Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference
Abstract
1. Introduction
2. Materials and Methods
2.1. Swiss Cheese Volatile Flavor Compound and Odor Activity Value (OAV) Analyses
2.2. Descriptive Sensory Analysis
2.3. Consumer Testing
2.4. Statistical Analysis
3. Results and Discussion
3.1. Variability and Correlation of Swiss Cheese Volatile Compounds
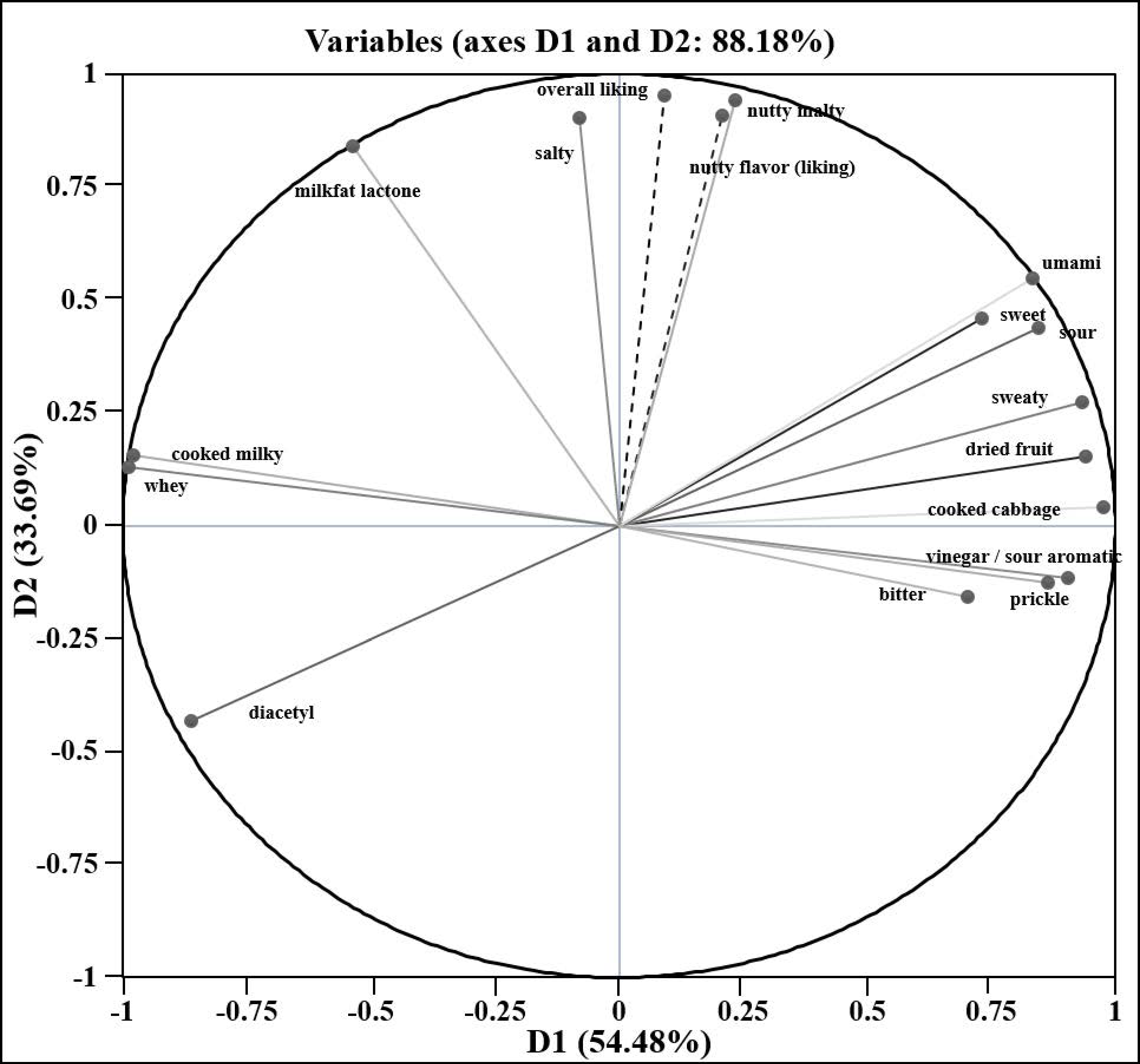
3.2. Correlation of Volatile Compounds with Sensory Attributes
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Young, N.; Drake, M.; Lopetcharat, K.; McDaniel, M. Preference mapping of Cheddar cheeses. J. Dairy Sci. 2004, 87, 11–19. [Google Scholar] [CrossRef]
- Drake, S.; Carunchia Whetstine, M.; Drake, M.; Courtney, P.; Fligner, K.; Jenkins, J.; Pruitt, C. Sources of umami taste in Cheddar and Swiss cheeses. J. Food Sci. 2007, 72, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kocaoglu-Vurma, N.; Eliardi, A.; Drake, M.; Rodriguez-Saona, L.; Harper, W. Rapid Profiling of Swiss Cheese by Attenuated Total Reflectance (ATR) Infrared Spectroscopy and Descriptive Sensory Analysis. J. Food Sci. 2009, 74, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Langsrud, T.; Reinbold, G.W. Flavor development and microbiology of Swiss cheese—A review. I. Milk quality and treatments. J. Milk Food Technol. 1973, 36, 487–490. [Google Scholar] [CrossRef]
- McSweeney, P.L. Biochemistry of cheese ripening. Int. Dairy J. 2004, 57, 127–144. [Google Scholar] [CrossRef]
- Castada, H.Z.; Harper, W.J. Variability of volatile organic compounds during the manufacture of Swiss-type cheese using selected ion flow tube mass spectrometry. Bull. Int. Dairy Fed. 2014, 473, 29–50. [Google Scholar]
- Preininger, M.; Grosch, W. Evaluation of key odorants of the neutral volatiles of Emmentaler cheese by the calculation of odour activity values. Lebensmittel-Wissenschaft Und Technologie 1994, 27, 237–244. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.T.; Bachmann, H.P. Cheeses with propionic acid fermentation. In Cheese: Chemistry, Physics and Microbiology, 3rd ed.; Fox, P.F., McSweeney, P.H., Cogan, T.M., Guinee, T.P., Eds.; Elsevier Academic Press: London, UK, 2004; Volume 2, pp. 141–156. [Google Scholar]
- Taylor, K.; Wick, C.; Castada, H.Z.; Kent, K.; Harper, W.J. Discrimination of Swiss cheese from 5 different factories by high impact volatile organic compound profiles determined by odor activity value using Selected Ion Flow Tube Mass Spectrometry and odor threshold. J. Food Sci. 2013, 78, C1509–C1515. [Google Scholar] [CrossRef] [PubMed]
- Attaie, R. Quantification of volatile compounds in goat milk Jack cheese using static headspace gas chromatography. J. Dairy Sci. 2009, 92, 2435–2443. [Google Scholar] [CrossRef]
- Mulder, H. Taste and flavor-forming substances in cheese. Neth Milk Dairy J. 1952, 6, 157–167. [Google Scholar]
- Kosikowski, F.; Mocquot, G. Advances in Cheese Technology; Food and Agriculture Organization: Rome, Italy, 1958; Volume 38, p. 236. [Google Scholar]
- Langsrud, T.; Reinbold, G.W. Flavor development and microbiology of Swiss cheese—A review. III. Ripening and flavor production. J. Milk Food Technol. 1973, 36, 593–609. [Google Scholar] [CrossRef]
- Flament, I. Coffee Flavor Chemistry; John Wiley & Sons Ltd.: West Sussex, UK, 2002. [Google Scholar]
- Belitz, H.-D.; Grosch, W.; Schieberle, P. Aroma Compounds. In Food Chemistry; Belitz, H.-D., Grosch, W., Schieberle, P., Eds.; Springer: Heidelberg, Germany, 2009; pp. 340–402. [Google Scholar]
