Substitution Effects of NaCl by KCl and CaCl2 on Lipolysis of Salted Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments, Material and Additives
2.2. Salted Meat Processing
2.3. Lipid Oxidation
2.4. Instrumental Color
2.5. Lipolysis Analysis
2.5.1. Formalin-Fixed Paraffin-Embedded (FFPE) Sample Preparation
2.5.2. Lipids Extraction
2.5.3. Electrospray Ionization Mass Spectrometer (ESI-MS) Analysis
2.5.4. Volatile Compound Profile
2.5.5. Free Fatty Acids Profile
2.5.6. Statistical Analysis
3. Results
3.1. Lipid Oxidation (TBARs)
3.2. Lipolysis
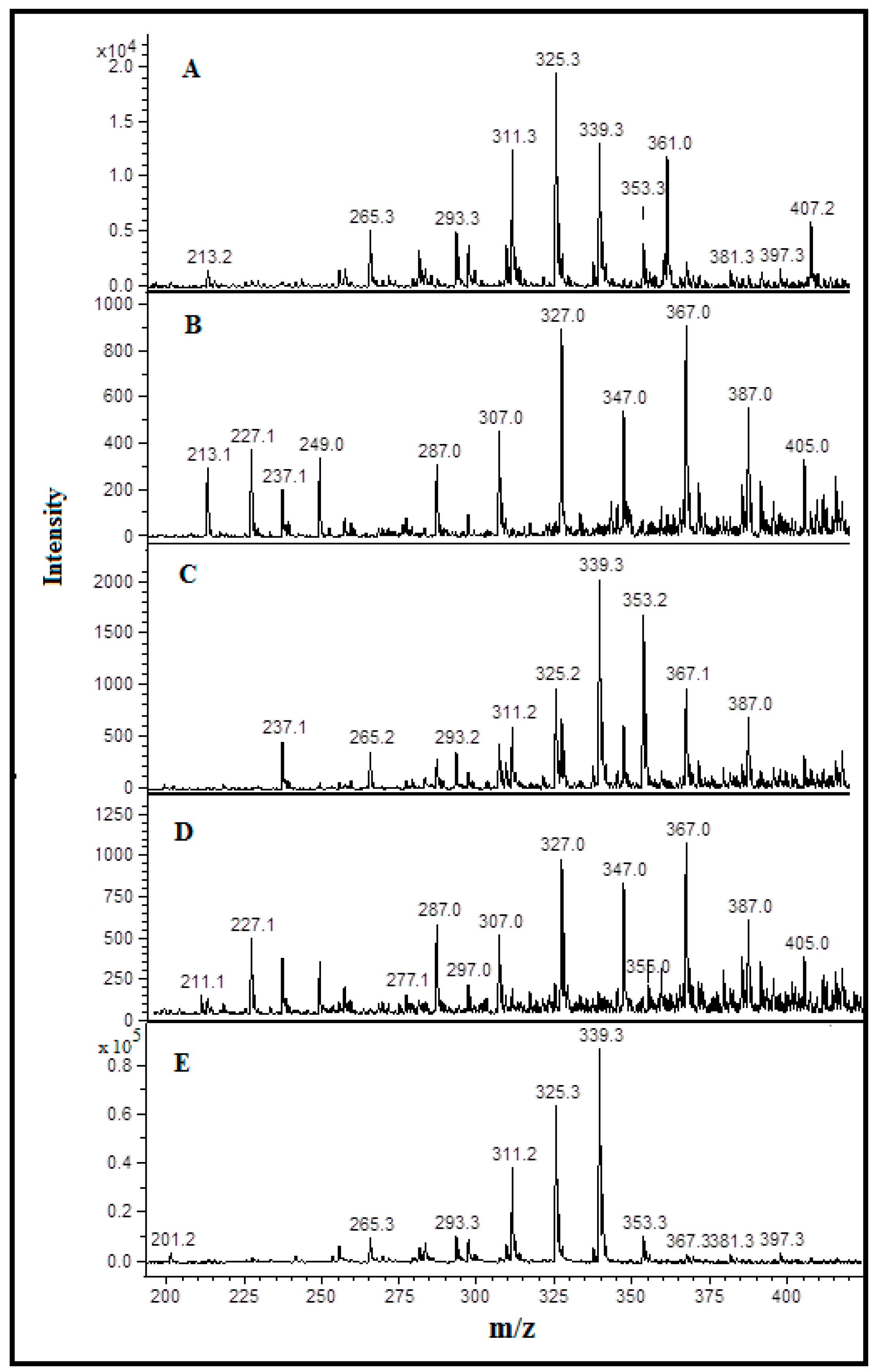
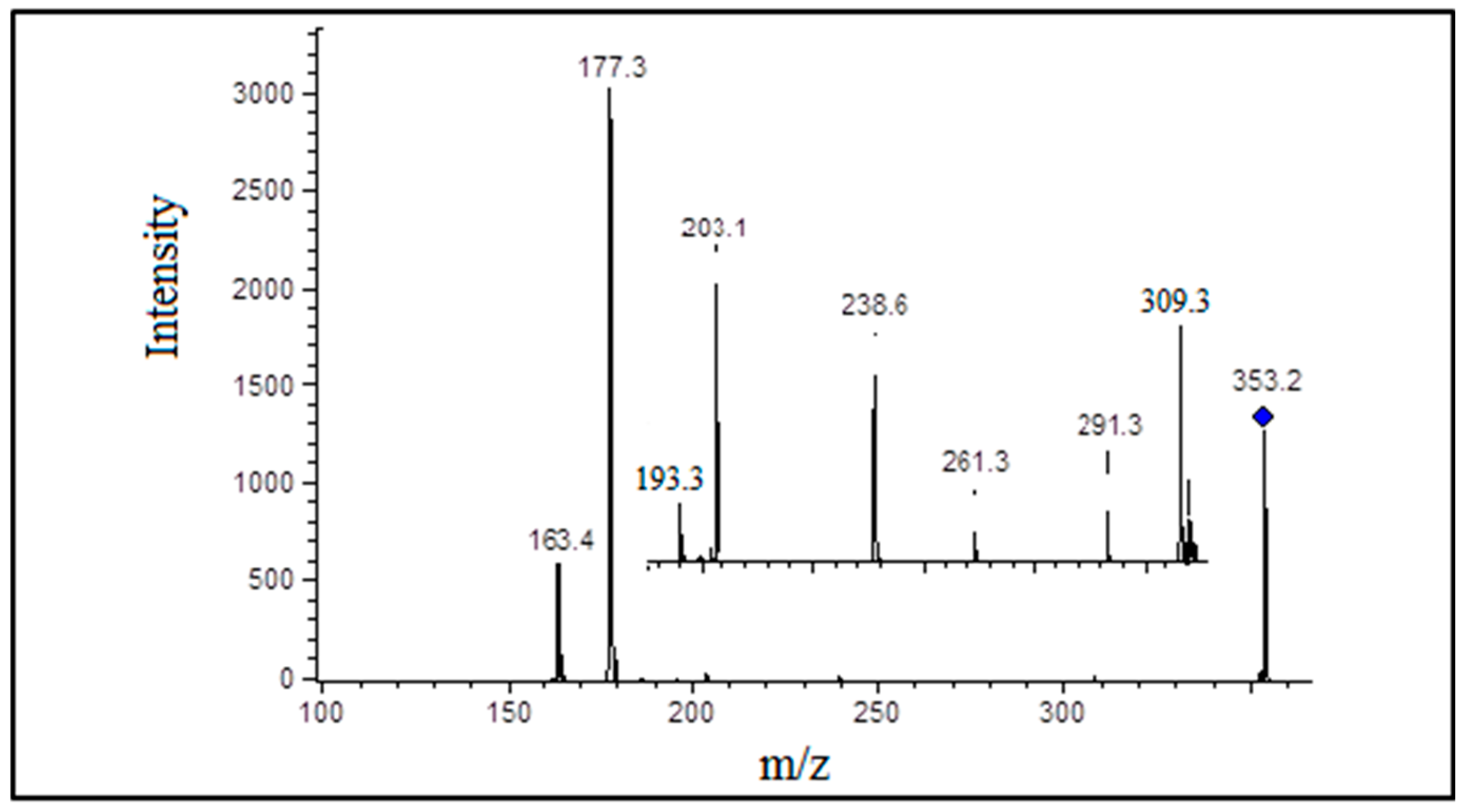
3.3. Detection and Identification of Oxidized Phospholipids
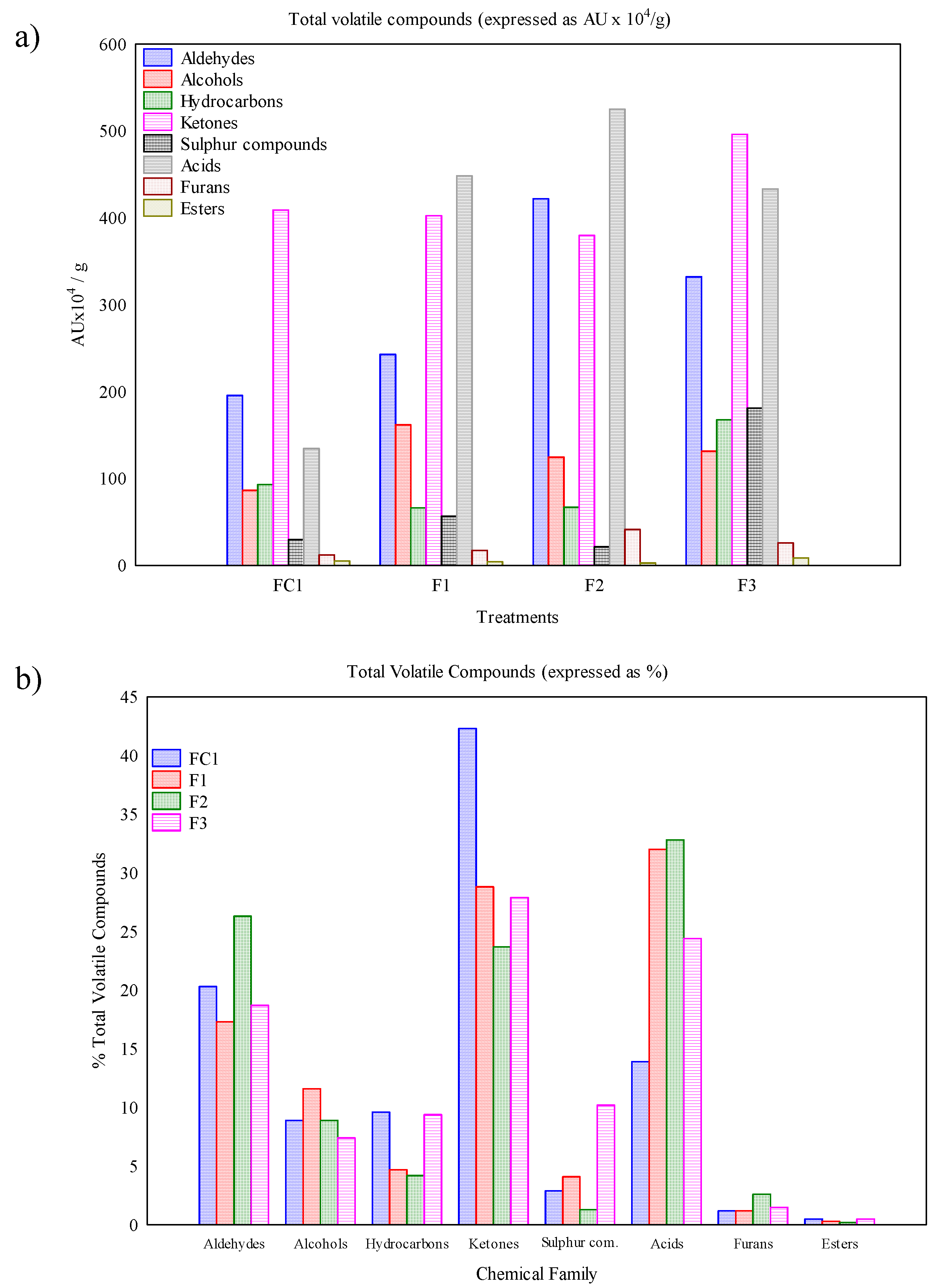
3.4. Volatile Compound Profile
3.5. Free Fatty Acids Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Pu, H.; Sun, D.-W.; Wang, L.; Zeng, X.-A. Combination of spectra and texture data of hyperspectral imaging for prediction of pH in salted meat. Food Chem. 2014, 160, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Vidal, V.A.S.; Biachi, J.P.; Paglarini, C.S.; Pinton, M.B.; Campagnol, P.C.B.; Esmerino, E.A.; da Cruz, A.G.; Morgano, M.A.; Pollonio, M.A.R. Reducing 50% sodium chloride in healthier jerked beef: An efficient design to ensure suitable stability, technological and sensory properties. Meat Sci. 2019, 152, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Picone, G.; De Noni, I.; Ferranti, P.; Nicolai, M.A.; Alamprese, C.; Trimigno, A.; Brodkorb, A.; Portmann, R.; Pihlanto, A.; El, S.N.; et al. Monitoring molecular composition and digestibility of ripened bresaola through a combined foodomics approach. Food Res. Int. 2019, 115, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Molinero, C.; Martínez, B.; Rubio, B.; Rovira, J.; Jaime, I. The effects of extended curing on the microbiological, physicochemical and sensorial characteristics of Cecina de León. Meat Sci. 2008, 80, 370–379. [Google Scholar] [CrossRef]
