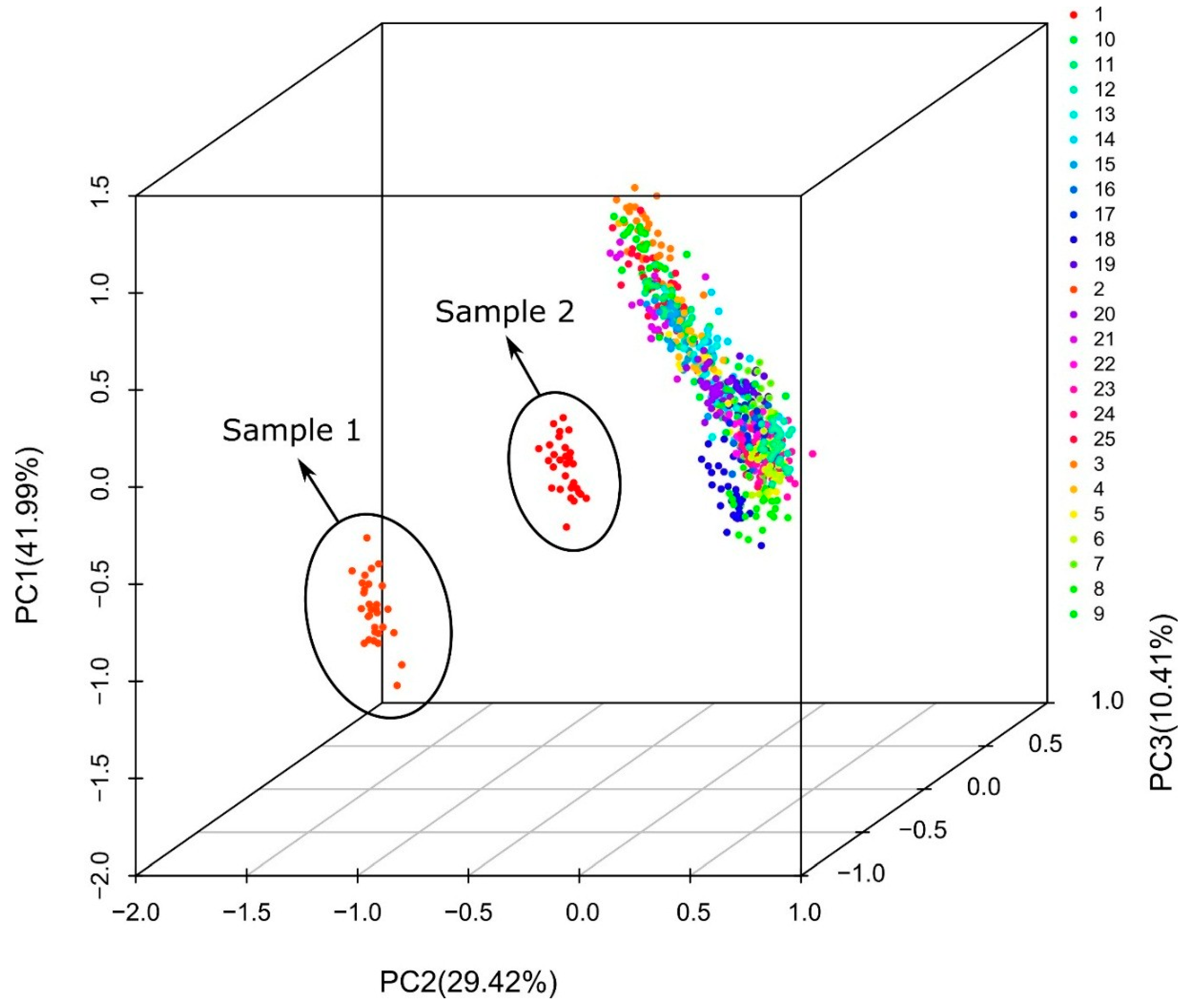
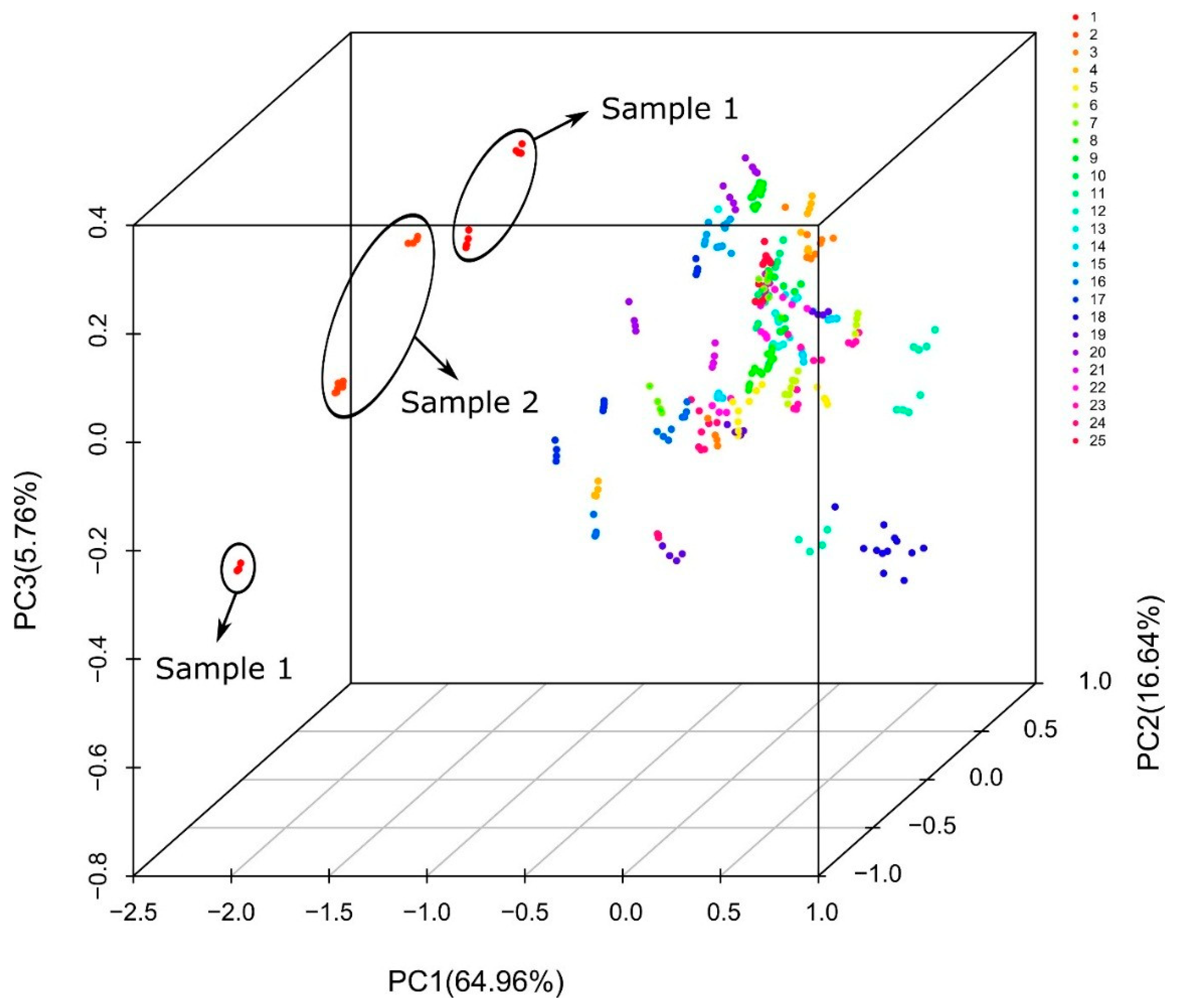
2.4. Multivariate Analysis
2.5. Pesticides Residues
3. Results and Discussion
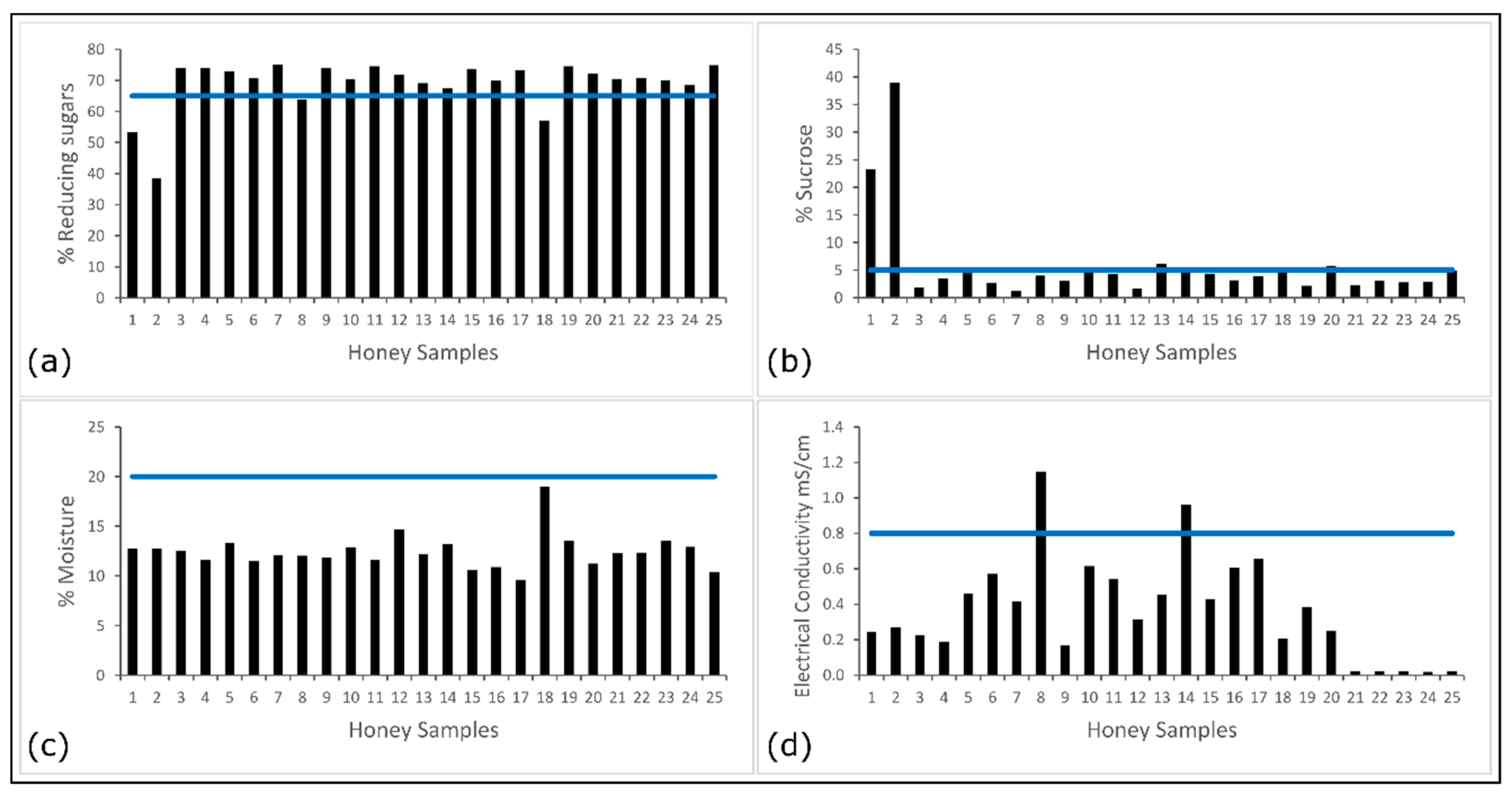
3.1. Basic Characterization of Honey Samples
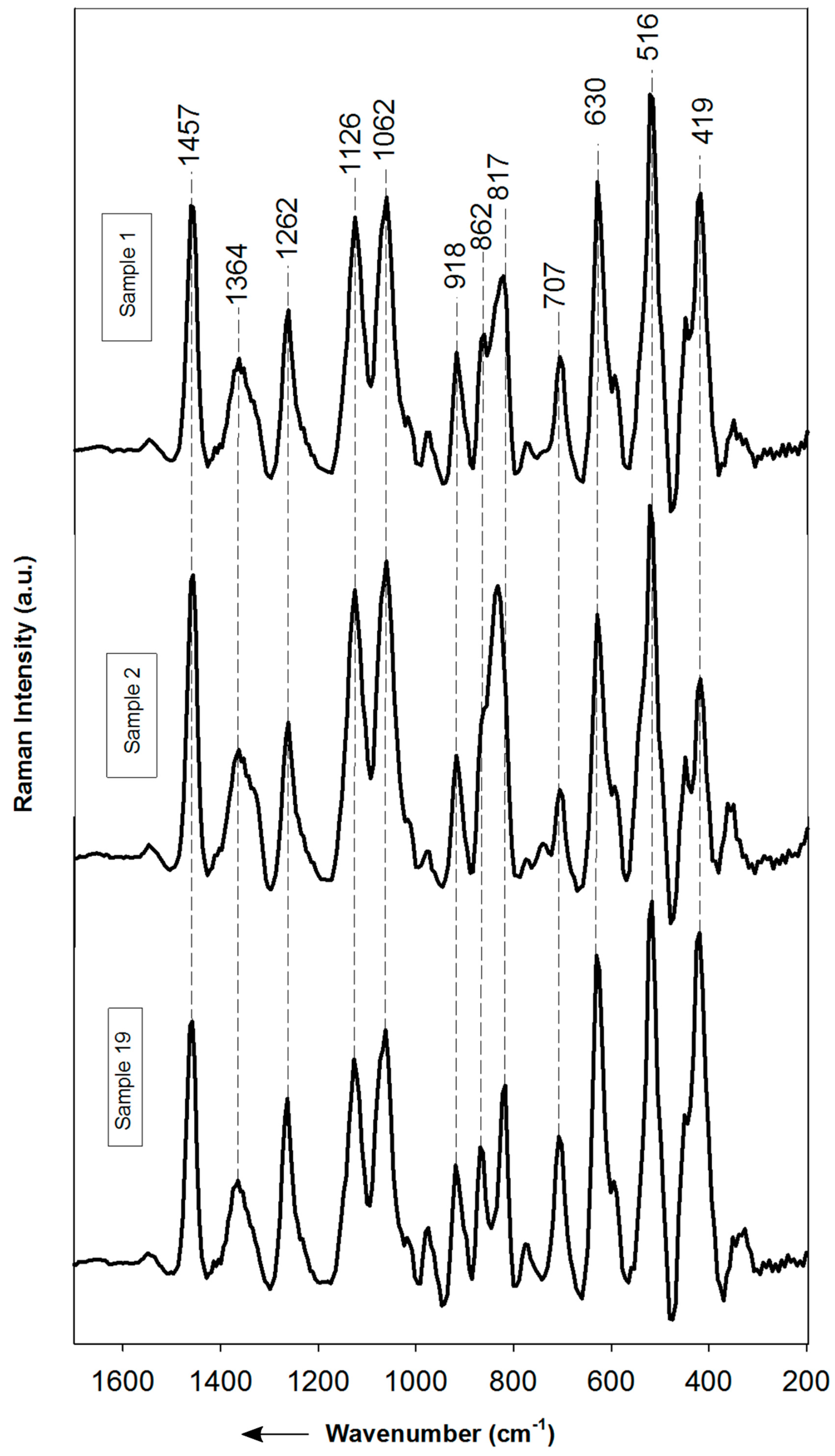
3.2. Raman and Infrared Spectra
3.3. Pesticides Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luchese, R.H.; Prudêncio, E.R.; Guerra, A.F. Honey as a functional food. In Honey Analysis; Toledo, V.A.A., Ed.; IntechOpen: London, UK, 2017; pp. 287–307. [Google Scholar]
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Bardy, J.; Slevin, N.J.; Mais, K.L.; Molassiotis, A. A systematic review of honey uses and its potential value within oncology care. J. Clin. Nurs. 2008, 17, 2604–2623. [Google Scholar] [CrossRef] [PubMed]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.E.; Clarke, A.M.; Ndip, N. An overview of honey: Therapeutic properties and contribution in nutrition and human health. Afr. J. Microbiol. Res. 2011, 5, 844–852. [Google Scholar]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Cimpoiu, C.; Hosu, A.; Miclaus, V.; Puscas, A. Determination of the floral origin of some romanian honeys on the basis of physical and biochemical properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 100, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, C.C. Aplicación de la Voltametría de Pulsos en la Detección de la Presencia de Jarabes en Miel de Girasol. Master’s Thesis, Universitat Politècnica de València, València, Spain, 2016. [Google Scholar]
- Rios-Corripio, M.A.; Rojas-López, M.; Delgado-Macuil, R. Analysis of adulteration in honey with standard sugar solutions and syrups using attenuated total reflectance-fourier transform infrared spectroscopy and multivariate methods. CyTA J. Food 2012, 10, 119–122. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Shan, Y.; Su, D.; Ma, Q.; Wen, R.; Li, J. Qualitative and quantitative detection of honey adulterated with high-fructose corn syrup and maltose syrup by using near-infrared spectroscopy. Food Chem. 2017, 218, 231–236. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Ghojoghi, A.; Ghajarbeygi, P. Honey safety hazards and public health. J. Chem. Health Risks 2016, 6, 249–267. [Google Scholar]
- Tosun, M. Detection of adulteration in honey samples added various sugar syrups with 13c/12c isotope ratio analysis method. Food Chem. 2013, 138, 1629–1632. [Google Scholar] [CrossRef]
- Codex_Alimentarius. Standard for Honey—Codex Stan 12-1981; CODEX: Roma, Italy, 2001; pp. 1–8. [Google Scholar]
- INEN, Servicio Ecuatoriano de Normalización. Miel de Abejas. Requisitos—Nte Inen 1572; INEN: Quito, Ecuador, 2016; Volume NTE INEN 1572. [Google Scholar]
- Bázár, G.; Romvári, R.; Szabó, A.; Somogyi, T.; Éles, V.; Tsenkova, R. Nir detection of honey adulteration reveals differences in water spectral pattern. Food Chem. 2016, 194, 873–880. [Google Scholar] [CrossRef]
- Bougrini, M.; Tahri, K.; Saidi, T.; El Alami El Hassani, N.; Bouchikhi, B.; El Bari, N. Classification of honey according to geographical and botanical origins and detection of its adulteration using voltammetric electronic tongue. Food Anal. Methods 2016, 9, 2161–2173. [Google Scholar] [CrossRef]
- Downey, G.; Fouratier, V.; Kelly, J.D. Detection of honey adulteration by addition of fructose and glucose using near infrared transflectance spectroscopy. J. Near Infrared Spectrosc. 2003, 11, 447–456. [Google Scholar] [CrossRef]
- Kelly, J.F.D.; Downey, G.; Fouratier, V. Initial study of honey adulteration by sugar solutions using midinfrared (mir) spectroscopy and chemometrics. J. Agric. Food Chem. 2004, 52, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.-U. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Sivakesava, S.; Irudayaraj, J. A rapid spectroscopic technique for determining honey adulteration with corn syrup. J. Food Sci. 2001, 66, 787–791. