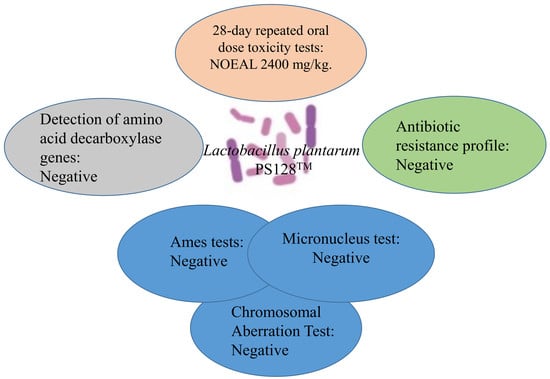
Toxicity Studies of Lactobacillus plantarum PS128TM Isolated from Spontaneously Fermented Mustard Greens
Abstract
1. Introduction
2. Materials and Methods
2.1. PS128TM Preparation
2.2. The Ames Salmonella/Microsome Mutagenicity Assay
2.3. Cell Viability Assay
2.4. Chromosome Aberration Test
2.5. In Vivo Micronucleus Assay
2.6. 28 Day Subacute Toxicity Study
2.7. Serum Biochemical and Hematological Analysis
2.8. Gross Necropsy
2.9. Histopathology
2.10. Ethics Statement
2.11. Antibiotic Resistance Profile
2.12. Detection of Amino Acid Decarboxylase Genes
2.13. Statistical Analysis
3. Results and Discussion
3.1. PS128TM Treatment Was Negative to Mutagenicity and Clastogenicity Evaluation
3.2. PS128TM did not Induce Adverse Effects in the 28 Day Subacute Toxicity Test
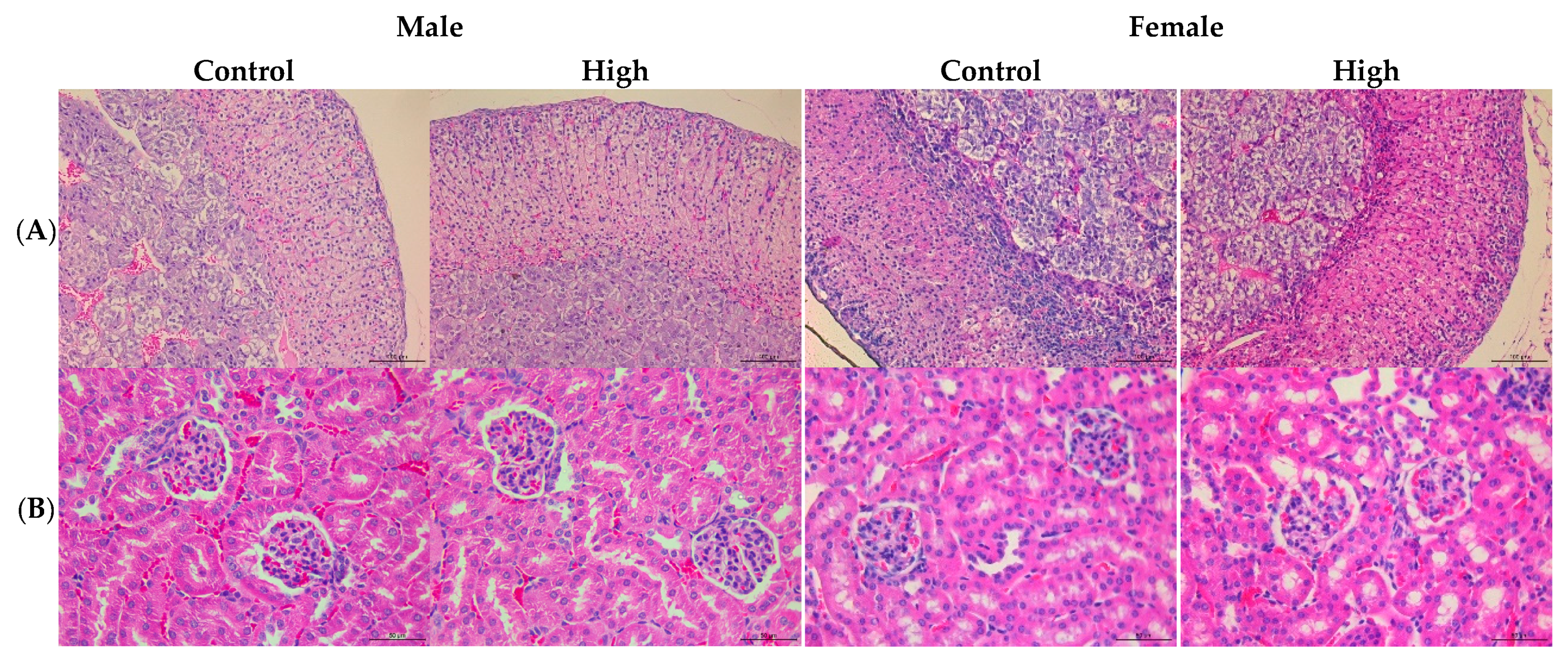
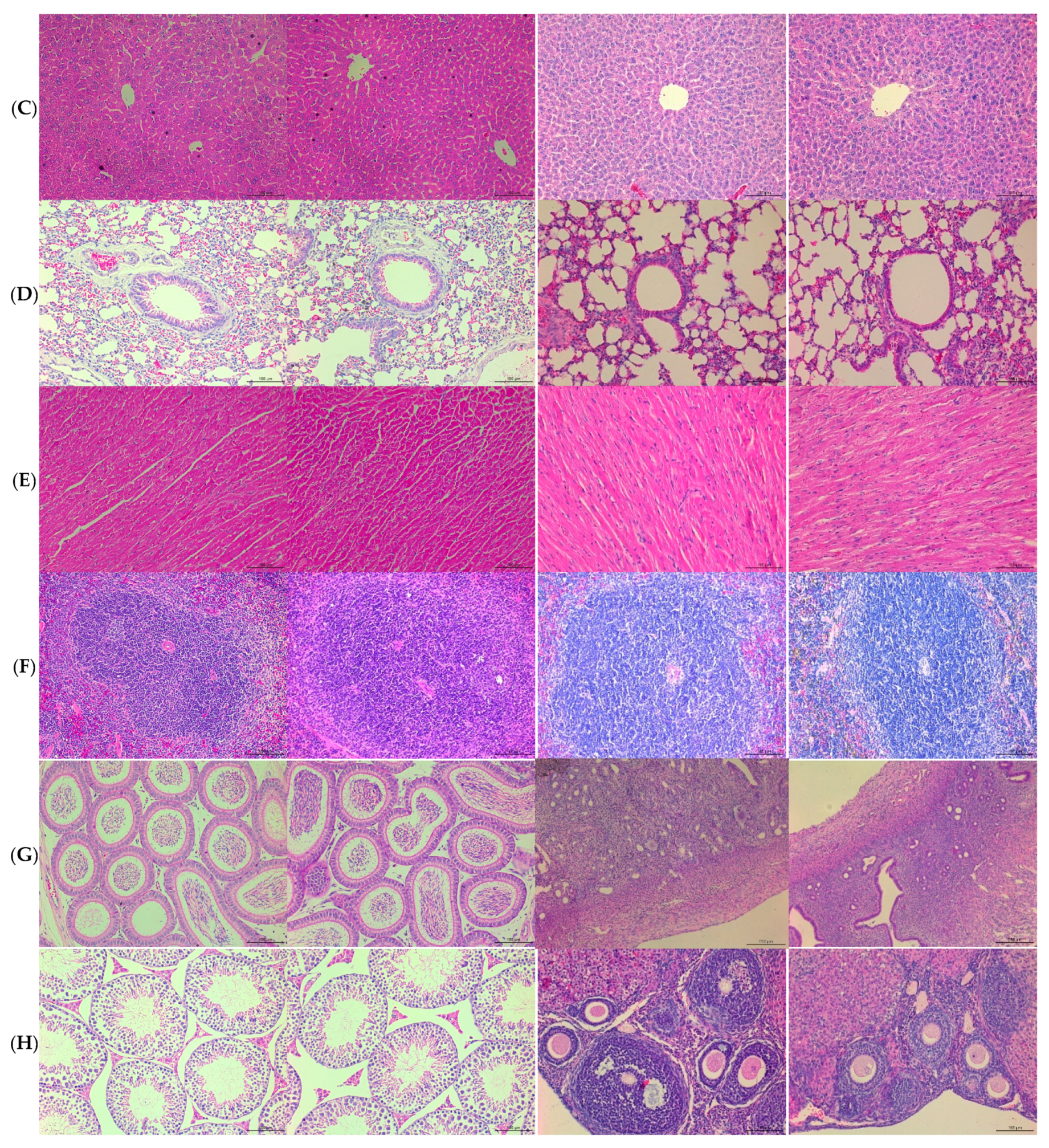
3.3. PS128TM did not Cause Macropathological or Histopathological Lesions in the 28 Day Subacute Toxicity Test
3.4. PS128TM Was Sensitive to Antibiotics
3.5. No Genes Related to the Production of Biogenic Amines Were Found in PS128TM
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO/WHO. Joint FAO/WHO Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2002; pp. 1–11. [Google Scholar]
- Vyas, U.; Ranganathan, N. Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterol. Res. Pract. 2012, 2012, 872716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Su, Y.W.; Ong, W.K.; Cheng, T.H.; Tsai, Y.C. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int. Immunopharmacol. 2011, 11, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.C.; Ngan, B.Y.; McKay, D.M.; Soderholm, J.D.; Perdue, M.H.; Sherman, P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Fitzgerald, P.; Dinan, T.G.; Cryan, J.F.; Ross, R.P.; Quigley, E.M.; Shanahan, F.; Kiely, B.; Fitzgerald, G.F.; O’Toole, P.W.; et al. Bifidobacterium breve with alpha-linolenic acid and linoleic acid alters fatty acid metabolism in the maternal separation model of irritable bowel syndrome. PLoS ONE 2012, 7, e48159. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, S.; Villena, J.; Shimazu, T.; Tohno, M.; Fujie, H.; Chiba, E.; Shimosato, T.; Aso, H.; Suda, Y.; Kawai, Y.; et al. Immunobiotic lactic acid bacteria beneficially regulate immune response triggered by poly(I:C) in porcine intestinal epithelial cells. Vet. Res. 2011, 42, 111. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.C.; Liu, Y.W.; Chiang, Y.C.; Chao, S.H.; Mei, N.W.; Tsai, Y.C. Immunomodulatory Activity of Lactococcus lactis A17 from Taiwan Fermented Cabbage in OVA-Sensitized BALB/c Mice. Evid. Based Complement. Altern. Med. 2013, 2013, 287803. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Sohn, W.; Lee, O.Y.; Lee, S.P.; Lee, K.N.; Jun, D.W.; Lee, H.L.; Yoon, B.C.; Choi, H.S.; Chung, W.S.; et al. Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014, 29, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Yang, C.H.; Lin, C.T.; Li, S.W.; Cheng, W.S.; Jiang, Y.P.; Wu, C.C.; Chang, C.H.; Tsai, Y.C. Genome architecture of Lactobacillus plantarum PS128, a probiotic strain with potential immunomodulatory activity. Gut Pathog. 2015, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Chuang, H.L.; Huang, Y.T.; Wu, C.C.; Chou, G.T.; Wang, S.; Tsai, Y.C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.F.; Cheng, Y.F.; Li, S.W.; Lee, W.T.; Hsu, C.C.; Wu, C.C.; Jeng, O.J.; Wang, S.; Tsai, Y.C. Lactobacillus plantarum PS128 ameliorates 2,5-Dimethoxy-4-iodoamphetamine-induced tic-like behaviors via its influences on the microbiota-gut-brain-axis. Brain Res. Bull. 2019, 153, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Pararajasingam, A.; Uwagwu, J. Lactobacillus: The not so friendly bacteria. BMJ Case Rep. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Liao, J.W.; Liao, P.L.; Huang, W.K.; Tse, L.S.; Lin, C.H.; Kang, J.J.; Cheng, Y.W. Evaluation of Acute 13-Week Subchronic Toxicity and Genotoxicity of the Powdered Root of Tongkat Ali (Eurycoma longifolia Jack). Evid. Based Complement. Altern. Med. 2013, 2013, 102987. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.L.; Li, C.H.; Tse, L.S.; Kang, J.J.; Cheng, Y.W. Safety assessment of the Cistanche tubulosa health food product Memoregain((R)): Genotoxicity and 28-day repeated dose toxicity test. Food Chem. Toxicol. 2018, 118, 581–588. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents; OECD: Paris, France, 2008. [Google Scholar]
- OECD. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents; OECD: Paris, France, 2018. [Google Scholar]
- Peggy, J.; Danneman, M.A.S.; Brayton, C. The Laboratory Mouse; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Bernardeau, M.; Vernoux, J.P.; Henri-Dubernet, S.; Gueguen, M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int. J. Food Microbiol. 2008, 126, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; de Las Rivas, B.; Marcobal, A.; Munoz, R. Molecular methods for the detection of biogenic amine-producing bacteria on foods. Int. J. Food Microbiol. 2007, 117, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- OECD. Test No. 473: In Vitro Mammalian Chromosomal Aberration Test; OECD: Paris, France, 2016. [Google Scholar]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test; OECD: Paris, France, 2016. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