- Cabrera, M.C.; Saadoun, A. An overview of the nutritional value of beef and lamb meat from South America. Meat Sci. 2014, 98, 435–444. [Google Scholar] [CrossRef]
- Desmond, E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006, 74, 188–196. [Google Scholar] [CrossRef]
- McGough, M.M.; Sato, T.; Rankin, S.A.; Sindelar, J.J. Reducing sodium levels in frankfurters using a natural flavor enhancer. Meat Sci. 2012, 91, 185–194. [Google Scholar] [CrossRef]
- Pires, M.A.; Munekata, P.E.S.; Baldin, J.C.; Rocha, Y.J.P.; Carvalho, L.T.; dos Santos, I.R.; Barros, J.C.; Trindade, M.A. The effect of sodium reduction on the microstructure, texture and sensory acceptance of Bologna sausage. Food Struct. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Ripollés, S.; Campagnol, P.C.B.; Armenteros, M.; Aristoy, M.C.; Toldrá, F. Influence of partial replacement of NaCl with KCl, CaCl2 and MgCl2 on lipolysis and lipid oxidation in dry-cured ham. Meat Sci. 2011, 89, 58–64. [Google Scholar] [CrossRef]
- Mapiye, C.; Aldai, N.; Turner, T.D.; Aalhus, J.L.; Rolland, D.C.; Kramer, J.K.G.; Dugan, M.E.R. The labile lipid fraction of meat: From perceived disease and waste to health and opportunity. Meat Sci. 2012, 92, 210–220. [Google Scholar] [CrossRef]
- Valsta, L.M.; Tapanainen, H.; Männistö, S. Meat fats in nutrition. Meat Sci. 2005, 70, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Chizzolini, R.; Novelli, E.; Zanardi, E. Oxidation in traditional mediterranean meat products. Meat Sci. 1998, 49, S87–S99. [Google Scholar] [CrossRef]
- Ordóñez, J.A.; Hierro, E.M.; Bruna, J.M.; Hoz, L.D.L. Changes in the Components of Dry-Fermented Sausages during Ripening. Crit. Rev. Food Sci. Nutr. 1999, 39, 329–367. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, D. Meat Fermentation: Principles and Applications; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Zm, X. Minimizing rancidity in muscle food. Food Technol. 1998, 52, 54–59. [Google Scholar]