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Wang, L.; Lin, L.; Shi, H.; Cao, H.; Cao, B. Detection of honey adulteration with starch syrup by high performance liquid chromatography. Food Chem. 2015, 172, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Trifković, J.; Andrić, F.; Ristivojević, P.; Guzelmeric, E.; Yesilada, E. Analytical methods in tracing honey authenticity. J. AOAC Int. 2017, 100, 827–839. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Scattareggia, C.; Chiofalo, V. Botanical origin identification of sicilian honeys based on artificial senses and multi-sensor data fusion. Eur. Food Res. Technol. 2018, 244, 117–125. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Marino, A.M.F.; Leone, F.; Corpina, G.G.; Giunta, R.P.; Chiofalo, V. Characterization of sicilian honeys pollen profiles using a commercial e-tongue and melissopalynological analysis for rapid screening: A pilot study. Sensors 2018, 18, 4065. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Cheli, F.; Chiofalo, V. Novel approach for the characterisation of sicilian honeys based on the correlation of physico-chemical parameters and artificial senses. Ital. J. Anim. Sci. 2018, 1–9. [Google Scholar] [CrossRef]
- Schievano, E.; Zuccato, V.; Finotello, C.; Vit, P. Authenticity of ecuadorian commercial honeys. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng 2015, 9. [Google Scholar] [CrossRef]
- Padovan, G.J.; De Jong, D.; Rodrigues, L.P.; Marchini, J.S. Detection of adulteration of commercial honey samples by the 13c/12c isotopic ratio. Food Chem. 2003, 82, 633–636. [Google Scholar] [CrossRef]
- Ureña Varela, M.; Arrieta Bolaños, E.; Umaña, E.; Zamora, L.G.; Arias Echandi, M.L. Evaluation of the potential adulteration of commercial honey distributed in costa rica compared with artesanian honey samples coming from specific apiaries. Arch Lat. Nutr 2007, 57, 63–68. [Google Scholar]