| TA97 | TA98 | TA100 | TA102 | TA1535 | |
|---|---|---|---|---|---|
| Without S9 Metabolic Activation (Number/Plate) | |||||
| Negative 1 | 37 ± 6 | 15 ± 7 | 129 ± 15 | 7 ± 0 | 17 ± 3 |
| Positive 2 | 467 ± 64 * | 2208 ± 200 * | 1249 ± 76 * | 413 ± 21 * | 227 ± 7 * |
| PS128 (mg/Plate) | |||||
| 0.3125 | 26 ± 11 | 18 ± 4 | 167 ± 2 | 5 ± 2 | 11 ± 2 |
| 0.625 | 34 ± 8 | 18 ± 1 | 177 ± 6 | 4 ± 1 | 17 ± 2 |
| 1.25 | 36 ± 5 | 19 ± 4 | 170 ± 1 | 8 ± 2 | 14 ± 2 |
| 2.5 | 35 ± 5 | 20 ± 2 | 147 ± 11 | 11 ± 7 | 12 ± 2 |
| 5 | 21 ± 4 | 15 ± 2 | 120 ± 7 | 6 ± 1 | 12 ± 5 |
| With S9 Metabolic Activation (Number/Plate) | |||||
| Negative 1 | 100 ± 13 | 24 ± 8 | 37 ± 7 | 17 ± 1 | 18 ± 13 |
| Positive 2 | 1220 ± 73 * | 296 ± 7 * | 2004 ± 178 * | 518 ± 19 * | 168 ± 29 * |
| PS128 (mg/Plate) | |||||
| 0.3125 | 93 ± 12 | 29 ± 3 | 33 ± 4 | 16 ± 1 | 10 ± 1 |
| 0.625 | 82 ± 2 | 25 ± 4 | 34 ± 5 | 17 ± 4 | 8 ± 3 |
| 1.25 | 85 ± 6 | 28 ± 5 | 33 ± 5 | 20 ± 4 | 15 ± 9 |
| 2.5 | 79 ± 14 | 31 ± 2 | 40 ± 4 | 17 ± 4 | 11 ± 1 |
| 5 | 95 ± 25 | 18 ± 4 | 34 ± 5 | 16 ± 1 | 14 ± 5 |
| Aberrant Cell (%) 4 | Number of Cells with Structural Aberrations (%) 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| With Gap | Without Gap | G | B | D | g | b | e | |
| 3 h without S9 Metabolic Activation | ||||||||
| Negative 1 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Positive 2 | 7.5 ± 1.9 *** | 5.3 ± 1.0 *** | 1.3 ± 0.5 *** | 0.8 ± 0.5 ** | 0.8 ± 1.0 | 1.0 ± 0.8 | 1.0 ± 0.8 * | 1.3 ± 1.0 ** |
| PS128 (mg/mL) | ||||||||
| 0.3125 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 0.625 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 1.25 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 |
| 3 h with S9 Metabolic Activation | ||||||||
| Negative 1 | 0.5 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Positive 2 | 7.5 ± 1.3 *** | 5.5 ± 1.3 *** | 1.3 ± 1.0 ** | 0.3 ± 0.5 | 1.3 ± 1.0 ** | 0.8 ± 0.5 | 1.3 ± 1.0 * | 1.5 ± 1.0 ** |
| PS128 (mg/mL) | ||||||||
| 0.3125 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 0.625 | 0.5 ± 1.0 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.0 ± 0.0 |
| 1.25 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 24 h without S9 Metabolic Activation | ||||||||
| Negative 1 | 0.8 ± 1.0 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 1.0 | 0.3 ± 0.5 | 0.0 ± 0.0 |
| Positive 2 | 8.0 ± 2.2 *** | 5.8 ± 1.7 *** | 1.3 ± 0.5 *** | 0.5 ± 0.6 | 1.5 ± 1.3 * | 1.0 ± 0.8 | 1.0 ± 0.8 | 2.0 ± 0.8 *** |
| PS128 (mg/mL) | ||||||||
| 0.3125 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 |
