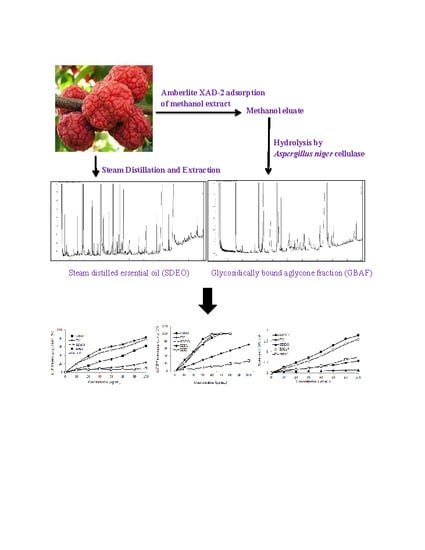
Chemical Composition and Antioxidant Activity of Steam-Distilled Essential Oil and Glycosidically Bound Volatiles from Maclura Tricuspidata Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Materials
2.3. Isolation of Steam-Distilled Essential Oil
2.4. Isolation of Free Volatiles and Glycosidically Bound Volatiles
2.5. Gas Chromatography (GC) and GC–Mass Spectrometry (GC–MS) Analysis
2.6. Determination of Total Phenolic Content
2.7. Antioxidant Activity
2.7.1. Preparation of Sample
2.7.2. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Free Radical-Scavenging Activity
2.7.3. ABTS (2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid)) Free Radical-Scavenging Activity
2.7.4. Ferric-Reducing Antioxidant Power (FRAP)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the Steam-Distilled Essential Oil (SDEO) Fraction
3.2. Chemical Composition of Glycosidically Bound Aglycone Fraction (GBAF)
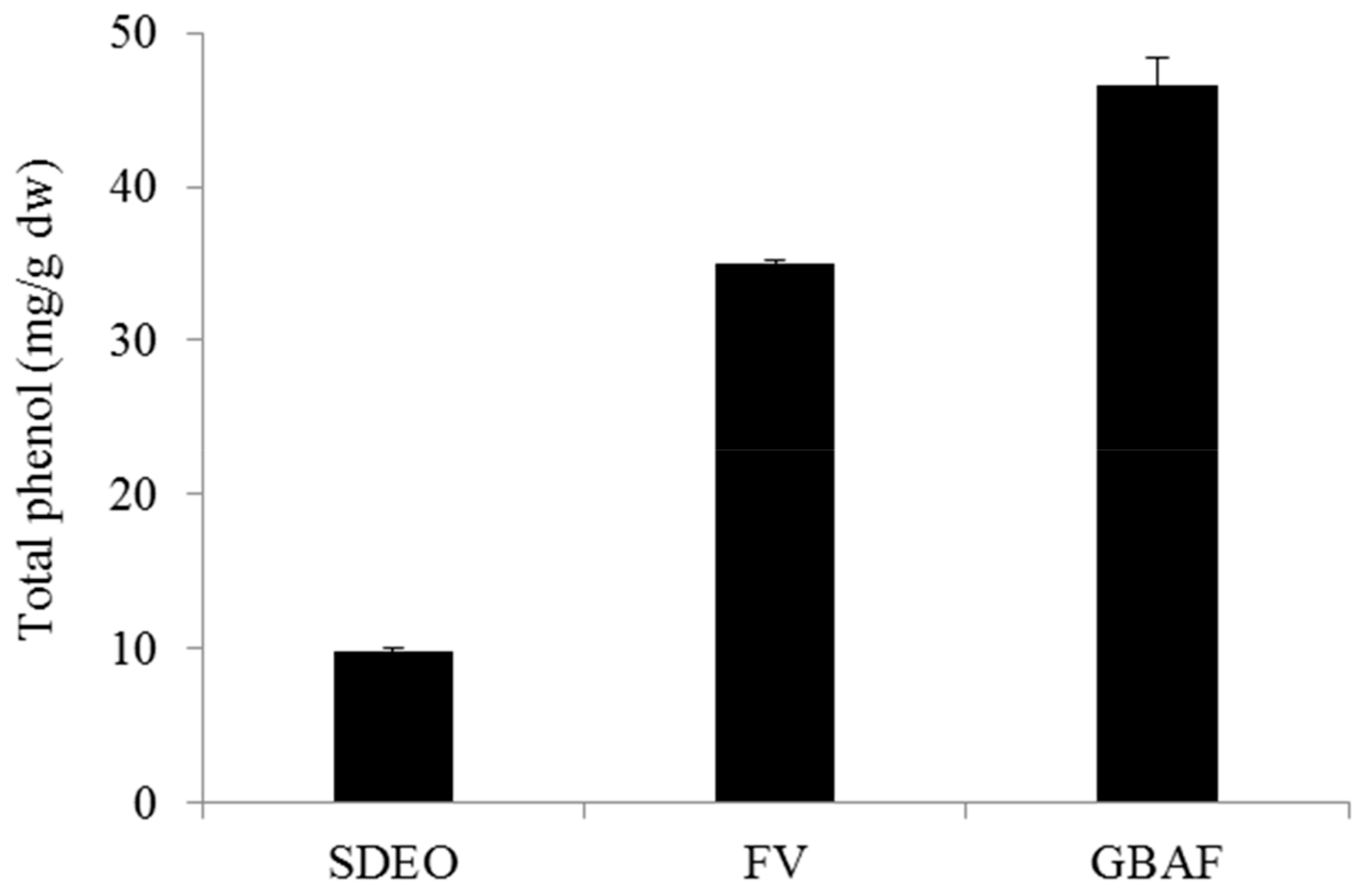
3.3. Total Phenol Contents of Fractions
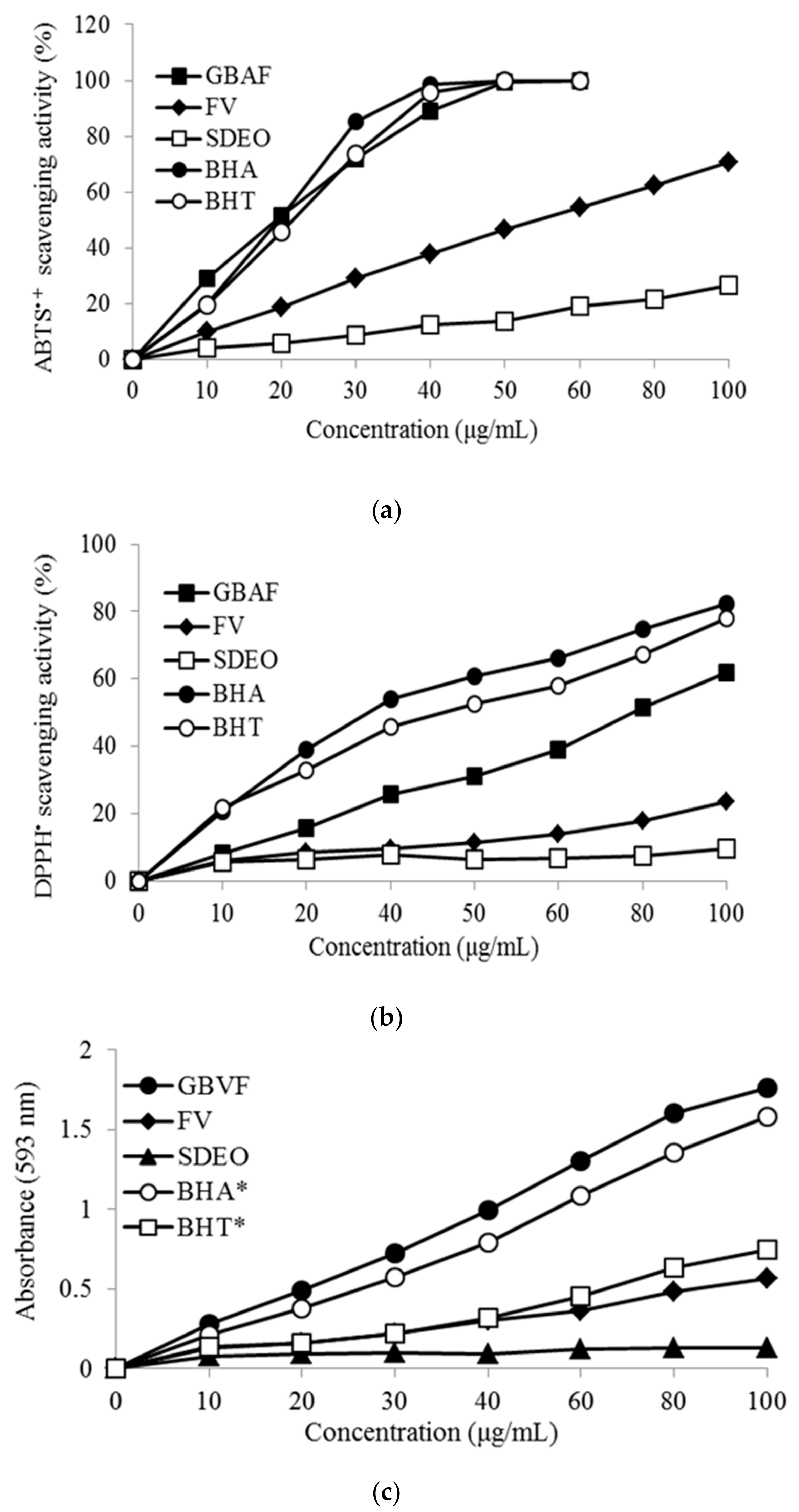
3.4. Antioxidant Activity of SDEO and GBAF
3.5. Antioxidant Activity of Individual Phenolic Compounds in GBAF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Sendra, E. Essential Oils in Foods: From Ancient Times to the 21st Century. Foods 2016, 5, 43. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phyther. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Wang, H.F.; Yih, K.H.; Yang, C.H.; Huang, K.F. Anti-oxidant activity and major chemical component analyses of twenty-six commercially available essential oils. J. Food Drug Anal. 2017, 25, 881–889. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between Free Radical Scavenging and Antioxidant Activity in Foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Williams, G.M.; Iatropoulos, M.J.; Whysner, J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-Based Delivery Systems for Lipophilic Bioactive Components. J. Food Sci. 2007, 72, R109–R124. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Hiep, N.T.; Kwon, J.; Kim, D.W.; Hong, S.; Guo, Y.; Hwang, B.Y.; Kim, N.; Mar, W.; Lee, D. Neuroprotective constituents from the fruits of Maclura tricuspidata. Tetrahedron 2017, 73, 2747–2759. [Google Scholar] [CrossRef]
- Seo, W.G.; Pae, H.O.; Oh, G.S.; Chai, K.Y.; Yun, Y.G.; Chung, H.T.; Jang, K.K.; Kwon, T.O. Ethyl acetate extract of the stem bark of cudrania tricuspidata induces apoptosis in human leukemia HL-60 cells. Am. J. Chin. Med. 2001, 29, 313–320. [Google Scholar] [CrossRef]