- Karunathilaka, S.R.; Yakes, B.J.; He, K.; Brückner, L.; Mossoba, M.M. First use of handheld Raman spectroscopic devices and on-board chemometric analysis for the detection of milk powder adulteration. Food Control 2018, 92, 137–146. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Ciobotă, V.; Salinas, W.; Kampe, B.; Aponte, P.M.; Rösch, P.; Popp, J.; Ramos, L.A. Distinction of ecuadorian varieties of fermented cocoa beans using Raman spectroscopy. Food Chem. 2016, 211, 274–280. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Gualpa, F.; Ramos, L.A.; Ciobotă, V. Adulteration of clove essential oil: Detection using a handheld Raman spectrometer. Flavour Fragr. J. 2018, 33, 184–190. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Torrico-Vallejos, S.; Mendieta-Brito, S.; Ramos, L.A.; Ciobotă, V. Detection of counterfeit stevia products using a handheld Raman spectrometer. Vib. Spectrosc. 2016, 83, 126–131. [Google Scholar] [CrossRef]
- Hladik, M.L.; Vandever, M.; Smalling, K.L. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci. Total Environ. 2016, 542, 469–477. [Google Scholar] [CrossRef]
- Klein, S.; Cabirol, A.; Devaud, J.-M.; Barron, A.B.; Lihoreau, M. Why bees are so vulnerable to environmental stressors. Trends Ecol. Evol. 2017, 32, 268–278. [Google Scholar] [CrossRef]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; van Engelsdorp, D. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yañez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid pesticides severely affect honey bee queens. Sci. Rep. 2015, 5, 14621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 2017, 356, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Development-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Autria, 2008. [Google Scholar]