- Kristensen, L.; Purslow, P.P. The effect of processing temperature and addition of mono- and di-valent salts on the heme- nonheme-iron ratio in meat. Food Chem. 2001, 73, 433–439. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Carballo, J.; Cofrades, S. Healthier meat and meat products: Their role as functional foods. Meat Sci. 2001, 59, 5–13. [Google Scholar] [CrossRef]
- Bruna, J.M.; Ordóñez, J.A.; Fernández, M.; Herranz, B.; de la Hoz, L. Microbial and physico-chemical changes during the ripening of dry fermented sausages superficially inoculated with or having added an intracellular cell-free extract of Penicillium aurantiogriseum. Meat Sci. 2001, 59, 87–96. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M.; Jones, M.L.; Totty, B.A. Theory and Practice of Histological Techniques. In Connective Tissues and Stains, 15th ed.; Churchill Livingstone Publications: London, UK, 2004; pp. 139–200. [Google Scholar]
- Wojakowska, A.; Marczak, Ł.; Jelonek, K.; Polanski, K.; Widlak, P.; Pietrowska, M. An Optimized Method of Metabolite Extraction from Formalin-Fixed Paraffin-Embedded Tissue for GC/MS Analysis. PLoS ONE 2015, 10, e0136902. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Bio. Chem. 1957, 226, 497–509. [Google Scholar]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of Volatile Compounds of Dry-Cured Meat Products Using HS-SPME-GC/MS Technique. Food Anal. Method 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Regueiro, J.A.G.; Gibert, J.; Díaz, I. Determination of neutral lipids from subcutaneous fat of cured ham by capillary gas chromatography and liquid chromatography. J. Chromatogr. A 1994, 667, 225–233. [Google Scholar] [CrossRef]