| 0.625 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 1.25 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Negative | PS128 (g/kg Body Weight) | Positive | |||
|---|---|---|---|---|---|
| Distilled Water | 0.5 | 1.0 | 2.0 | Cyclophosphamide 200 mg/kg | |
| Number of Micronuclei (%) | |||||
| Day 1 | 0.4 ± 0.5 | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.4 ± 0.5 | 2.8 ± 0.4 *** |
| Day 2 | 0.4 ± 0.5 | 0.2 ± 0.4 | 0.6 ± 0.5 | 0.6 ± 0.5 | 3.4 ± 0.5 *** |
| Day 3 | 0.6 ± 0.5 | 0.4 ± 0.5 | 0.6 ± 0.5 | 0.4 ± 0.5 | 3.2 ± 0.8 *** |
| Reticuloctes (%) | |||||
| Day 1 | 5.2 ± 0.8 | 5.4 ± 0.5 | 4.8 ± 0.8 | 5.6 ± 0.5 | 3.4 ± 0.5 *** |
| Day 2 | 5.2 ± 1.3 | 5.4 ± 0.9 | 6.0 ± 1.0 | 5.2 ± 1.1 | 2.8 ± 0.8 *** |
| Day 3 | 5.8 ± 1.1 | 5.2 ± 1.3 | 5.6 ± 1.3 | 5.2 ± 1.3 | 3.6 ± 1.1 *** |
| Gender | Male | |||
| PS128 (mg/kg (Body Weight/Day)) | 0 mg/kg Control | 40 mg/kg Low | 400 mg/kg Middle | 2400 mg/kg High |
| Number of Animals | 10 | 10 | 10 | 10 |
| Mean of Body Weight per Day in Different Week (g) | ||||
| Week 0 | 33.3 ± 1.5 | 34.6 ± 1.3 | 34.5 ± 1.9 | 34.4 ± 1.8 |
| Week 1 | 35.0 ± 2.2 | 35.4 ± 1.2 | 34.7 ± 1.6 | 34.4 ± 2.0 |
| Week 2 | 36.1 ± 2.4 | 35.8 ± 1.4 | 35.3 ± 1.6 | 34.8 ± 2.1 |
| Week 3 | 36.4 ± 2.4 | 35.9 ± 1.8 | 35.9 ± 2.0 | 35.9 ± 3.0 |
| Week 4 | 36.3 ± 2.3 | 36.2 ± 1.8 | 36.3 ± 2.2 | 36.6 ± 3.2 |
| Gender | Female | |||
| PS128 (mg/kg (Body Weight/Day)) | 0 mg/kg Control | 40 mg/kg Low | 400 mg/kg Middle | 2400 mg/kg High |
| Number of Animals | 10 | 10 | 10 | 10 |
| Mean of Body Weight per Day in Different Week (g) | ||||
| Week 0 | 26.1 ± 1.4 | 27.2 ± 1.8 | 27.1 ± 1.3 | 27.6 ± 1.7 |
| Week 1 | 26.3 ± 1.5 | 27.3 ± 2.0 | 27.2 ± 1.0 | 27.3 ± 1.2 |
| Week 2 | 27.3 ± 1.5 | 27.8 ± 1.9 | 28.1 ± 1.5 | 28.2 ± 1.3 |
| Week 3 | 27.9 ± 1.3 | 29.4 ± 2.6 | 29.0 ± 1.7 | 29.1 ± 1.8 |
| Week 4 | 28.6 ± 1.7 | 29.7 ± 2.0 | 30.4 ± 2.5 | 29.7 ± 1.8 |
| Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 40 | 400 | 2400 | 0 | 40 | 400 | 2400 | ||
| Red blood cell count (RBC) | 106/dL | 9.10 ± 1.32 | 9.82 ± 0.66 | 9.59 ± 0.22 | 8.84 ± 2.55 | 9.15 ± 0.32 | 9.30 ± 0.48 | 9.30 ± 0.53 | 8.67 ± 2.79 |
| Hematocrit (HCT) | % | 45.30 ± 6.10 | 46.68 ± 2.77 | 45.99 ± 1.78 | 42.53 ± 12.03 | 45.91 ± 1.64 | 46.06 ± 1.78 | 47.12 ± 2.14 | 46.02 ± 1.23 |
| Hemoglobin (Hb) | g/L | 145.69 ± 12.89 | 147.40 ± 9.73 | 146.00 ± 4.71 | 135.50 ± 41.05 | 144.10 ± 4.04 | 146.00 ± 6.48 | 149.10 ± 6.40 | 150.60 ± 6.90 |
| Mean corpuscular hemoglobin (MCH) | Pg | 16.01 ± 2.89 | 15.02 ± 0.50 * | 15.22 ± 0.42 * | 15.05 ± 1.24 | 15.75 ± 0.24 | 15.73 ± 0.76 | 16.06 ± 0.52 | 15.33 ± 1.75 |