- Kwon, S.-B.; Kim, M.-J.; Yang, J.M.; Lee, H.P.; Hong, J.T.; Jeong, H.S.; Kim, E.S.; Yoon, D.Y. Cudrania tricuspidata Stem Extract Induces Apoptosis via the Extrinsic Pathway in SiHa Cervical Cancer Cells. PLoS ONE 2016, 11, e0150235. [Google Scholar] [CrossRef]
- Chang, S.H.; Jung, E.J.; Lim, D.G.; Oyungerel, B.; Lim, K.I.; Her, E.; Choi, W.S.; Jun, M.H.; Choi, K.D.; Han, D.J.; et al. Anti-inflammatory action of Cudrania tricuspidata on spleen cell and T lymphocyte proliferation. J. Pharm. Pharmacol. 2008, 60, 1221–1226. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, J.-W.; Youn, K.-S. Antioxidant Activities of Extracts from Fermented Mulberry (Cudrania tricuspidata) Fruit and Inhibitory Actions on Elastase and Tyrosinase. Korean J. Food Preserv. 2011, 18, 236–243. [Google Scholar] [CrossRef]
- Jeong, C.-H.; Choi, G.-N.; Kim, J.-H.; Kwak, J.-H.; Heo, H.-J.; Shim, K.-H.; Cho, B.-R.; Bae, Y.-I.; Choi, J.-S. In vitro Antioxidative Activities and Phenolic Composition of Hot Water Extract from Different Parts of Cudrania tricuspidata. Prev. Nutr. Food Sci. 2009, 14, 283–289. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.; Chung, Y.W.; Kim, B.M.; Kim, H.; Kim, K.; Yang, K.M. Antiobesity and Antidiabetes Effects of a Cudrania tricuspidata Hydrophilic Extract Presenting PTP1B Inhibitory Potential. Biomed Res. Int. 2016. [Google Scholar] [CrossRef]
- Han, X.H.; Hong, S.S.; Jin, Q.; Li, D.; Kim, H.K.; Lee, J.; Kwon, S.H.; Lee, D.; Lee, C.K.; Lee, M.K.; et al. Prenylated and Benzylated Flavonoids from the Fruits of Cudrania tricuspidata. J. Nat. Prod. 2009, 72, 164–167. [Google Scholar] [CrossRef]
- Hwang, J.H.; Hong, S.S.; Han, X.H.; Hwang, J.S.; Lee, D.; Lee, H.; Yun, Y.P.; Kim, Y.; Ro, J.S.; Hwang, B.Y. Prenylated Xanthones from the Root Bark of Cudrania tricuspidata. J. Nat. Prod. 2007, 70, 1207–1209. [Google Scholar] [CrossRef]
- Xin, L.T.; Yue, S.J.; Fan, Y.C.; Wu, J.S.; Yan, D.; Guan, H.S.; Wang, C.Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017, 7, 31807–31832. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.; Lee, S.J.; Ham, I.; Whang, W.K. Antioxidant activities of new flavonoids from Cudrania tricuspidata root bark. Arch. Pharm. Res. 2009, 32, 195–200. [Google Scholar] [CrossRef]
- Song, S.-H.; Ki, S.; Park, D.-H.; Moon, H.S.; Lee, C.D.; Yoon, I.S.; Cho, S.S. Quantitative Analysis, Extraction Optimization, and Biological Evaluation of Cudrania tricuspidata Leaf and Fruit Extracts. Molecules 2017, 22, 1489. [Google Scholar] [CrossRef]