- Weerawatanakorn, M.; Wu, J.-C.; Pan, M.-H.; Ho, C.-T. Reactivity and stability of selected flavor compounds. J. Food Drug Anal. 2015, 23, 176–190. [Google Scholar] [CrossRef]
- Castada, H.Z.; Park, C.; Harper, W.J.; Barringer, S.A. Suppression of propanoic acid, acetic acid and 3-methylbutanoic acid production by other volatiles in a Swiss cheese curd slurry system. Int. Dairy J. 2016, 54, 29–32. [Google Scholar] [CrossRef]
- Marsili, R.T. Comparing sensory and analytical chemistry flavor analysis. In Sensory-Directed Flavor Analysis; Marsili, R.T., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 1–22. [Google Scholar]
- Drake, M.; Miracle, R.; Caudle, A.D.; Cadwallader, K.R. Relating sensory and instrumental analyses. In Sensory-Directed Flavor Analysis; Marsili, R.T., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 23–55. [Google Scholar]
- Drake, M. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E., IV; Koppel, K. Associations of volatile compounds with sensory aroma and flavor: The complex nature of flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Castada, H.Z.; Wick, C.; Harper, W.J.; Barringer, S. Headspace quantification of pure and aqueous solutions of binary mixtures of key volatile organic compounds in Swiss cheeses using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Španěl, P. Application of ion chemistry and the SIFT technique to the quantitative analysis of trace gases in air and on breath. Int. Rev. Phys. Chem. 1996, 15, 231–271. [Google Scholar] [CrossRef]
- Spanel, P.; Smith, D. Selected ion flow tube studies of the reactions of H3O+, NO+, and O2+ with some organosulphur molecules. Int. J. Mass Spectrom. 1998, 176, 167–176. [Google Scholar] [CrossRef]
- Spanel, P.; Smith, D. Quantitative selected ion flow tube mass spectrometry: The influence of ionic diffusion and mass discrimination. J. Am. Soc. Mass Spectrom. 2001, 12, 863–872. [Google Scholar] [CrossRef]
- Milo, C.; Reineccius, G.A. Identification and quantification of potent odorants in regular-fat and low-fat mild Cheddar cheese. J. Agric. Food Chem. 1997, 45, 3590–3594. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners: Utrecht, The Netherlands, 2011. [Google Scholar]
- Drake, M.A.; Civille, G.V. Flavor lexicons. Compr. Rev. Food Sci. Food Saf. 2003, 2, 33–40. [Google Scholar] [CrossRef]
- Singh, T.K.; Drake, M.A.; Cadwallader, K.R. Flavor of Cheddar cheese: A chemical and sensory perspective. Compr. Rev. Food Sci. Food Saf. 2003, 2, 166–189. [Google Scholar] [CrossRef]
- Liggett, R.E.; Drake, M.A.; Delwiche, J.F. Impact of flavor attributes on consumer liking of Swiss cheese. J. Dairy Sci. 2008, 91, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- Drake, M.A.; McIngvale, S.C.; Gerard, P.D.; Cadwallader, K.R.; Civille, G.V. Development of a descriptive language for Cheddar cheese. J. Food Sci. 2001, 66, 1422–1427. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in facot analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Yang, H. Factor Loadings. In Encyclopedia of Research Design; Salkind, N.J., Ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2010; pp. 481–483. [Google Scholar]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- Heiman, G.W. Basic Statistics for the Behavioral Sciences; Wadsworth: Belmont, CA, USA, 2011. [Google Scholar]
- Mevik, B.-H.; Wehrens, R. The pls Package: Principal Componnt and Partial Least Squares Regression in R. J. Stat. Softw. 2007, 18, 1–23. [Google Scholar] [CrossRef]