| MCH concentration (MCHC) | g/dL | 31.40 ± 5.39 | 31.58 ± 1.29 | 31.75 ± 0.66 | 31.14 ± 2.93 | 31.41 ± 1.18 | 31.72 ± 1.03 | 31.66 ± 0.68 | 31.45 ± 4.08 |
| Mean corpuscular volume (MCV) | fL | 50.36 ±2.30 | 47.60 ± 1.70 * | 47.96 ± 1.33 * | 48.44 ± 2.26 * | 50.25 ± 2.53 | 49.64 ± 2.37 | 50.76 ± 1.92 | 48.93 ±2.41 |
| RBC distribution width coefficient of variation (RDW-CV) | % | 19.39 ± 2.01 | 19.87 ± 1.10 | 19.33 ± 0.45 | 18.95 ± 2.06 | 19.24 ± 0.49 | 19.33 ± 0.86 | 19.13 ± 0.69 | 18.69 ± 2.15 |
| RBC distribution width standard deviation (RDW-SD) | fL | 31.39 ± 2.46 | 29.60 ± 2.16 | 29.28 ± 0.90 | 28.93 ± 2.13 | 30.72 ± 1.46 | 30.30 ± 1.44 | 30.80 ± 1.96 | 28.67 ± 2.45 |
| Platelet distribution width (PDW) | fL | 6.77 ± 0.32 | 7.15 ± 0.34 | 6.77 ± 0.24 | 6.75 ± 0.54 | 6.79 ± 0.35 | 6.89 ± 0.20 | 7.09 ± 0.52 | 6.84 ± 0.34 |
| Mean platelet volume (MPV) | fL | 6.62 ± 0.36 | 6.69 ± 0.27 | 6.41 ± 0.21 | 6.51 ± 0.15 | 6.59 ± 0.28 | 6.58 ± 0.21 | 6.63 ± 0.36 | 6.59 ± 0.24 |
| White blood cell count (WBC) | 103/μL | 5.74 ± 2.40 | 5.46 ± 2.38 | 5.13 ± 1.30 | 5.86 ± 3.09 | 3.38 ± 0.56 | 5.30 ± 1.59 * | 5.29 ± 1.83 * | 5.97 ± 2.26 * |
| Lymphocytes | % | 82.91 ± 4.44 | 80.84 ± 8.29 | 85.87 ± 4.03 | 82.92 ± 3.07 | 79.90 ± 5.07 | 84.72 ± 5.86 | 85.37 ± 4.18 | 85.61 ± 6.32 |
| Neutrophils | % | 13.79 ± 4.31 | 15.53 ± 6.09 | 11.78 ± 3.23 | 14.07 ± 2.94 | 16.43 ± 3.09 | 12.38 ± 4.84 | 12.02 ± 4.37 * | 11.44 ± 6.07 * |
| Monocytes | % | 1.45 ± 0.55 | 1.03 ± 0.41 | 0.83 ± 0.42 | 1.10 ± 0.49 | 0.44 ± 0.20 | 1.04 ± 0.80 | 0.99 ± 0.43 | 0.96 ± 0.51 |
| Eosinophil | % | 1.54 ± 1.14 | 1.53 ± 0.69 | 1.38 ± 0.67 | 1.77 ± 1.31 | 1.25 ± 0.79 | 1.75 ± 0.70 | 1.57 ± 0.62 | 1.84 ± 1.74 |
| Basophils | % | 0.11 ± 0.12 | 0.08 ± 0.10 | 0.14 ± 0.13 | 0.14 ± 0.13 | 0.30 ± 0.48 | 0.11 ± 0.25 | 0.05 ± 0.11 | 0.14 ± 0.12 |
| Platelets count (PLT) | 106/μL | 1.23 ± 0.56 | 1.48 ± 0.41 | 1.59 ± 0.11 | 1.39 ± 0.49 | 1.29 ± 0.30 | 1.41 ± 0.22 | 1.37 ± 0.26 | 1.31 ± 0.30 |
| Platelet large cell ratio (P-LCR) | % | 4.94 ± 1.67 | 5.24 ± 1.61 | 3.84 ± 0.87 | 4.35 ± 1.03 | 4.46 ± 1.68 | 4.53 ± 1.08 | 4.68 ± 1.72 | 4.33 ± 1.09 |
| Plateletcrit (PCT) | % | 0.81 ± 0.38 | 0.99 ± 0.27 | 1.02 ± 0.08 | 0.91 ± 0.32 | 0.85 ± 0.19 | 0.93 ± 0.15 | 0.91 ± 0.19 | 0.80 ± 0.30 |
| Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Daily Dose (g/kg (b.w/day)) | 0 | 0.04 | 0.4 | 2.4 | 0 | 0.04 | 0.4 | 2.4 | |
| Calcium | mg/dL | 7.88 ± 0.68 | 8.09 ± 1.08 | 8.35 ± 0.47 | 8.78 ± 0.58 | 8.30 ± 0.60 | 8.53 ± 0.55 | 8.59 ± 0.55 | 8.88 ± 0.73 * |
| Chloride | mmol/L | 84.80 ± 1.30 | 84.30 ± 1.34 | 83.60 ± 2.12 | 85.40 ± 1.65 | 85.20 ± 2.74 | 84.40 ± 2.17 | 84.50 ± 1.90 | 84.50 ± 1.08 |