- Alfaia, C.M.M.; Alves, S.P.; Lopes, A.F.; Fernandes, M.J.E.; Costa, A.S.H.; Fontes, C.M.G.A.; Castro, M.L.F.; Bessa, R.J.B.; Prates, J.A.M. Effect of cooking methods on fatty acids, conjugated isomers of linoleic acid and nutritional quality of beef intramuscular fat. Meat Sci. 2010, 84, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Broncano, J.M.; Petrón, M.J.; Parra, V.; Timón, M.L. Effect of different cooking methods on lipid oxidation and formation of free cholesterol oxidation products (COPs) in Latissimus dorsi muscle of Iberian pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- Ma, H.J.; Ledward, D.A.; Zamri, A.I.; Frazier, R.A.; Zhou, G.H. Effects of high pressure/thermal treatment on lipid oxidation in beef and chicken muscle. Food Chem. 2007, 104, 1575–1579. [Google Scholar] [CrossRef]
- Schweiger, M.; Eichmann, T.O.; Taschler, U.; Zimmermann, R.; Zechner, R.; Lass, A. Chapter Ten—Measurement of Lipolysis. In Methods in Enzymology; MacDougald, O.A., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 538, pp. 171–193. [Google Scholar]
- Armenteros, M.; Aristoy, M.-C.; Barat, J.M.; Toldrá, F. Biochemical and Sensory Properties of Dry-Cured Loins as Affected by Partial Replacement of Sodium by Potassium, Calcium, and Magnesium. J. Agric. Food Chem. 2009, 57, 9699–9705. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Carballo, J. Changes in physico-chemical properties and volatile compounds throughout the manufacturing process of dry-cured foal loin. Meat Sci. 2015, 99, 44–51. [Google Scholar] [CrossRef]
- Muriel, E.; Andres, A.I.; Petron, M.J.; Antequera, T.; Ruiz, J. Lipolytic and oxidative changes in Iberian dry-cured loin. Meat Sci. 2007, 75, 315–323. [Google Scholar] [CrossRef]
- Martín, L.; Córdoba, J.J.; Ventanas, J.; Antequera, T. Changes in intramuscular lipids during ripening of Iberian dry-cured ham. Meat Sci. 1999, 51, 129–134. [Google Scholar] [CrossRef]