- Gonzalez, I.; Le Cao, K.-A.; Davis, M.J.; Dejean, S. Visualising associations between paired ‘omics’ data sets. BioData Min. 2012, 5, 1–23. [Google Scholar] [CrossRef]
- Engels, W.J.; Visser, S. Isolation and comparative characterization of components that contribute to the flavour of different types of cheese. Neth. Milk Dairy J. 1994, 48, 127–140. [Google Scholar]
- Molimard, P.; Spinnler, H.E. Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. J. Dairy Sci. 1996, 79, 169–184. [Google Scholar] [CrossRef]
- Holland, R.; Liu, S.Q.; Crow, V.L.; Delabre, M.L.; Lubbers, M.; Bennet, M.; Norris, G. Esterases of lactic acid bacteria and cheese flavour: Milk fat hydrolysis, alcoholysis and esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, P.L.; Sousa, M.J. Biochemical pathways for the production of flavour compounds in cheeses during ripening: A review. Lait 2000, 80, 293–324. [Google Scholar] [CrossRef]
- Castada, H.Z.; Harper, W.J.; Barringer, S.A. Volatile organic compounds of a Swiss cheese slurry system with and without added reduced glutathione, compared with commercial Swiss cheese. Int. Dairy J. 2015, 49, 72–77. [Google Scholar] [CrossRef]
- Fox, P.F.; Wallace, J.M. Formation of flavour compounds in cheese. Adv. Appl. Microbiol. 1997, 45, 17–85. [Google Scholar] [PubMed]
- Collins, Y.F.; McSweeney, P.L.; Wilkinson, M.G. Lipolysis and free fatty acid catabolism in cheese: A review of current knowledge. Int. Dairy J. 2003, 13, 841–866. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Springer: New York, NY, USA, 2017. [Google Scholar]
- Downey, W.K. Lipid oxidation as a source of off-flavour development during the storage of dairy products. J. Soc. Dairy Technol. 1969, 22, 154–161. [Google Scholar] [CrossRef]
- Langsrud, T.; Reinbold, G.W. Flavor development and microbiology of Swiss cheese - A review. J. Milk Food Technol. 1974, 37, 26–41. [Google Scholar] [CrossRef]
- Kristensen, D.; Orlien, V.; Mortensen, G.; Brockhoff, P.; Skibsted, L.H. Light-induced oxidation in sliced Havarti cheese packaged in modified atmosphere. Int. Dairy J. 2000, 10, 95–103. [Google Scholar] [CrossRef]
- Wold, J.P.; Jørgensen, K.; Lundby, F. Nondestructive measurement of light-induced oxidation in dairy products by fluorescence spectroscopy and imaging. J. Dairy Sci. 2002, 85, 1693–1704. [Google Scholar] [CrossRef]
- Dirinck, P.; De Winne, A. Flavour characterisation and classification of cheeses by gas chromatographic-mass spectrometric profiling. J. Chromatogr. A 1999, 847, 203–208. [Google Scholar] [CrossRef]
- Alewijn, M.; Smit, B.A.; Sliwinski, E.L.; Wouters, J.M. The formation mechanism of lactones in Gouda cheese. Int. Dairy J. 2007, 17, 59–66. [Google Scholar] [CrossRef]
- Stadhouders, J.; Veringa, H.A. Fat hydrolysis by lactic acid bacteria in cheese. Neth Milk Dairy J. 1973, 27, 77–91. [Google Scholar]
- Liu, S.-Q.; Holland, R.; Crow, V.L. Esters and their biosynthesis in fermented dairy products: A review. Int. Dairy J. 2004, 14, 923–945. [Google Scholar] [CrossRef]
- Chamba, J.-F.; Perreard, E. Contribution of propionic acid bacteris to lipolyis of Emmental cheese. Lait 2002, 82, 33–44. [Google Scholar] [CrossRef]
- Thierry, A.; Collins, Y.F.; Abeijon Mukdsi, M.C.; McSweeney, P.L.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and metabolism of fatty acids in cheese. In Cheese: Chemistry, Physics and Microbiology. Vol 1: General Aspects, 4th ed.; McSweeney, P.L., Fox, P.F., Cotter, P.D., Everetts, D.W., Eds.; Elsevier Ltd: London, UK, 2017; Volume 1, pp. 423–444. [Google Scholar]