| Phosphorus | mg/dL | 6.50 ± 1.05 | 6.10 ± 1.02 | 6.01 ± 1.16 | 6.67 ± 0.75 | 6.79 ± 0.51 | 6.31 ± 0.74 | 6.44 ± 0.63 | 6.86 ± 0.97 |
| Potassium | mmol/L | 4.24 ± 0.57 | 4.01 ± 0.50 | 4.13 ± 0.36 | 4.40 ± 0.49 | 4.02 ± 0.30 | 4.39 ± 0.26 | 4.33 ± 0.32 | 4.17 ± 0.52 |
| Sodium | mmol/L | 130.40 ± 1.34 | 129.90 ± 1.37 | 129.90 ± 1.73 | 130.10 ± 0.74 | 132.60 ± 2.17 | 132.50 ± 2.99 | 132.40 ± 1.51 | 132.10 ± 2.08 |
| Glucose | mg/dL | 125.00 ± 61.53 | 75.40 ± 41.40 * | 98.50 ± 28.27 | 87.10 ± 21.05 * | 119.40 ± 22.32 | 140.60 ± 43.39 | 155.30 ± 36.95 | 150.20 ± 37.32 |
| Total bilirubin (TBIL) | mg/dL | 0.12 ± 0.04 | 0.24 ± 0.18 | 0.24 ± 0.11 | 0.20 ± 0.05 | 0.41 ± 0.11 | 0.37 ± 0.11 | 0.44 ± 0.13 | 0.41 ± 0.14 |
| Alanine aminotransferase (ALT) | U/L | 68.80 ± 60.14 | 66.40 ± 31.02 | 51.80 ± 37.95 | 49.50 ± 51.60 | 40.10 ± 18.02 | 27.90 ± 9.36 | 35.89 ± 13.17 | 30.10 ± 9.89 |
| Aspartate aminotransferase (AST) | U/L | 102.40 ± 22.21 | 87.50 ± 25.89 | 76.60 ± 17.71 | 76.60 ± 28.58 | 109.90 ± 27.31 | 76.80 ± 12.35 * | 87.40 ± 18.81 * | 82.00 ± 18.35 * |
| Alkaline phosphatase (ALP) | U/L | 238.40 ± 89.90 | 210.50 ± 69.02 | 228.10 ± 51.36 | 265.70 ± 65.13 | 340.40 ± 55.86 | 354.80 ± 126.63 | 303.80 ± 67.18 | 365.40 ± 82.67 |
| Creatinine | mg/dL | 0.24 ± 0.05 | 0.24 ± 0.10 | 0.16 ± 0.08 | 0.20 ± 0.09 | 0.23 ± 0.05 | 0.19 ± 0.07 | 0.18 ± 0.04 | 0.19 ± 0.06 |
| Blood urea nitrogen (BUN) | mg/dL | 34.28 ± 3.44 | 35.86 ± 5.53 | 30.99 ± 1.66 | 32.04 ± 4.38 | 23.58 ± 4.03 | 26.61 ± 5.39 | 27.01 ± 7.29 | 29.69 ± 8.24 |
| Albumin | g/dL | 2.88 ± 0.23 | 3.00 ± 0.38 | 3.15 ± 0.24 | 3.23 ± 0.19 | 3.28 ± 0.52 | 3.34 ± 0.35 | 2.93 ± 1.04 | 3.15 ± 0.33 |
| Total protein | g/dL | 5.79 ± 0.42 | 5.93 ± 0.40 | 6.00 ± 0.33 | 6.30 ± 0.37 | 6.15 ± 0.73 | 6.37 ± 0.30 | 6.22 ± 0.33 | 6.09 ± 0.41 |
| Cholesterol | mg/dL | 109.40 ± 17.80 | 126.40 ± 16.29 | 128.80 ± 31.00 | 140.25 ± 22.48 | 104.00 ± 14.16 | 111.70 ± 16.80 | 97.90 ± 22.11 | 119.20 ± 24.54 |
| Triglycerides | mg/dL | 106.40 ± 15.19 | 96.90 ± 23.52 | 128.70 ± 35.81 | 143.80 ± 47.87 | 86.70 ± 27.54 | 118.10 ± 52.60 | 123.00 ± 33.11 * | 78.30 ± 31.70 |
| Lactate dehydrogenase (LDH) | U/L | 499.40 ± 246.70 | 525.00 ± 274.47 | 470.40 ± 284.01 | 355.70 ± 223.76 | 276.33 ± 28.74 | 293.30 ± 96.82 | 334.70 ± 104.52 | 303.60 ± 87.05 |
| Amylase | U/L | 1034.20 ± 95.16 | 973.70 ± 124.57 | 882.20 ± 272.25 | 1119.00 ± 222.90 | 885.00 ± 152.56 | 1082.20 ± 289.52 | 857.30 ± 128.63 | 819.10 ± 196.25 |
| Male | Female | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Daily Dose (mg/kg) | 0 | 40 | 400 | 2400 | 0 | 40 | 400 | 2400 | |
| Adrenals | |||||||||