- Shin, G.R.; Lee, S.; Lee, S.; Do, S.G.; Shin, E.; Lee, C.H. Maturity stage-specific metabolite profiling of Cudrania tricuspidata and its correlation with antioxidant activity. Ind. Crops Prod. 2015, 70, 322–331. [Google Scholar] [CrossRef]
- Kim, D.-W.; Lee, W.-J.; Asmelash Gebru, Y.; Choi, H.S.; Yeo, S.H.; Jeong, Y.J.; Kim, S.; Kim, Y.H.; Kim, M.K. Comparison of Bioactive Compounds and Antioxidant Activities of Maclura tricuspidata Fruit Extracts at Different Maturity Stages. Molecules 2019, 24, 567. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K. Antioxidant efficacy, lipid peroxidation inhibition and phenolic content of essential oil of fruits of cudrania tricuspidata. Bangladesh J. Bot. 2017, 46, 1015–102046. [Google Scholar]
- Stahl-Biskup, E.; Intert, F.; Holthuijzen, J.; Stengele, M.; Schulz, G. Glycosidically bound volatiles—A review 1986–1991. Flavour Fragr. J. 1993, 8, 61–80. [Google Scholar] [CrossRef]
- Winterhalter, P.; Skouroumounis, G.K. Glycoconjugated aroma compounds: Occurrence, role and biotechnological transformation. Adv. Biochem. Eng. Biotechnol. 1997, 55, 73–105. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Maric, S.; Jukic, M.; Katalinic, V.; Milos, M. Comparison of Chemical Composition and Free Radical Scavenging Ability of Glycosidically Bound and Free Volatiles from Bosnian Pine (Pinus heldreichii Christ. var. leucodermis). Molecules 2007, 12, 283–289. [Google Scholar] [CrossRef]
- Schultz, T.H.; Flath, R.A.; Mon, T.R.; Eggling, S.B.; Teranishi, R. Isolation of Volatile Components from a Model System. J. Agric. Food Chem. 1977, 25, 446–449. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. A 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Aubert, C.; Ambid, C.; Baumes, R.; Günata, Z. Investigation of Bound Aroma Constituents of Yellow-Fleshed Nectarines (Prunus persica L. Cv. Springbright). Changes in Bound Aroma Profile during Maturation. J. Agric. Food Chem. 2003, 51, 6280–6286. [Google Scholar] [CrossRef]
- Aubert, C.; Günata, Z.; Ambid, C.; Baumes, R. Changes in Physicochemical Characteristics and Volatile Constituents of Yellow- and White-Fleshed Nectarines during Maturation and Artificial Ripening. J. Agric. Food Chem. 2003, 51, 3083–3091. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Available online: http://www.hindawin.com/journals/ecam/2014/253875/abs/ (accessed on 23 March 2014).