- Curtin, A.C.; McSweeney, P.L. Catabolism of amino acids in cheese during ripening. In Cheese: Chemistry, Physics and Microbiology. Vol 1: General Aspects, 3rd ed.; Fox, P.F., McSweeney, P.L.T., Cogan, M., Guinee, T.P., Eds.; Elsevier: London, UK, 2004; pp. 435–454. [Google Scholar]
- Marilley, L.; Casey, M.G. Flavours of cheese products: Metabolic pathways, analytical tools and identification of producing strains. Int. J. Food Microbiol. 2004, 90, 139–159. [Google Scholar] [CrossRef]
- Harper, W.; Kocaoglu-Vurma, N.A.; Wick, C.; Elekes, K.; Langford, V. Analysis of volatile sulfur compounds in Swiss cheese using selected ion flow tube mass spectrometry (SIFT-MS). In Volatile Sulfur Compounds in Food; Qian, M.C., Fan, X., Mahattanatawee, K., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 153–181. [Google Scholar]
- Aston, J.W.; Douglas, K. The production of volatile sulphur compounds in cheddar cheeses during accelerated ripening. Aust. J. Dairy Technol. 1983, 38, 66–70. [Google Scholar]
- Urbach, G. Contribution of lactic acid bacteria to flavour compound formation in dairy products. Int. Dairy J. 1995, 5, 877–903. [Google Scholar] [CrossRef]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Lawlor, J.B.; Delahunty, C.M.; Wilkinson, M.G.; Sheehan, J. Swiss-type and Swiss-Cheddar hybrid-type cheeses: Effects of manufacture on sensory character and relationships between the sensory attributes and volatile compounds and gross compositional constituents. Int. J. Dairy Technol. 2003, 56, 39–51. [Google Scholar] [CrossRef]
- Calbert, H.E.; Price, W.V. A study of the diacetyl in cheese. I. Diacetyl content and flavor of Cheddar cheese. J. Dairy Sci. 1949, 32, 515–520. [Google Scholar] [CrossRef]
- Bradley, R.L.; Smukowski, M. Butter. In The Sensory Evaluation of Dairy Products; Clark, S., Costello, M., Drake, M.A., Bodyfelt, F., Eds.; Springer: New York, NY, USA, 2009; pp. 135–165. [Google Scholar]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Thierry, A.; Maillard, M.-B.; Richoux, R.; Lortal, S. Ethyl ester formation is enhanced by ethanol addition in mini Swiss cheese with and without added propionibacteria. J. Agric. Food Chem. 2006, 54, 6819–6824. [Google Scholar] [CrossRef] [PubMed]
- Richoux, R.; Maillard, M.-B.; Kerjean, J.; Lortal, S.; Thierry, A. Enhancement of ethyl ester and flavour formation in Swiss cheese by ethanol addition. Int. Dairy J. 2008, 18, 1140–1145. [Google Scholar] [CrossRef]
- Lawlor, J.B.; Delahunty, C.M.; Wilkinson, M.G.; Sheehan, J. Relationships between the sensory characteristics, neutral volatile composition and gross composition of ten cheese varieties. Lait 2001, 81, 487–507. [Google Scholar] [CrossRef]
- Bills, D.D.; Morgan, M.E.; Libbey, L.M.; Day, E.A. Identification of compounds responsible for fruity flavor defect of experimental Cheddar cheeses. J. Dairy Sci. 1965, 48, 1168–1173. [Google Scholar] [CrossRef]
- Avsar, Y.K.; Karagul-Yuceer, Y.; Drake, M.A.; Singh, T.K.; Yoon, Y.; Cadwallader, K.R. Characterization of nutty flavor in Cheddar cheese. J. Dairy Sci. 2004, 87, 1999–2010. [Google Scholar] [CrossRef]
- Whetstine, M.C.; Drake, M.A.; Broadbent, J.R.; McMahon, D. Enhanced nutty flavor formation in Cheddar cheese made with a malty Lactococcus lactis adjunct culture. J. Dairy Sci. 2006, 89, 3277–3284. [Google Scholar] [CrossRef]