| Absolute Weight | mg | 5.09 ± 1.15 | 4.89 ± 0.89 | 4.81 ± 1.20 | 4.33 ± 0.71 | 14.17 ± 8.53 | 7.89 ± 2.05 | 9.78 ± 2.74 | 9.42 ± 2.47 |
| Ratio per Body Weight | (10−3) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.03 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Heart | |||||||||
| Absolute Weight | mg | 153.00 ± 9.49 | 162.00 ± 20.98 | 146.00 ± 11.74 | 140.00 ± 18.86 | 121.00 ± 11.01 | 133.00 ± 15.67 | 132.00 ± 11.35 | 129.00 ± 14.49 |
| Ratio per Body Weight | (10−3) | 0.41 ± 0.04 | 0.45 ± 0.06 | 0.40 ± 0.04 | 0.38 ± 0.05 | 0.42 ± 0.03 | 0.45 ± 0.05 | 0.43 ± 0.03 | 0.43 ± 0.05 |
| Kidneys | |||||||||
| Absolute Weight | mg | 517.00 ± 41.11 | 514.00 ± 60.22 | 488.00 ± 59.78 | 477.00 ± 46.20 | 365.00 ± 25.50 | 377.00 ± 35.61 | 385.00 ± 49.72 | 372.00 ± 23.48 |
| Ratio per Body Weight | (10−3) | 1.40 ± 0.09 | 1.42 ± 0.19 | 1.35 ± 0.13 | 1.31 ± 0.14 | 1.27 ± 0.11 | 1.27 ± 0.11 | 1.26 ± 0.12 | 1.25 ± 0.07 |
| Liver | |||||||||
| Absolute Weight | mg | 1703 ± 163.91 | 1509 ± 219.42 | 1423 ± 103.39 | 1443 ± 143.53 | 1200 ± 133.67 | 1240 ± 217.61 | 1354 ± 218.39 | 1279 ± 203.06 |
| Ratio per Body Weight | (10−3) | 4.59 ± 0.34 | 4.15 ± 0.45 | 3.94 ± 0.30 | 3.96 ± 0.45 | 4.17 ± 0.31 | 4.18 ± 0.75 | 4.41 ± 0.45 | 4.27 ± 0.44 |
| Spleen | |||||||||
| Absolute Weight | mg | 119.00 ± 32.81 | 106.00 ± 27.16 | 93.00 ± 22.14 | 78.00 ± 19.32 | 115.00 ± 21.21 | 95.00 ± 23.21 | 101.00 ± 18.53 | 104.00 ± 22.21 |
| Ratio per Body Weight | (10−3) | 0.32 ± 0.07 | 0.29 ± 0.06 | 0.26 ± 0.06 | 0.21 ± 0.05 | 0.40 ± 0.05 | 0.32 ± 0.08 | 0.33 ± 0.05 | 0.35 ± 0.06 |
| Testis/Ovary | |||||||||
| Absolute Weight | mg | 228.00 ± 22.01 | 217.00 ± 46.92 | 220.00 ± 35.28 | 222.00 ± 37.95 | 24.00 ± 5.16 | 22.00 ± 6.32 | 28.00 ± 7.89 | 27.00 ± 6.75 |
| Ratio per Body Weight | (10−3) | 0.62 ± 0.07 | 0.60 ± 0.14 | 0.61 ± 0.09 | 0.61 ± 0.13 | 0.08 ± 0.02 | 0.07 ± 0.02 | 0.09 ± 0.03 | 0.09 ± 0.02 |
| Epididymis/Uterus | |||||||||
| Absolute Weight | mg | 53.00 ± 9.49 | 54.00 ± 8.97 | 45.00 ± 5.27 | 50.00 ± 8.16 | 142.00 ± 72.23 | 153.00 ± 61.29 | 138.00 ± 56.53 | 125.00 ± 41.97 |
| Ratio per Body Weight | (10−3) | 0.14 ± 0.03 | 0.15 ± 0.06 | 0.12 ± 0.01 | 0.14 ± 0.03 | 0.49 ± 0.25 | 0.51 ± 0.19 | 0.45 ± 0.19 | 0.42 ± 0.13 |
| Lung | |||||||||
| Absolute Weight | mg | 195.00 ± 12.69 | 193.00 ± 18.29 | 183.00 ± 17.67 | 175.00 ± 17.16 | 176.00 ± 10.75 | 175.00 ± 8.50 | 176.00 ± 19.55 | 175.00 ± 14.34 |
| Ratio per Body Weight | (10−3) | 0.53 ± 0.05 | 0.53 ± 0.04 | 0.51 ± 0.05 | 0.48 ± 0.05 | 0.61 ± 0.05 | 0.59 ± 0.04 | 0.58 ± 0.04 | 0.59 ± 0.04 |
| Antibiotics | Cut-Off Values of L. plantarum a (mg/L) | PS128 | |
|---|---|---|---|
| MICs (mg/L) | Interpretation | ||