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Strauss, C.R.; Gooley, P.R.; Wilson, B.; Williams, P.J. Application of droplet countercurrent chromatography to the analysis of conjugated forms of terpenoids, phenols, and other constituents of grape juice. J. Agric. Food Chem. 1987, 35, 519–524. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Dugelay, I.; Sapis, J.C.; Baumes, R.; Bayonove, C. Role of enzymes in the use of the flavor potential from grape glycosides in winemaking. In Progress in Flavor Precursor Studies; 1994.
- Synthesis and enantiodifferentiation of isomeric theaspiranes. Available online: https://doi.org/10.1021/jf00019a022 (accessed on 1 July 1992).
- Zelena, K.; Hardebusch, B.; Hülsdau, B.; Berger, R.G.; Zorn, H. Generation of Norisoprenoid Flavors from Carotenoids by Fungal Peroxidases. J. Agric. Food Chem. 2009, 57, 9951–9955. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, H.; Ding, S.; Kropachev, K.; Schwaid, A.G.; Tang, Y.; Mu, H.; Wang, S.; Geacintov, N.E.; Zhang, Y.; et al. Free energy profiles of base flipping in intercalative polycyclic aromatic hydrocarbon-damaged DNA duplexes: Energetic and structural relationships to nucleotide excision repair susceptibility. Chem. Res. Toxicol. 2013, 26, 1115–1125. [Google Scholar] [CrossRef]
- Winterhalter, P.; Rouseff, R.L. (Eds.) Carotenoid-Derived Aroma Compounds; American Chemical Society: Washington, DC, USA, 2001; Volume 802. [Google Scholar] [CrossRef]
- Novruzov, E.N.; Agamirov, U.M. Carotinoids of Cudrania tricuspidata fruit. Chem. Nat. Compd. 2002, 38, 468–469. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Adedeji, J.; Hartman, T.G.; Lech, J.; Ho, C.T. Characterization of glycosidically bound aroma compounds in the African mango (Mangifera indica L.). J. Agric. Food Chem. 1992, 40, 659–661. [Google Scholar] [CrossRef]
- Kicel, A.; Wolbiś, M. Study on the phenolic constituents of the flowers and leaves of Trifolium repens L. Nat. Prod. Res. 2012, 26, 2050–2054. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Sun, Y.X.; Shahidi, F. Preparation and antioxidant activity of tyrosol and hydroxytyrosol esters. J. Funct. Foods. 2017, 37, 66–73. [Google Scholar] [CrossRef]
- Cho, B.R.; Ryu, D.R.; Lee, K.S.; Lee, D.K.; Bae, S.; Kang, D.G.; Ke, Q.; Singh, S.S.; Ha, K.S.; Kwon, Y.G.; et al. P-Hydroxybenzyl alcohol-containing biodegradable nanoparticle improves functional blood flow through angiogenesis in a mouse model of hindlimb ischemia. Biomaterials 2015, 53, 679–687. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Li, X.; Du, Y.; Zhang, L.; Ran, L.; Zhou, N.N. Protective effect and mechanism of p-hydroxybenzaldehyde on blood-brain barrier. Zhongguo Zhongyao Zazhi 2018, 43, 1021–1027. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Daliu, P.; Santini, A. Fruit-based juices: Focus on antioxidant properties—Study approach and update. Phyther. Res. 2019, 33. [Google Scholar] [CrossRef]