- Barbieri, G.; Bolzoni, L.; Careri, M.; Mangia, A.; Parolari, G.; Spagnoli, S.; Virgili, R. Study of volatile fraction of Parmesan cheese. J. Agric. Food Chem. 1994, 42, 1170–1176. [Google Scholar] [CrossRef]
- Preininger, M.; Warmke, R.; Grosch, W. Identification of character impacts flavor compounds of Swiss cheese by sensory studies of models. Z Lebensm Unters Forsch 1996, 202, 30–34. [Google Scholar] [CrossRef]
- Thierry, A.; Maillard, M.-B.; Le Quere, J.-L. Dynamic headspace analysis of Emmental aqueous phase as a method to quantify changes in volatile flavour compounds during ripening. Int. Dairy J. 1999, 9, 453–463. [Google Scholar] [CrossRef]
- Rychlik, M.; Bosset, J.O. Flavour and off-flavour compounds of Swiss Gruyere cheese. Evaluation of potent odorants. Int. Dairy J. 2001, 11, 895–901. [Google Scholar] [CrossRef]
- Rychlik, M.; Bosset, J.O. Flavour and off-flavour compounds of Swiss Gruyere cheese. Identification of key odorants by quantitative instrumental and sensory studies. Int. Dairy J. 2001, 11, 903–910. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G.A. Identification of aroma compounds in Parmigianno Reggiano by gas chromatography-olfactometry. J. Dairy Sci. 2002, 85, 1362–1369. [Google Scholar] [CrossRef]
- Andres, A.I.; Cava, R.; Ruiz, J. Monitoring volatile compounds during dry-cured ham ripening by solid-phase microextraction coupled to a new direct-extraction device. J. Chromatogr. A 2002, 963, 83–88. [Google Scholar] [CrossRef]
- Andres, A.I.; Ventanas, S.; Ventanas, J.; Cava, R.; Ruiz, J. Physicochemical changes throughout the ripening of dry cured hams with different salt content and processing conditions. Eur. Food Res. Technol. 2005, 221, 30–35. [Google Scholar] [CrossRef]
- Song, H.; Cadwallader, K.R.; Singh, T.K. Odour-active compounds of Jinhua ham. Flavour Fragr. J. 2008, 23, 1–6. [Google Scholar] [CrossRef]
- Bossett, J.O.; Gauch, R. Comparison of the volatile flavour compounds of six European ‘AOC’ cheeses by using a new dynamic headspace GC-MS method. Int. Dairy J. 1993, 3, 359–377. [Google Scholar] [CrossRef]
- Engels, W.J.; Dekker, R.; de Jong, C.; Neeter, R.; Visser, S. A comparative study of volatile compounds in the water-soluble fraction of various types of ripened cheese. Int. Dairy J. 1997, 7, 255–263. [Google Scholar] [CrossRef]
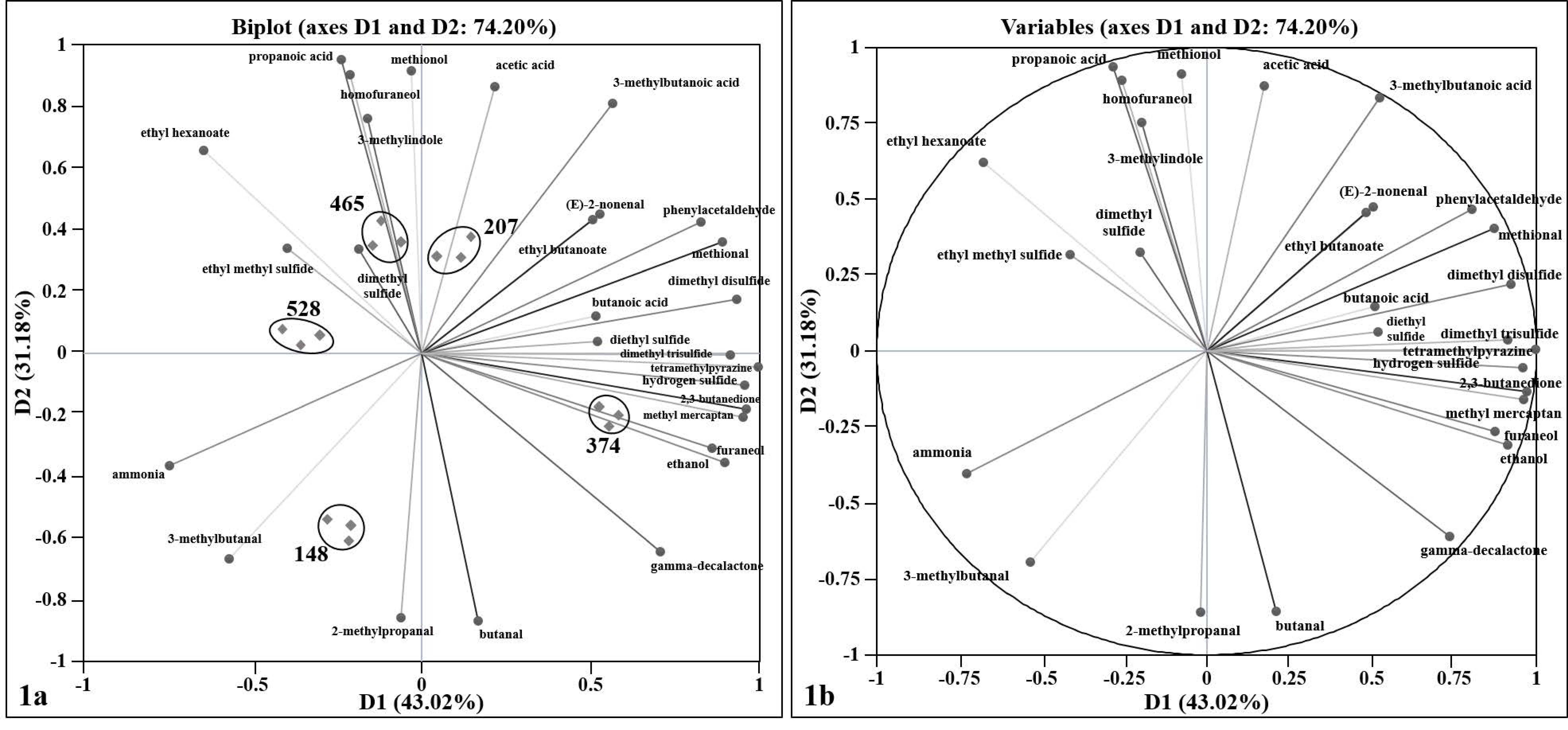
| Factory | Cheese Age (Days) at the Time of Packaging (Post-Curing and Ripening) | Streptococcus (S.)a | Propionibacterium (P.) a | Primary Lactobacillus (L.) a | Adjunct Lactobacillus (L.) a |
|---|---|---|---|---|---|
| 148 | 30 | S. thermophilus | P. freudenreichii | L. delbrueckii | L. casei |
| 207 | 54 | S. thermophilus | P. freudenreichii | L. helveticus | Lactobacillus |
| 374 | 35–36 | S. thermophilus | P. freudenreichii | L. helveticus | L. rhamnosus |
| 465 | 32 | S. thermophilus | P. freudenreichii | L. helveticus | None |
| 528 | 31 | S. thermophilus | P. freudenreichii | L. delbrueckii var lactis | L. casei |
| Compound | Reagent | Reaction Rate, k (cm3/s) | Branching Ratio (%) | Mass-to-Charge Ratio (m/z) | Product |
|---|---|---|---|---|---|
| (E)-2-nonenal | H3O+ | 4.8 × 10−9 | 100 | 141 | C9H17O+ |
| H3O+ | 159 | C9H17O+•H2O | |||
| NO+ | 3.8 × 10−9 | 80 | 139 | C9H15O+ | |
| NO+ | 20 | 169 | C9H15O+•NO+ | ||
| O2+ | 3.7 × 10−9 | 18 | 83 | C5H7O+ | |
| 84 | C5H8O+ | ||||
| 96 | C7H12+ | ||||
| 2,3-butanedione | H3O+ | 1.7 × 10−9 | 100 | 87 | C4H7O2+ |
| NO+ | 1.3 × 10−9 | 35 | 43 | C2H3O+ | |
| NO+ | 65 | 86 | C4H6O2+ | ||
| 2-methylpropanal | O2+ | 3.0 × 10−9 | 70 | 72 | C4H8O+ |