- Funaro, A.; Cardenia, V.; Petracci, M.; Rimini, S.; Rodriguez-Estrada, M.T.; Cavani, C. Comparison of meat quality characteristics and oxidative stability between conventional and free-range chickens. Poult. Sci. 2014, 93, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.A.; Campagnol, P.C.B.; Fagundes, M.B.; Wagner, R.; Pollonio, M.A.R. Adding Blends of NaCl, KCl, and CaCl2 to Low-Sodium Dry Fermented Sausages: Effects on Lipid Oxidation on Curing Process and Shelf Life. J. Food Qual. 2017, 2017, 7085798. [Google Scholar] [CrossRef]
- dos Santos, B.A.; Campagnol, P.C.B.; Fagundes, M.B.; Wagner, R.; Pollonio, M.A.R. Generation of volatile compounds in Brazilian low-sodium dry fermented sausages containing blends of NaC1, KC1, and CaC12 during processing and storage. Food Res. Int. 2015, 74, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Cheeseman, K.H.; Dianzani, M.U.; Poli, G.; Slater, T.F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem. J. 1982, 208, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible mutagens derived from lipids and lipid precursors. Mutat. Res. Rev. Genet. Toxicol. 1990, 238, 223–233. [Google Scholar] [CrossRef]
- Esterbauer, H.; Lang, J.; Zadravec, S.; Slater, T.F. Detection of Malonaldehyde by High-Performance Liquid Chromatography. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 319–328. [Google Scholar]
- Benedetti, A.; Comporti, M.; Esterbauer, H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta 1980, 620, 281–296. [Google Scholar] [CrossRef]
- Spickett, C.M.; Pitt, A.R. Oxidative lipidomics coming of age: Advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid. Redox Signal. 2015, 22, 1646–1666. [Google Scholar] [CrossRef]
- Motilva, M.-J.; Toldrá, F.; Nieto, P.; Flores, J. Muscle lipolysis phenomena in the processing of dry-cured ham. Food Chem. 1993, 48, 121–125. [Google Scholar] [CrossRef]
- Coutron-Gambotti, C.; Gandemer, G.; Rousset, S.; Maestrini, O.; Casabianca, F. Reducing salt content of dry-cured ham: Effect on lipid composition and sensory attributes. Food Chem. 1999, 64, 13–19. [Google Scholar] [CrossRef]