- Parada, F.; Duque, C.; Fujimoto, Y. Free and Bound Volatile Composition and Characterization of Some Glucoconjugates as Aroma Precursors in Melón de Olor Fruit Pulp (Sicana o dorifera). J. Agric. Food Chem. 2000, 48, 6200–6204. [Google Scholar] [CrossRef]
- Choi, J.; Yeo, S.; Kim, M.; Lee, H.; Kim, S. p-Hydroxybenzyl alcohol inhibits four obesity-related enzymes in vitro. J. Biochem. Mol. Toxicol. 2018, 32, e22223. [Google Scholar] [CrossRef]
- Coelho, E.; Genisheva, Z.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Vinegar production from fruit concentrates: Effect on volatile composition and antioxidant activity. J. Food Sci. Technol. 2017, 54, 4112–4122. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Kashanian, S.; Ezzati Nazhad Dolatabadi, J. In vitro studies on calf thymus DNA interaction and 2-tert-butyl-4-methylphenol food additive. Eur. Food Res. Technol. 2010, 230, 821–825. [Google Scholar] [CrossRef]
- Fagali, N.; Catalá, A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH{radical dot} techniques. Biophys. Chem. 2008, 137, 56–62. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH-assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Lee, Y.; Oh, J.; Jeong, Y.S. Lactobacillus plantarum-mediated conversion of flavonoid glycosides into flavonols, quercetin, and kaempferol in Cudrania tricuspidata leaves. Food Sci. Biotechnol. 2015, 24, 1817–1821. [Google Scholar] [CrossRef]

| PeakNo | tR (min) | Compounds | RI 1) | RI 2) | Concentration (μg/100 g dw) 3) | |
|---|---|---|---|---|---|---|
| SDEO | GBAF | |||||
| Alcohols | ||||||
| 1 | 5.363 | 2-Methyl-1-butanol | 737 | 1206 | 3.03 ± 0.25 | 1036.0 ± 124.6 |
| 5 | 7.735 | trans-2-Hexen-1-ol | 862 | 1405 | 7.33 ± 1.53 | − 5) |
| 8 | 10.318 | 5-Methyl-2-furfuryl alcohol | 956 | − 4) | 3.17 ± 0.76 | - |
| 20 | 19.585 | 3,4-Dimethylcyclohexanol 6) | 1109 | - | 15.67 ± 2.08 | - |
| Aldehydes and ketones | ||||||
| 3 | 6.644 | Furfural | 819 | 1459 | 53.67 ± 6.03 | - |
| 4 | 7.378 | trans-2-Hexenal | 848 | 1201 | 10.33 ± 3.06 | - |
| 2 | 6.284 | n-Hexanal | 804 | 1097 | 3.00 ± 0.80 | - |
| 7 | 8.777 | 2-Acetyl furan | 903 | 1493 | 5.33 ± 1.53 | - |
| 9 | 10.513 | 5-Methylfufural | 966 | 1508 | 4.13 ± 0.81 | - |