| 3-methylbutanal | NO+ | 3.0 × 10−9 | 100 | 85 | C5H9O+ |
| H3O+ | 3.6 × 10−9 | 30 | 65 | C5H6+ | |
| 3-methylbutanoic acid | H3O+ | 3.0 × 10−9 | 95 | 103 | C5H11O2+ |
| NO+ | 2.5 × 10−9 | 70 | 132 | C5H10O2+•NO+ | |
| 3-methylindole | H3O+ | 3.3 × 10−9 | 100 | 132 | C9H10N+ |
| NO+ | 2.5 × 10−9 | 100 | 131 | C9H10N+ | |
| O2+ | 2.4 × 10−9 | 20 | 130 | C9H10N+ | |
| 75 | 131 | C9H10N+ | |||
| acetic acid | H3O+ | 2.6 × 10−9 | 100 | 61 | CH3COOH2+ |
| 79 | CH3COOH2+•H2O | ||||
| 97 | CH3COOH2+•2H2O | ||||
| ammonia | H3O+ | 2.6 × 10−9 | 100 | 18 | NH4+ |
| 2.6 × 10−9 | 36 | NH4+•H2O | |||
| O2+ | 2.6 × 10−9 | 100 | 17 | NH3+ | |
| butanal | NO+ | 3.5 × 10−9 | 100 | 71 | C4H7O+ |
| butanoic acid | H3O+ | 2.9 × 10−9 | 90 | 89 | C3H7COOH2+ |
| 107 | C3H7COOH2+•2H2O | ||||
| NO+ | 1.9 × 10−9 | 50 | 118 | NO+•C3H7COOH | |
| diethyl sulfide | O2+ | 2.5 × 10−9 | 30 | 75 | C3H7S+ |
| 2.5 × 10−9 | 35 | 90 | C4H10S+ | ||
| dimethyl disulfide | NO+ | 2.4 × 10−9 | 100 | 94 | (CH3)2S2+ |
| O2+ | 2.3 × 10−9 | 80 | 94 | (CH3)2S2+ | |
| dimethyl sulfide | H3O+ | 2.5 × 10−9 | 100 | 63 | (CH3)2S.H+ |
| NO+ | 2.2 × 10−9 | 100 | 62 | (CH3)2S+ | |
| O2+ | 2.2 × 10−9 | 25 | 47 | CH3S+ | |
| dimethyl trisulfide | H3O+ | 2.8 × 10−9 | 100 | 127 | C2H6S3H+ |
| NO+ | 1.9 × 10−9 | 100 | 126 | C2H6S3+ | |
| O2+ | 2.2 × 10−9 | 15 | 111 | CH3S3+ | |
| O2+ | 2.2 × 10−9 | 45 | 126 | C2H6S3+ | |
| ethanol | H3O+ | 2.7 × 10−9 | 100 | 47 | C2H7O+ |
| 2.7 × 10−9 | 65 | C2H7O+•H2O | |||
| 2.7 × 10−9 | 83 | C2H7O+•(H2O)2 | |||
| NO+ | 1.2 × 10−9 | 100 | 45 | C2H5O+ | |
| 1.2 × 10−9 | 63 | C2H5O+•H2O | |||
| 1.2 × 10−9 | 81 | C2H5O+•(H2O)2 | |||
| O2+ | 2.3 × 10−9 | 75 | 45 | C2H5O+ | |
| 2.3 × 10−9 | 63 | C2H5O+•H2O | |||
| 2.3 × 10−9 | 81 | C2H5O+•2H2O | |||
| ethyl butanoate | H3O+ | 3.0 × 10−9 | 80 | 117 | C6H12O2•H+ |
| 135 | C6H13O2+•H2O | ||||
| NO+ | 2.4 × 10−9 | 30 | 146 | C6H12O2+•NO+ | |
| ethyl hexanoate | H3O+ | 3.0 × 10−9 | 100 | 145 | C8H16O2•H+ |
| H3O+ | 3.0 × 10−9 | 163 | C8H16O2•H+•H2O | ||
| NO+ | 2.5 × 10−9 | 95 | 174 | C8H16O2+•NO+ | |
| ethyl methyl sulfide | H3O+ | 2.4 × 10−9 | 100 | 77 | CH3SHC2H5+ |
| furaneol | H3O+ | 4.0 × 10−9 | 100 | 129 | C6H8O3•H+ |
| 4.0 × 10−9 | 147 | C6H8O3•H3O+ | |||
| NO+ | 2.5 × 10−9 | 95 | 128 | C6H8O3+ | |
| O2+ | 3.0 × 10−9 | 100 | 128 | C6H8O3+ | |
| γ-decalactone | H3O+ | 3.0 × 10−9 | 100 | 171 | C10H18O2•H+ |
| NO+ | 2.5 × 10−9 | 100 | 200 | C10H18O2•NO+ | |
| homofuraneol | H3O+ | 3.0 × 10−9 | 100 | 143 | C7H10O3•H+ |
| H3O+ | 3.0 × 10−9 | 161 | C7H10O3•H+•H2O | ||
| NO+ | 2.5 × 10−9 | 100 | 142 | C7H10O3+ | |
| O2+ | 2.5 × 10−9 | 100 | 142 | C7H10O3+ | |
| hydrogen sulfide | H3O+ | 1.6 × 10−9 | 100 | 35 | H3S+ |
| 1.6 × 10−9 | 53 | H3S+•H2O | |||
| O2+ | 1.4 × 10−9 | 100 | 34 | H2S+ | |
| methional | O2+ | 2.5 × 10−9 | 75 | 104 | C4H8OS+ |
| methionol | NO+ | 2.5 × 10−9 | 100 | 106 | C4H10OS+ |
| O2+ | 2.5 × 10−9 | 30 | 89 | C4H9S+ | |
| 2.5 × 10−9 | 40 | 106 | C4H10OS+ | ||
| methyl mercaptan (methanethiol) | H3O+ | 1.8 × 10−9 | 100 | 49 | CH4S.H+ |
| 1.8 × 10−9 | 67 | CH4S.H+•H2O | |||
| phenylacetaldehyde | H3O+ | 3.0 × 10−9 | 100 | 121 | C8H8O•H+ |
| H3O+ | 3.0 × 10−9 | 157 | C8H8O•H+•2H2O+ | ||
| NO+ | 2.5 × 10−9 | 15 | 91 | C7H7+ | |
| NO+ | 2.5 × 10−9 | 60 | 120 | C8H8O+ | |
| NO+ | 2.5 × 10−9 | 25 | 150 | C8H8O•NO+ | |
| O2+ | 2.5 × 10−9 | 40 | 91 | C7H7+ | |
| O2+ | 2.5 × 10−9 | 40 | 92 | C7H8+ | |
| O2+ | 2.5 × 10−9 | 20 | 120 | C8H8+ | |
| O2+ | 2.5 × 10−9 | 121 | C8H8O•H+ | ||