- Morháč, M. An algorithm for determination of peak regions and baseline elimination in spectroscopic data. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2009, 600, 478–487. [Google Scholar] [CrossRef]
- Mol, H.G.; Plaza-Bolanos, P.; Zomer, P.; de Rijk, T.C.; Stolker, A.A.; Mulder, P.P. Toward a generic extraction method for simultaneous determination of pesticides, mycotoxins, plant toxins, and veterinary drugs in feed and food matrixes. Anal. Chem. 2008, 80, 9450–9459. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Łukasiewicz, M.; Berski, W. Applicability of physico-chemical parameters of honey for identification of the botanical origin. Acta Sci. Polonorum. Technol. Aliment. 2013, 12, 51–59. [Google Scholar]
- Tornuk, F.; Karaman, S.; Ozturk, I.; Toker, O.S.; Tastemur, B.; Sagdic, O.; Dogan, M.; Kayacier, A. Quality characterization of artisanal and retail turkish blossom honeys: Determination of physicochemical, microbiological, bioactive properties and aroma profile. Ind. Crops Prod. 2013, 46, 124–131. [Google Scholar] [CrossRef]
- Solares Leal, C.K. Estudio Comparativo de los Niveles de Sacarosa y Azúcares Reductores (Glucosa+ fructosa) de la Miel de Abeja (Apis mellifera). Ph.D. Thesis, Universidad de San Carlos de Guatemala, Guatemala City, Guatemala, 2014. [Google Scholar]
- Kahraman, T.; Buyukunal, S.K.; Vural, A.; Altunatmaz, S.S. Physico-chemical properties in honey from different regions of turkey. Food Chem. 2010, 123, 41–44. [Google Scholar] [CrossRef]
- Velásquez Giraldo, A.M.; Vélez Acosta, L.M.; Zuluaga Gallego, R. Physicochemical and microbiological characterization of apis mellifera sp. Honey from southwest of antioquia in colombia. Ingeniería y Ciencia 2013, 9, 61–74. [Google Scholar]
- Ruoff, K.; Bogdanov, S. Authenticity of honey and other bee products. Apiacta 2004, 38, 317–327. [Google Scholar]