| 10 | 12.102 | Benzaldehyde | 971 | 1508 | 6.33 ± 1.53 | - |
| 11 | 12.491 | 6-Methyl-5-hepten-2-one | 989 | 1326 | 6.33 ± 1.53 | - |
| 12 | 13.267 | 1-(2-Furanyl)-3-butanone 6) | 1006 | - | 4.03 ± 0.55 | - |
| 16 | 15.179 | Phenylacetaldehyde | 1039 | 1629 | 44.33 ± 3.51 | 5.33 ± 1.04 |
| 18 | 18.958 | n-Nonanal | 1104 | 1388 | 140.3 ± 20.5 | - |
| 23 | 22.487 | 10-Undecenal 6) | 1146 | - | 6.67 ± 2.52 | - |
| 24 | 23.306 | 2,4-Dimethylbenzaldehyde 6) | 1158 | 1712 | 7.03 ± 1.55 | - |
| 44 | 41.734 | Genanyl acetone | 1451 | 1860 | 17.33 ± 2.52 | - |
| 52 | 44.505 | 2-Tridecanone | 1493 | - | 23.67 ± 5.51 | - |
| Terpenoids | ||||||
| 36 | 35.657 | Ylangene | 1356 | 1464 | 10.93 ± 3.10 | - |
| 37 | 36.379 | α-Copaene | 1368 | 1477 | 62.33 ± 51.47 | - |
| 41 | 39.05 | β-Caryophyllene | 1409 | 1565 | 145.7 ± 10.5 | - |
| 43 | 39.533 | α-Bergamotene | 1416 | 1575 | 5.67 ± 0.58 | - |
| 45 | 41.982 | β-Humulene | 1454 | - | 10.33 ± 2.52 | - |
| 53 | 45.905 | δ-Cadinene | 1517 | 1754 | 147.7 ± 7.5 | - |
| 58 | 50.101 | Caryophyllene oxide | 1588 | 1968 | 56.33 ± 3.51 | - |
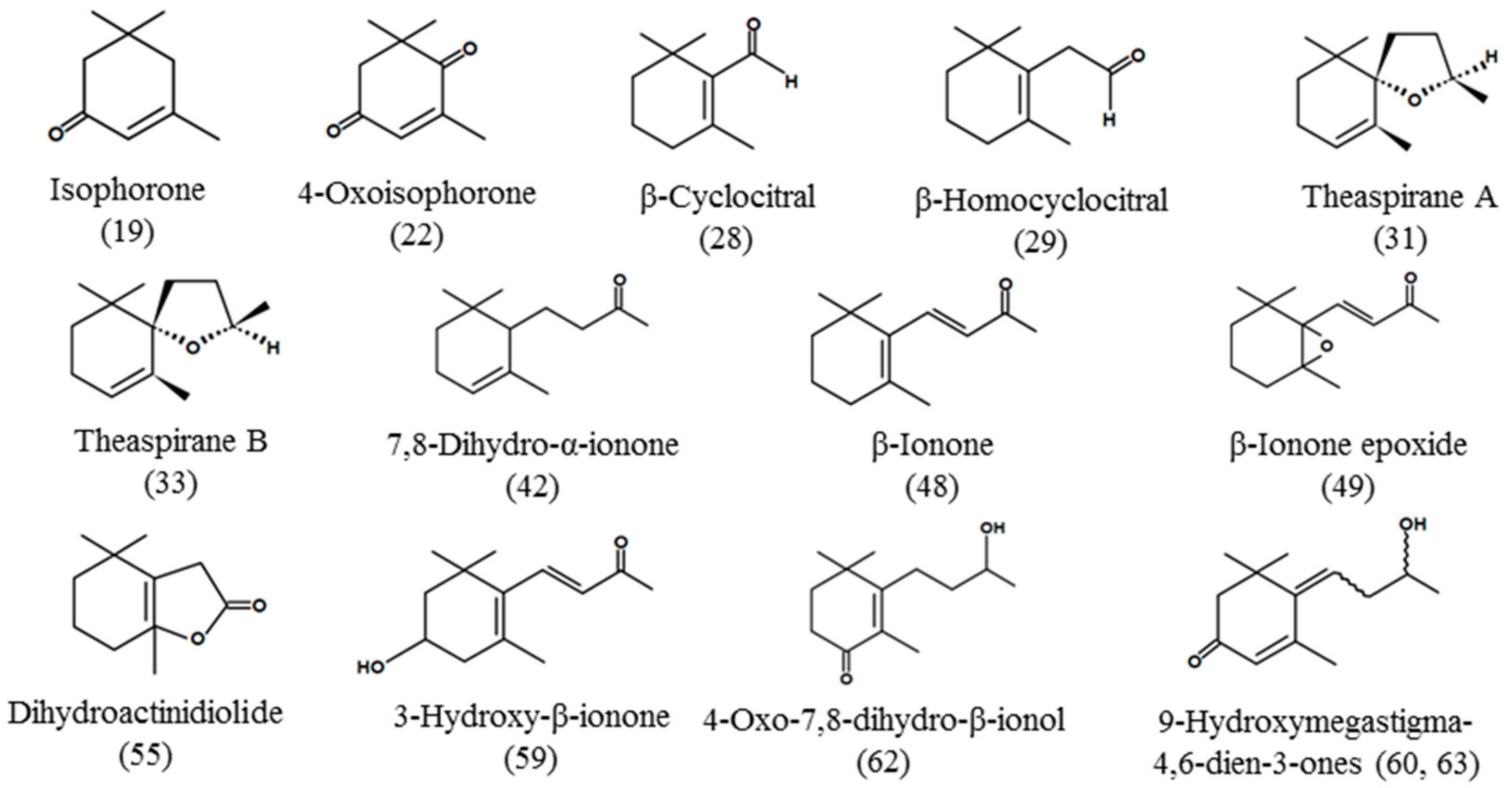
| Carotenoid-derived compounds | ||||||
| 14 | 15.079 | 2,2,6-Trimethylcyclohexanone 6) | 1037 | 1300 | 5.57 ± 0.51 | - |
| 19 | 19.303 | Isophorone | 1119 | 1578 | 7.10 ± 1.85 | - |
| 22 | 21.727 | 4-Oxoisophorone 6) | 1115 | 1674 | 5.33 ± 0.58 | - |
| 28 | 26.163 | β-Cyclocitral | 1214 | 1603 | 17.10 ± 1.85 | - |
| 29 | 28.870 | β-Homocyclocitral 6) | 1254 | - | 15.10 ± 0.85 | - |
| 31 | 31.253 | Theaspirane A | 1289 | 1482 | 121.3 ± 4.5 | - |
| 33 | 32.447 | Theaspirane B | 1306 | 1522 | 99.67 ± 9.02 | |
| 42 | 39.454 | 7,8-Dihydro-α-ionone 6) | 1415 | 1825 | - | 30.33 ± 2.52 |
| 48 | 43.389 | β-Ionone | 1480 | 1907 | 141.0 ± 4.4 | - |
| 49 | 43.637 | β-Ionone epoxide | 1483 | 1957 | 92.33 ± 9.71 | - |
| 55 | 46.692 | Dihydroactinidiolide 6) | 1530 | 2291 | 10.67 ± 5.51 | - |
| 59 | 56.486 | 3-Hydroxy-β-ionone 6) | 1698 | 2646 | - | 160.7 ± 30.0 |
| 60 | 57.969 | 9-Hydroxymegastigma-4,6-dien-3-one (isomer #1) 6) | 1705 | 2677 | - | 197.67 ± 9.45 |
| 61 | 58.525 | 4-Oxo-7,8-dihydro-β-ionol | 1725 | 2694 | - | 76.00 ± 11.00 |
| 63 | 61.311 | 9-Hydroxymegastigma-4,6-dien-3-one (isomer #2) 6) | 1786 | 2846 | - | 234.3 ± 24.5 |
| Aromatic and phenolic compounds | ||||||
| 15 | 15.292 | Benzyl alcohol | 1040 | 1864 | - | 883.7 ± 29.8 |
| 17 | 18.294 | p-Cresol | 1092 | 2074 | 393.5 ± 17.7 | 43.00 ± 7.55 |
| 21 | 19.694 | 2-Phenylethyl alcohol | 1113 | 1892 | - | 58.85 ± 4.58 |
| 26 | 25.427 | Pyrocatechol 7) | 1203 | 2646 | - | 20.33 ± 5.51 |