| propanoic acid | H3O+ | 2.7 × 10−9 | 90 | 75 | C2H5COOH2 + |
| NO+ | 1.5 × 10−9 | 30 | 57 | C2H5CO+ | |
| O2+ | 2.2 × 10−9 | 80 | 74 | C2H5COOH+ | |
| tetramethylpyrazine | H3O+ | 3.0 × 10−9 | 100 | 137 | C8H12N2•H+ |
| NO+ | 2.5 × 10−9 | 100 | 136 | C8H12N2 + | |
| O2+ | 2.5 × 10−9 | 100 | 136 | C8H12N2 + |
| Swiss Cheeses Sensory Descriptor | Definition |
|---|---|
| Bitter | Fundamental taste sensation elicited by various compounds |
| Cooked cabbage | Aromatics associated with cooked cabbage |
| Cooked/milky | Aromatics associated with cooked milk |
| Diacetyl (buttery) | Aromatics associated with diacetyl |
| Dried fruit | Aromatics associated with dried fruits, specifically peaches and apricots |
| Milk fat | Aromatics associated with milk fat |
| Nutty | Nutlike aromatic associated with different nuts |
| Prickle | Chemical feeling factor of which the sensation of carbonation on the tongue is typical |
| Salty | Fundamental taste sensation elicited by salts |
| Sour | Fundamental taste sensation elicited by acids |
| Sweaty | Aromatic associated with human sweat |
| Sweet | Fundamental taste sensation elicited by sugars |
| Umami | Chemical feeling factor elicited by certain peptides and nucleotides |
| Vinegar | Aromatics associated with vinegar |
| Whey | Aromatics associated with Cheddar cheese whey |
| Sensory Attribute | Compounds with Positive Correlation | Compounds with Negative Correlation | Sensory Attribute | Compounds with Positive Correlation | Compounds with Negative Correlation | ||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Score, r | Compound | Score, r | Compound | Score, r | Compound | Score, r | ||
| bitter | ethyl hexanoate | 0.87 | furaneol | −0.90 | sweaty | ethyl hexanoate | 0.78 | ethanol | −0.94 |
| dimethyl sulfide | 0.65 | diethyl sulfide | −0.82 | homofuraneol | 0.78 | gamma-decalactone | −0.93 | ||
| butanoic acid | −0.79 | propanoic acid | 0.74 | 2,3-butanedione | −0.89 | ||||
| butanal | −0.73 | methyl mercaptan | −0.89 | ||||||
| gamma-decalactone | −0.71 | hydrogen sulfide | −0.83 | ||||||
| cooked cabbage | ethyl hexanoate | 0.96 | gamma-decalactone | −0.98 | tetramethylpyrazine | −0.78 | |||
| propanoic acid | 0.80 | furaneol | −0.87 | furaneol | −0.74 | ||||
| homofuraneol | 0.78 | ethanol | −0.85 | dimethyl disulfide | −0.71 | ||||
| methyl mercaptan | −0.79 | sweet | ethyl methyl sulfide | 0.79 | ethanol | −0.93 | |||
| 2,3-butanedione | −0.79 | 3-methylindole | 0.73 | methyl mercaptan | −0.90 | ||||
| butanal | −0.77 | ethyl hexanoate | 0.71 | hydrogen sulfide | −0.88 | ||||
| tetramethylpyrazine | −0.75 | propanoic acid | 0.71 | 2,3-butanedione | −0.87 | ||||
| hydrogen sulfide | −0.71 | dimethyl trisulfide | −0.79 | ||||||
| cooked milky | gamma-decalactone | 0.98 | ethyl hexanoate | −0.96 | tetramethylpyrazine | −0.77 | |||
| furaneol | 0.85 | homofuraneol | −0.76 | gamma-decalactone | −0.75 | ||||
| butanal | 0.81 | propanoic acid | −0.69 | furaneol | −0.72 | ||||
| 2-methylpropanal | 0.71 | umami | ethyl hexanoate | 0.70 | 2,3-butanedione | −0.99 | |||
| diacetyl | 2,3-butanedione | 0.93 | ethyl hexanoate | −0.68 | homofuraneol | 0.50 | methyl mercaptan | −0.98 | |
| ethanol | 0.91 | homofuraneol | −0.59 | ethanol | −0.97 | ||||
| methyl mercaptan | 0.91 | hydrogen sulfide | −0.96 | ||||||
| hydrogen sulfide | 0.87 | tetramethylpyrazine | −0.94 | ||||||
| tetramethylpyrazine | 0.87 | dimethyl disulfide | −0.91 | ||||||
| gamma-decalactone | 0.86 | gamma-decalactone | −0.84 | ||||||