- Yücel, Y.; Sultanog, P. Characterization of honeys from hatay region by their physicochemical properties combined with chemometrics. Food Biosci. 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Soria, A.C.; González, M.; de Lorenzo, C.; Martínez-Castro, I.; Sanz, J. Characterization of artisanal honeys from madrid (central spain) on the basis of their melissopalynological, physicochemical and volatile composition data. Food Chem. 2004, 85, 121–130. [Google Scholar] [CrossRef]
- Belay, A.; Solomon, W.K.; Bultossa, G.; Adgaba, N.; Melaku, S. Physicochemical properties of the harenna forest honey, bale, ethiopia. Food Chem. 2013, 141, 3386–3392. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Paduret, S. Honey adulteration detection using Raman spectroscopy. Food Anal. Methods 2018, 11, 959–968. [Google Scholar] [CrossRef]
- Ilaslan, K.; Boyaci, I.H.; Topcu, A. Rapid analysis of glucose, fructose and sucrose contents of commercial soft drinks using Raman spectroscopy. Food Control 2015, 48, 56–61. [Google Scholar] [CrossRef]
- Özbalci, B.; Boyaci, İ.H.; Topcu, A.; Kadılar, C.; Tamer, U. Rapid analysis of sugars in honey by processing Raman spectrum using chemometric methods and artificial neural networks. Food Chem. 2013, 136, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, M.; Luu, D.V. Laser-Raman spectra of d-fructose in aqueous solution. Carbohydr. Res. 1980, 78, 225–233. [Google Scholar] [CrossRef]
- Mathlouthi, M.; Vinh Luu, D. Laser-Raman spectra of d-glucose and sucrose in aqueous solution. Carbohydr. Res. 1980, 81, 203–212. [Google Scholar] [CrossRef]
- Goodacre, R.; Radovic, B.S.; Anklam, E. Progress toward the rapid nondestructive assessment of the floral origin of european honey using dispersive Raman spectroscopy. Appl. Spectrosc. 2002, 56, 521–527. [Google Scholar] [CrossRef]
- Paradkar, M.M.; Irudayaraj, J. Discrimination and classification of beet and cane inverts in honey by ft-Raman spectroscopy. Food Chem. 2002, 76, 231–239. [Google Scholar] [CrossRef]