| 30 | 31.225 | Resorcinol | 1288 | - | - | 57.33 ± 10.50 |
| 32 | 31.523 | Carvacrol | 1293 | 2213 | 19.37 ± 3.46 | - |
| 34 | 34.006 | α-Methoxy-p-cresol 7) | 1331 | 2490 | - | 2783.0 ± 143.0 |
| 35 | 34.981 | p-Vinylguaiacol | 1346 | 2181 | - | 17.33 ± 3.51 |
| 25 | 24.925 | Methyl chavicol | 1171 | 1658 | 66.67 ± 9.02 | - |
| 38 | 37.539 | 2,4,6-Trihydroxybenzaldehyde | 1386 | - | 9.33 ± 1.53 | - |
| 39 | 38.473 | p-Hydroxybenzyl alcohol 7) | 1400 | 2952 | 17.67 ± 3.06 | 468.1 ± 30.9 |
| 40 | 38.977 | p-Hydroxybenzaldehyde 7) | 1408 | 2964 | - | 170.0 ± 19.5 |
| 46 | 42.529 | Tyrosol 7) | 1463 | 2969 | - | 68.67 ± 4.51 |
| 47 | 43.524 | p-Methylsalicylaldehyde 7) | 1478 | - | 43.00 ± 10.82 | 4088.0 ± 147.8 |
| 50 | 44.116 | Methyl p-hydroxybenzoate 7) | 1487 | 1969 | - | 289.3 ± 12.5 |
| 51 | 44.439 | Vanillyl alcohol 7) | 1492 | - | - | 30.67 ± 3.27 |
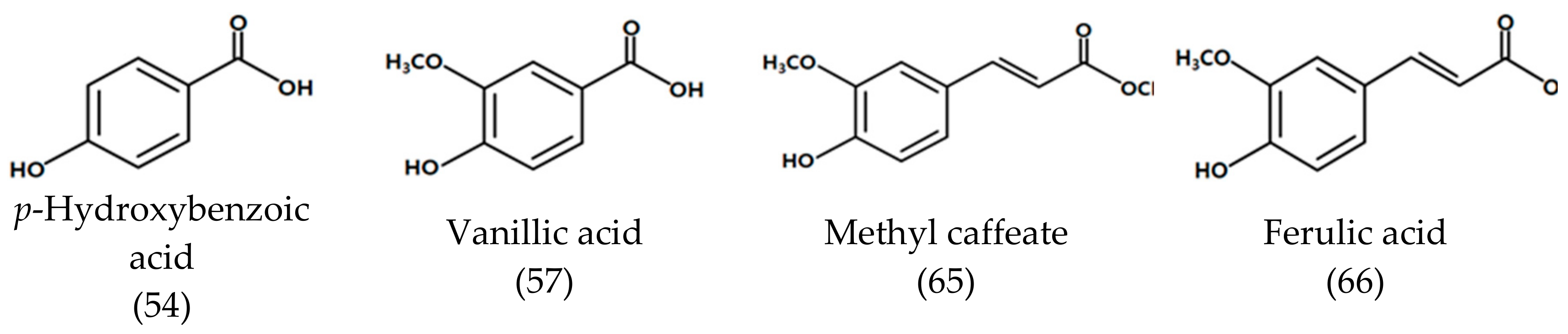
| 54 | 46.293 | p-Hydroxybenzoic acid 7) | 1523 | - | - | 20.33 ± 4.51 |
| 56 | 46.955 | Methyl caffeate 7) | 1532 | 2593 | - | 31.33 ± 4.51 |
| 57 | 48.027 | Vanillic acid 7) | 1583 | - | - | 22.67 ± 4.04 |
| 65 | 64.199 | Methyl ferulate | 1844 | - | - | 92.00 ± 28.62 |
| 66 | 65.320 | Ferulic acid 7) | 1865 | - | - | 383.0 ± 26.6 |
| 76 | 79.425 | p-(p-Hydroxybenzyl)phenol 6) | 2166 | - | - | 133.1 ± 12.9 |
| Aliphatic acids and esters | ||||||
| 62 | 61.180 | Myristic acid | 1775 | 2694 | 124.2 ± 10.3 | - |
| 64 | 61.871 | Ethyl myristate | 1798 | 2041 | 9.33 ± 1.53 | - |
| 67 | 65.807 | Pentadecanoic acid | 1875 | 2822 | 9.33 ± 2.52 | - |
| 68 | 68.480 | Methyl palmitate | 1928 | 2212 | 55.75 ± 6.23 | - |
| 69 | 71.900 | Palmitic acid | 1986 | 2953 | 813.1 ± 39.5 | - |
| 70 | 72.100 | Ethyl palmitate | 2002 | 2277 | 291.7 ± 29.0 | - |
| 71 | 76.479 | Methyl linoleate | 2120 | 2485 | 58.16 ± 8.23 | - |
| 72 | 76.776 | Methyl linolenate | 2119 | 2484 | 55.35 ± 10.53 | - |
| 73 | 79.580 | Linoleic acid | 2169 | - | 363.7 ± 39.0 | - |
| 74 | 79.897 | Linolenic acid | 2175 | - | 176.0 ± 22.5 | - |
| 75 | 80.583 | Ethyl linolenate | 2187 | 2585 | 9.33 ± 1.53 | - |
| Miscellaneous | - | |||||
| 6 | 7.987 | p-Xylene | 836 | 1279 | 4.17 ± 0.76 | - |
| 13 | 14.526 | 2-Acetylthiazole 6) | 1027 | - | 4.03 ± 0.35 | - |
| 27 | 25.537 | 2,3-Dihydrobenzofuran | 1205 | 2381 | 3.77 ± 0.68 | 316.0 ± 29.0 |
| Samples | DPPH 1 | ABTS +1 | FRAP 2 |
|---|---|---|---|
| SDEO | 17,065.22 ± 146.27 a | 1921.81 ± 49.45 a | 10,638.56 ± 223.33 a |
| FV | 2507.18 ± 24.21 b | 660.72 ± 7.18 b | 1963.48 ± 10.97 b |
| GBAF | 835.33 ± 6.97 d | 317.09 ± 1.99 d | 529.6 ± 4.73 d |