- Buscailhon, S.; Berdagué, J.L.; Monin, G. Time-related changes in volatile compounds of lean tissue during processing of French dry-cured ham. J. Agric. Food Chem. 1993, 63, 69–75. [Google Scholar] [CrossRef]
- Careri, M.; Mangia, A.; Barbieri, G.; Bouoni, L.; Virgili, R.; Parolari, G. Sensory property relationships to chemical data of italian-type dry-cured ham. J. Food Sci. 1993, 58, 968–972. [Google Scholar] [CrossRef]
- Flores, M.; Ingram, D.A.; Bett, K.L.; Toldrá, F.; Spanier, A.M. Sensory characteristics of spanish “serrano” dry-cured ham. J. Sens. Stud. 1997, 12, 169–179. [Google Scholar] [CrossRef]
- Andres, A.I.; Cava, R.; Martin, D.; Ventanas, J.; Ruiz, J. Lipolysis in dry-cured ham: Influence of salt content and processing conditions. Food Chem. 2005, 90, 523–533. [Google Scholar] [CrossRef]
- Armenteros, M.; Toldrá, F.; Aristoy, M.C.; Ventanas, J.; Estévez, M. Effect of the Partial Replacement of Sodium Chloride by Other Salts on the Formation of Volatile Compounds during Ripening of Dry-Cured Ham. J. Agric. Food Chem. 2012, 60, 7607–7615. [Google Scholar] [CrossRef]
- Domínguez, R.; Munekata, P.; Cittadini, A.; Lorenzo Rodriguez, J.M. Effect of the partial NaCl substitution by other chloride salts on the volatile profile during the ripening of dry-cured lacón. Grasas Aceites 2016, 67, e128. [Google Scholar]
- Marušić, N.; Vidaček, S.; Janči, T.; Petrak, T.; Medić, H. Determination of volatile compounds and quality parameters of traditional Istrian dry-cured ham. Meat Sci. 2014, 96, 1409–1416. [Google Scholar] [CrossRef]
- Petričević, S.; Marušić Radovčić, N.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Ventanas, S.; Estevez, M.; Andrés, A.I.; Ruiz, J. Analysis of volatile compounds of Iberian dry-cured loins with different intramuscular fat contents using SPME–DED. Meat Sci. 2008, 79, 172–180. [Google Scholar] [CrossRef]