| dimethyl disulfide | 0.85 | furaneol | −0.81 | ||||||
| furaneol | 0.74 | methional | −0.75 | ||||||
| dried fruit | ethyl hexanoate | 0.77 | gamma-decalactone | −0.93 | dimethyl trisulfide | −0.75 | |||
| homofuraneol | 0.76 | ethanol | −0.83 | vinegar/sour aromatic | propanoic acid | 0.93 | gamma-decalactone | −0.91 | |
| propanoic acid | 0.63 | 2,3-butanedione | −0.81 | ethyl hexanoate | 0.90 | butanal | −0.85 | ||
| methyl mercaptan | −0.79 | homofuraneol | 0.87 | ethanol | −0.75 | ||||
| tetramethylpyrazine | −0.74 | methionol | 0.79 | 2-methylpropanal | −0.72 | ||||
| hydrogen sulfide | −0.72 | 3-methylindole | 0.72 | furaneol | −0.72 | ||||
| furaneol | −0.72 | whey | gamma-decalactone | 0.99 | ethyl hexanoate | −0.95 | |||
| milkfat lactone | 2-methylpropanal | 0.93 | (E)-2-nonenal | −0.85 | butanal | 0.82 | homofuraneol | −0.85 | |
| 3-methylbutanal | 0.84 | 3-methylbutanoic acid | −0.69 | furaneol | 0.80 | propanoic acid | −0.81 | ||
| butanal | 0.84 | ethyl butanoate | −0.66 | ethanol | 0.75 | methionol | −0.62 | ||
| homofuraneol | −0.63 | 2-methylpropanal | 0.71 | ||||||
| nutty malty | 3-methylbutanal | 0.94 | dimethyl disulfide | −0.94 | 2,3-butanedione | 0.69 | |||
| 2-methylpropanal | 0.83 | ethyl butanoate | −0.93 | methyl mercaptan | 0.68 | ||||
| 3-methylbutanoic acid | −0.83 | tetramethylpyrazine | 0.64 | ||||||
| hydrogen sulfide | −0.82 | ||||||||
| 2,3-butanedione | −0.77 | ||||||||
| methyl mercaptan | −0.76 | ||||||||
| methional | −0.74 | ||||||||
| tetramethylpyrazine | −0.72 | ||||||||
| prickle | ethyl hexanoate | 0.77 | gamma-decalactone | −0.85 | |||||
| homofuraneol | 0.58 | diethyl sulfide | −0.83 | ||||||
| butanoic acid | −0.79 | ||||||||
| furaneol | −0.73 | ||||||||
| salty | 3-methylbutanal | 0.94 | ethyl butanoate | −0.98 | |||||
| 2-methylpropanal | 0.82 | 3-methylbutanoic acid | −0.76 | ||||||
| butanal | 0.70 | dimethyl disulfide | −0.74 | ||||||
| sour | homofuraneol | 0.68 | ethanol | −0.94 | |||||
| ethyl hexanoate | 0.65 | 2,3-butanedione | −0.92 | ||||||
| propanoic acid | 0.61 | methyl mercaptan | −0.91 | ||||||
| hydrogen sulfide | −0.88 | ||||||||
| gamma-decalactone | −0.84 | ||||||||
| dimethyl disulfide | −0.81 | ||||||||
| tetramethylpyrazine | −0.80 | ||||||||
| Overall Consumer Preference | Sensory Attributes with Positive Correlation | |
|---|---|---|
| Attribute | Score, r | |
| overall liking | nutty malty | 0.86 |
| milkfat lactone | 0.75 | |
| salty | 0.73 | |
| umami | 0.58 | |
| sweet | 0.56 | |
| nutty flavor (liking) | nutty malty | 0.85 |
| umami | 0.66 | |
| salty | 0.66 | |
| milkfat lactone | 0.64 | |
| sweet | 0.58 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castada, H.Z.; Hanas, K.; Barringer, S.A. Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference. Foods 2019, 8, 78. https://doi.org/10.3390/foods8020078
Castada HZ, Hanas K, Barringer SA. Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference. Foods. 2019; 8(2):78. https://doi.org/10.3390/foods8020078
Chicago/Turabian StyleCastada, Hardy Z., Kaitlyn Hanas, and Sheryl Ann Barringer. 2019. "Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference" Foods 8, no. 2: 78. https://doi.org/10.3390/foods8020078
APA StyleCastada, H. Z., Hanas, K., & Barringer, S. A. (2019). Swiss Cheese Flavor Variability Based on Correlations of Volatile Flavor Compounds, Descriptive Sensory Attributes, and Consumer Preference. Foods, 8(2), 78. https://doi.org/10.3390/foods8020078