- European_Commission. Commission Regulation (eu) 2017/623 of 30 March 2017 Amending Annexes II and III to Regulation (ec) no 396/2005 of the European Parliament and of the Council as Regards maximum Residue Levels for Acequinocyl, Amitraz, Coumaphos, Diflufenican, Flumequine, Metribuzin, Permethrin, Pyraclostrobin and Streptomycin in or on Certain Products. Off. J. Eur. Union 2017, 93, 1–29. [Google Scholar]
- Calatayud-Vernich, P.; Calatayud, F.; Simó, E.; Picó, Y. Pesticide residues in honey bees, pollen and beeswax: Assessing beehive exposure. Environ. Pollut. 2018, 241, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; L’Hostis, M.; Wiest, L.; Buleté, A.; Delbac, F.; Pouliquen, H. Widespread occurrence of chemical residues in beehive matrices from apiaries located in different landscapes of western france. PLoS ONE 2013, 8, e67007. [Google Scholar] [CrossRef] [PubMed]
- Souza Tette, P.A.; Rocha Guidi, L.; de Abreu Glória, M.B.; Fernandes, C. Pesticides in honey: A review on chromatographic analytical methods. Talanta 2016, 149, 124–141. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, L.; Guijarro, M.; Rubio, D.; Aucatoma, B.; Guillén, T.; Vargas Jentzsch, P.; Ciobotă, V.; Stolker, L.; Ulic, S.; Vásquez, L.; et al. Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador. Foods 2019, 8, 105. https://doi.org/10.3390/foods8030105
Salvador L, Guijarro M, Rubio D, Aucatoma B, Guillén T, Vargas Jentzsch P, Ciobotă V, Stolker L, Ulic S, Vásquez L, et al. Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador. Foods. 2019; 8(3):105. https://doi.org/10.3390/foods8030105
Chicago/Turabian StyleSalvador, Lorena, Michelle Guijarro, Daniela Rubio, Bolívar Aucatoma, Tanya Guillén, Paul Vargas Jentzsch, Valerian Ciobotă, Linda Stolker, Sonia Ulic, Luis Vásquez, and et al. 2019. "Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador" Foods 8, no. 3: 105. https://doi.org/10.3390/foods8030105