| ampicillin | 2 | <0.25 | S |
| vancomycin | n.r. | n.r. | n.r. |
| gentamicin | 16 | 2 | S |
| kanamycin | 64 | 32 | S |
| streptomycin | n.r. | n.r. | n.r. |
| erythromycin | 1 | 0.125 | S |
| clindamycin | 2 | 2 | S |
| tetracycline | 32 | 8 | S |
| chloramphenicol | 8 | 2 | S |
| Genes | Presence of Genes | Primer Sequence (5’–3’) |
|---|---|---|
| hdc | - | AGATGGTATTGTTTCTTATG |
| AGACCATACACCATAACCTT | ||
| tdc-1 | - | GAYATNATNGGNATNGGNYTNGAYCARG |
| CCRTARTCNGGNATAGCRAARTCNGTRTG | ||
| tdc-1 | - | CCACTGCTGCATCTGTTTG |
| CCRTARTCNGGNATAGCRAARTCNGTRTG | ||
| odc-2 | - | GTNTTYAAYGCNGAYAARACNTAYTTYGT |
| TACRCARAATACTCCNGGNGGRTANGG | ||
| odc-2 | - | GTNTTYAAYGCNGAYAARCANTAYTTYGT |
| ATNGARTTNAGTTCRCAYTTYTCNGG |
| Assay | Purpose | Reference | Results |
|---|---|---|---|
| The Ames Salmonella/microsome mutagenicity assay | To assess the potential mutagenic effect using the bacterial strain Salmonella typhimurium | [21] | Negative |
| Chromosome aberration test | To assess the potential mutagenic effect in vitro | [22] | Negative |
| Micronucleus assay | To assess the potential mutagenic effect in vivo | [23] | Negative |
| 28 day subacute toxicity study | To exam the possible health hazards likely to arise from repeated exposure of PS128 | [16] | Negative; NOAEL: more than 2.4 g/kg |
| Antibiotic resistance profile | To test the possibility of resistance to antibiotics | [24] | Negative |
| Detection of amino acid decarboxylase genes | To detect the possible biogenic amine-producing potential | [20] | Negative |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, P.-L.; Wu, C.-C.; Chen, T.-Y.; Tsai, Y.-C.; Peng, W.-S.; Yang, D.-J.; Kang, J.-J. Toxicity Studies of Lactobacillus plantarum PS128TM Isolated from Spontaneously Fermented Mustard Greens. Foods 2019, 8, 668. https://doi.org/10.3390/foods8120668
Liao P-L, Wu C-C, Chen T-Y, Tsai Y-C, Peng W-S, Yang D-J, Kang J-J. Toxicity Studies of Lactobacillus plantarum PS128TM Isolated from Spontaneously Fermented Mustard Greens. Foods. 2019; 8(12):668. https://doi.org/10.3390/foods8120668
Chicago/Turabian StyleLiao, Po-Lin, Chien-Chen Wu, Tai-Ying Chen, Ying-Chieh Tsai, Wu-Shun Peng, Deng-Jye Yang, and Jaw-Jou Kang. 2019. "Toxicity Studies of Lactobacillus plantarum PS128TM Isolated from Spontaneously Fermented Mustard Greens" Foods 8, no. 12: 668. https://doi.org/10.3390/foods8120668
APA StyleLiao, P.-L., Wu, C.-C., Chen, T.-Y., Tsai, Y.-C., Peng, W.-S., Yang, D.-J., & Kang, J.-J. (2019). Toxicity Studies of Lactobacillus plantarum PS128TM Isolated from Spontaneously Fermented Mustard Greens. Foods, 8(12), 668. https://doi.org/10.3390/foods8120668