| BHA | 466.79 ± 7.10 e | 89.15 ± 4.14 e | 129.46 ± 1.61 f |
| BHT | 535.75 ± 3.52 e | 108.62 ± 1.06 e | 331.26 ± 4.68 e |
| Compounds | EC50 (μg/mL) | ||
|---|---|---|---|
| DPPH 1 | ABTS 1 | FRAP 2 | |
| Pyrocatechol | 9.59 ± 1.22 e | 67.68 ± 2.47 jk | 74.45 ± 2.16 jk |
| α-Methoxy-p-cresol | 1114.09 ± 114.45 d | 59.55 ± 6.46 jk | 3298.92 ± 126.20 f |
| p-Hydroxybenzyl alcohol | 3357.55 ± 134.15 c | 377.85 ± 4.78 f | 2854.37 ± 43.04 g |
| p-Hydroxybenzaldehyde | 1765.90 ± 364.23 d | 1117.70 ± 7.01 c | 7906.18 ± 60.96 c |
| Tyrosol | 1331.74 ± 195.63 d | 287.36 ± 3.70 g | 92.64 ± 1.97 jk |
| p-Methylsalicylaldehyde | 1644.14 ± 365.52 d | 423.69 ± 3.13 e | 19,365.27 ± 81.38 b |
| Methyl p-hydroxybenzoate | 5241.03 ± 941.54 b | 12,735.03 ± 47.26a | 6789.61 ± 82.27 d |
| Vanillyl alcohol | 27.96 ± 1.65 e | 66.98 ± 1.99 jk | 5928.60 ± 90.87 e |
| p-Hydroxybenzoic acid | 10,906.51 ± 1103.69 a | 6921.86 ± 50.48 b | 1116.61 ± 11.69 h |
| Vanillic acid | 48.58 ± 2.50 e | 157.22 ± 4.83 h | 161.18 ± 4.25 jk |
| Methyl caffeate | 11.92 ± 0.48 e | 11.91 ± 1.29 l | 7.84 ± 0.28 k |
| Ferulic acid | 24.47 ± 2.59 e | 66.39 ± 2.11 jk | 138.98 ± 3.73 jk |
| BHA | 26.10 ± 0.42 e | 89.27 ± 4.01 ij | 129.46 ± 1.61 jk |
| BHT | 33.71 ± 1.04 e | 108.76 ± 3.93 i | 331.26 ± 4.68 j |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, G.-R.; Gebru, Y.A.; Kim, D.-W.; Kim, D.-H.; Han, H.-A.; Kim, Y.-H.; Kim, M.-K. Chemical Composition and Antioxidant Activity of Steam-Distilled Essential Oil and Glycosidically Bound Volatiles from Maclura Tricuspidata Fruit. Foods 2019, 8, 659. https://doi.org/10.3390/foods8120659
Yong G-R, Gebru YA, Kim D-W, Kim D-H, Han H-A, Kim Y-H, Kim M-K. Chemical Composition and Antioxidant Activity of Steam-Distilled Essential Oil and Glycosidically Bound Volatiles from Maclura Tricuspidata Fruit. Foods. 2019; 8(12):659. https://doi.org/10.3390/foods8120659
Chicago/Turabian StyleYong, Gyung-Rim, Yoseph Asmelash Gebru, Dae-Woon Kim, Da-Ham Kim, Hyun-Ah Han, Young-Hoi Kim, and Myung-Kon Kim. 2019. "Chemical Composition and Antioxidant Activity of Steam-Distilled Essential Oil and Glycosidically Bound Volatiles from Maclura Tricuspidata Fruit" Foods 8, no. 12: 659. https://doi.org/10.3390/foods8120659
APA StyleYong, G.-R., Gebru, Y. A., Kim, D.-W., Kim, D.-H., Han, H.-A., Kim, Y.-H., & Kim, M.-K. (2019). Chemical Composition and Antioxidant Activity of Steam-Distilled Essential Oil and Glycosidically Bound Volatiles from Maclura Tricuspidata Fruit. Foods, 8(12), 659. https://doi.org/10.3390/foods8120659