- Ramírez, R.; Cava, R. Volatile Profiles of Dry-Cured Meat Products from Three Different Iberian X Duroc Genotypes. J. Agric. Food Chem. 2007, 55, 1923–1931. [Google Scholar] [CrossRef]
- Pérez-Santaescolástica, C.; Carballo, J.; Fulladosa, E.; Garcia-Perez, J.V.; Benedito, J.; Lorenzo, J.M. Effect of proteolysis index level on instrumental adhesiveness, free amino acids content and volatile compounds profile of dry-cured ham. Food Res. Int. 2018, 107, 559–566. [Google Scholar] [CrossRef]
- Pugliese, C.; Sirtori, F.; Škrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Čandek-Potokar, M. The effect of ripening time on the chemical, textural, volatile and sensorial traits of Bicep femoris and Semimembranosus muscles of the Slovenian dry-cured ham Kraški pršut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Rivas, M.; Gallardo, E.; León-Camacho, M. Analysis of volatile compounds from Iberian hams: A review. Grasas Aceites 2012, 63, 432–454. [Google Scholar]
- Gomez, M.; Domínguez, R.; Fonseca, S.; Lorenzo Rodriguez, J.M. Effect of Finishing Diet on Physico-Chemical and Lipolytic Parameters and Volatile Compounds Throughout the Manufacture of Dry-Cured Foal “Cecina”. Austin J. Nut. Food Sci. 2015, 3, 1056. [Google Scholar]
- Lorenzo, J.M. Changes on physico-chemical, textural, lipolysis and volatile compounds during the manufacture of dry-cured foal “cecina”. Meat Sci. 2014, 96, 256–263. [Google Scholar] [CrossRef]
- Montanari, C.; Gatto, V.; Torriani, S.; Barbieri, F.; Bargossi, E.; Lanciotti, R.; Grazia, L.; Magnani, R.; Tabanelli, G.; Gardini, F. Effects of the diameter on physico-chemical, microbiological and volatile profile in dry fermented sausages produced with two different starter cultures. Food Biosci. 2018, 22, 9–18. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Fonseca, S.; Gómez, M.; Domínguez, R. Influence of the salting time on physico-chemical parameters, lipolysis and proteolysis of dry-cured foal “cecina”. LWT-Food Sci. Technol. 2015, 60, 332–338. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Cittadini, A.; Bermúdez, R.; Munekata, P.E.; Domínguez, R. Influence of partial replacement of NaCl with KCl, CaCl2 and MgCl2 on proteolysis, lipolysis and sensory properties during the manufacture of dry-cured lacón. Food Control 2015, 55, 90–96. [Google Scholar] [CrossRef]
- Wu, H.; Yan, W.; Zhuang, H.; Huang, M.; Zhao, J.; Zhang, J. Oxidative stability and antioxidant enzyme activities of dry-cured bacons as affected by the partial substitution of NaCl with KCl. Food Chem. 2016, 201, 237–242. [Google Scholar] [CrossRef]
- Flores, M.; Nieto, P.; Ferrer, J.M.; Flores, J. Effect of calcium chloride on the volatile pattern and sensory acceptance of dry-fermented sausages. Eur. Food Res. Technol. 2005, 221, 624. [Google Scholar] [CrossRef]
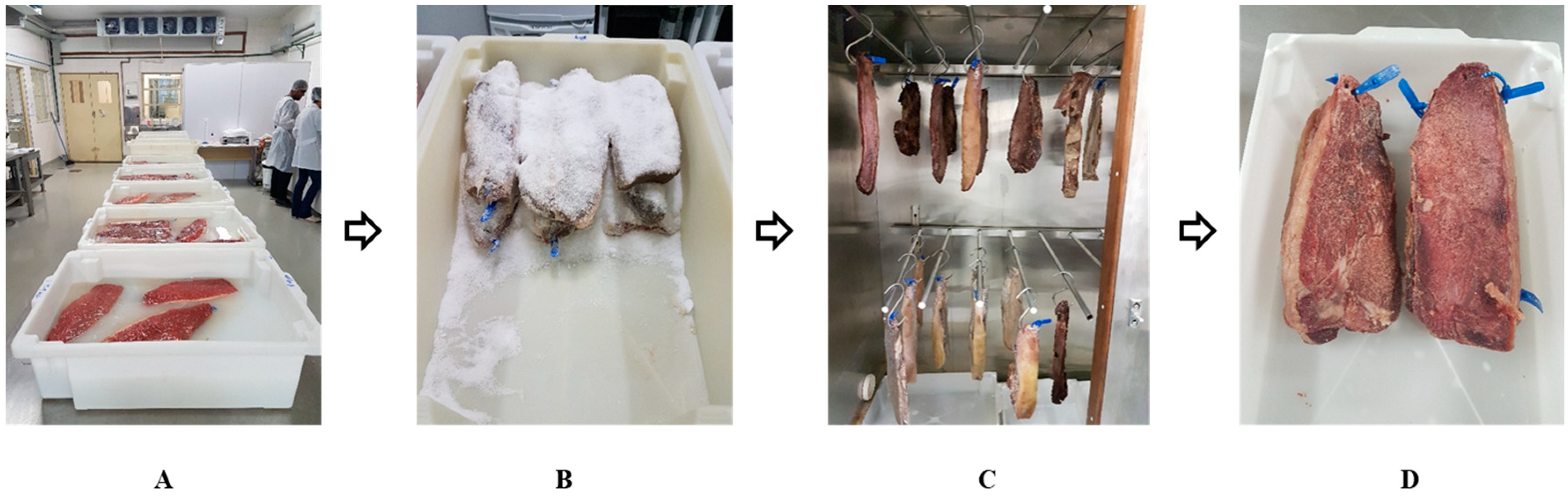
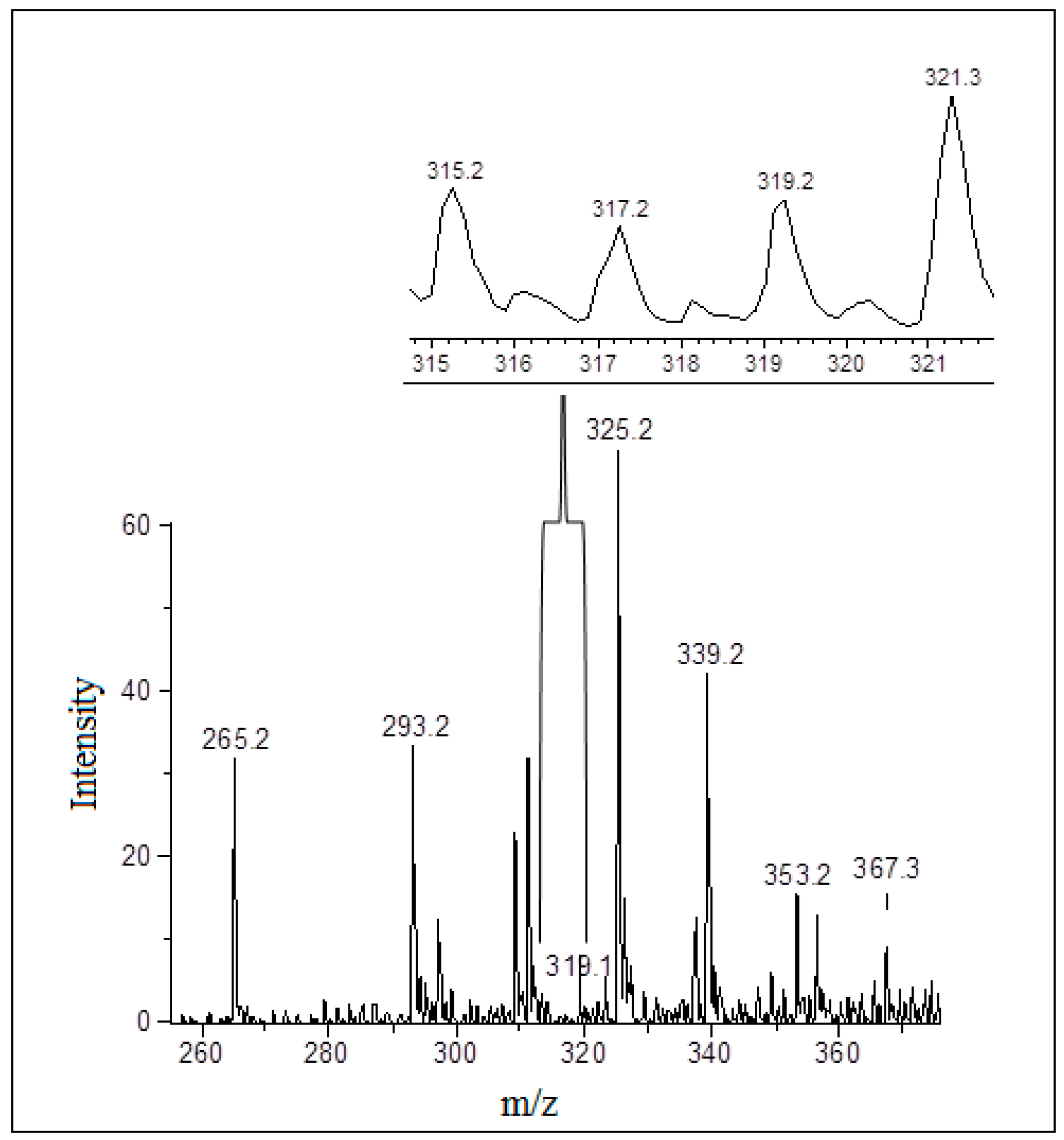
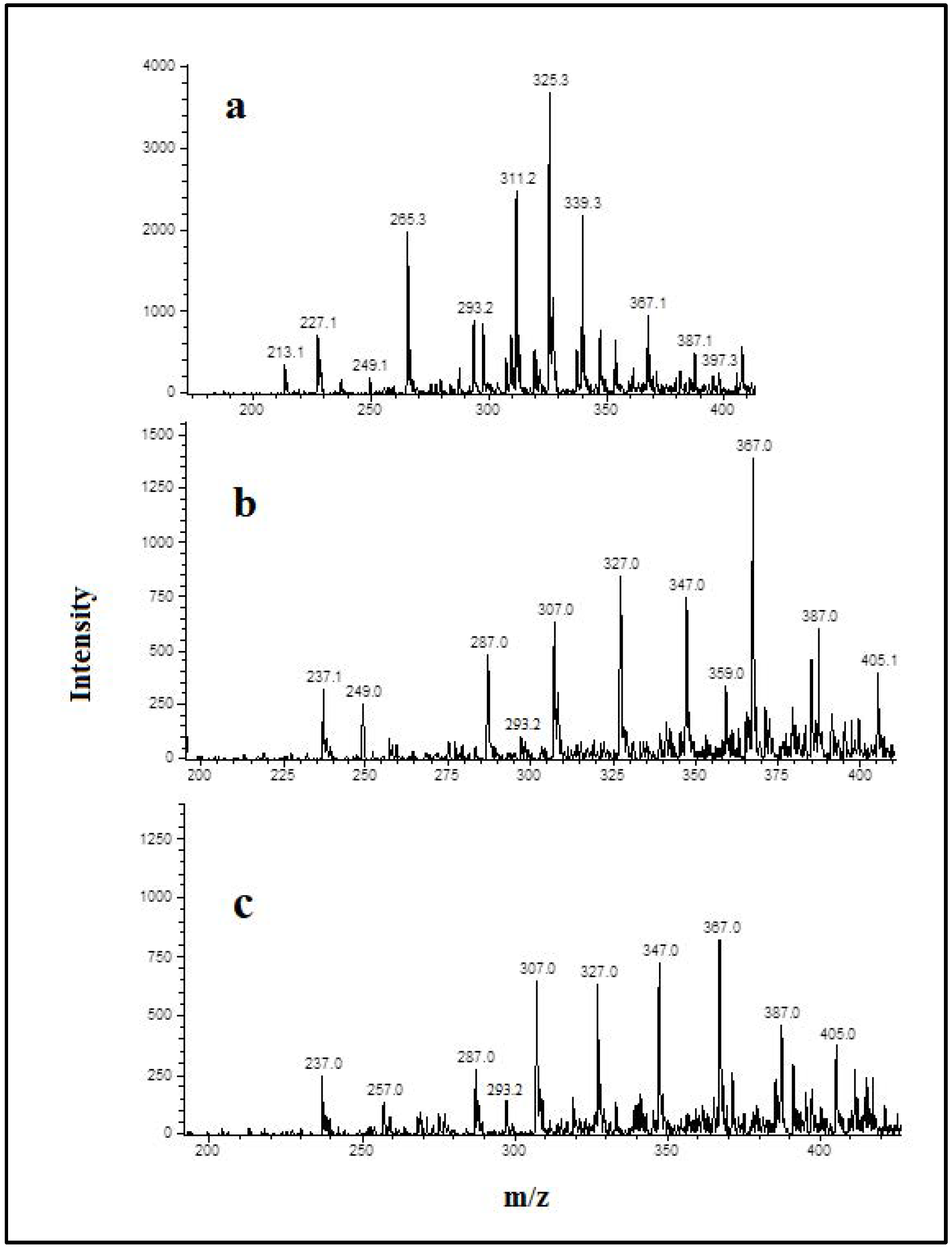
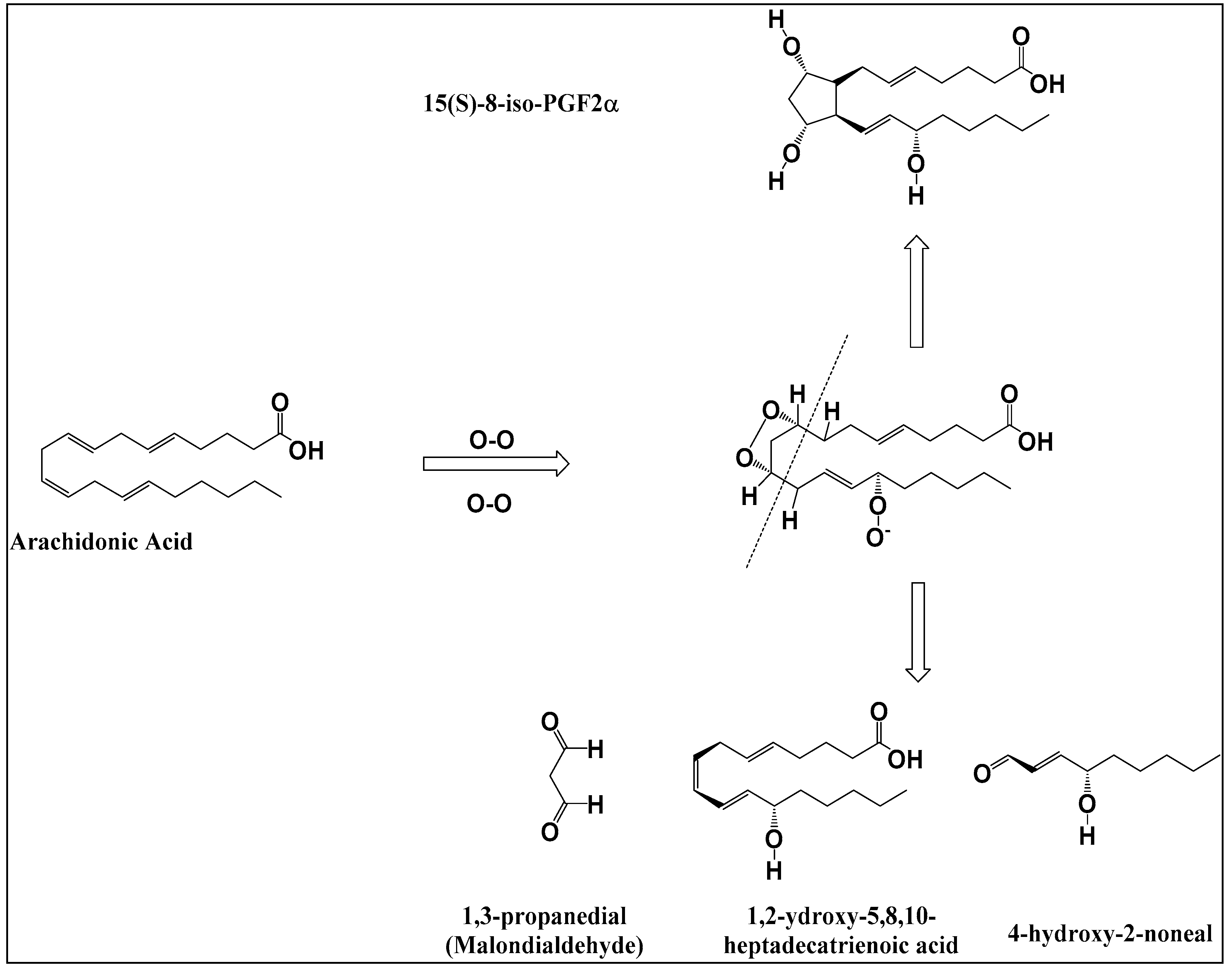
| Treatments | NaCl (%) | NaCl (mg) * | KCl (%) | KCl (mg) * | CaCl2 (%) | CaCl2 (mg) * |
|---|---|---|---|---|---|---|
| FC1 | 100 | 1000 | - | - | ||
| F1 | 50 | 441 | 50 | 560 | - | |
| F2 | 50 | 614 | - | - | 50 | 387 |
| F3 | 50 | 513 | 25 | 326 | 25 | 162 |
| Treatments | 0 Day | 45 Days | 90 Days | 135 Days | 180 Days |
|---|---|---|---|---|---|
| FC1 | 0.95 cC | 1.61 cA | 0.80 bC | 0.75 aC | 1.32 aB |
| F1 | 0.88 cB | 1.78 cA | 0.35 cC | 0.50 bC | 0.71 cB |
| F2 | 1.10 bB | 3.58 aA | 1.09 aB | 0.68 abC | 1.26 aB |
| F3 | 1.74 aB | 2.40 bA | 1.20 aC | 0.38 cD | 0.95 bC |
| Standard error | 0.06 | 0.15 | 0.06 | 0.03 | 0.05 |
| Carbon Annotation | Compound | Precursor (m/z) | Product (m/z) | Chemical Structure |
|---|---|---|---|---|
| 18:2 | LA | 279 | 261 | 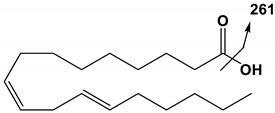 |
| 18:2 | 9-HODE | 295 | 171 | 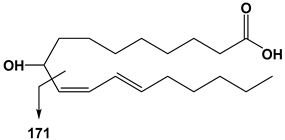 |
| 18:2 | 13-HODE | 295 | 195 | 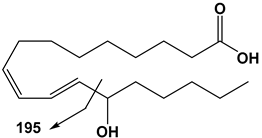 |
| 18:3 | 9-OxoODE | 293 | 185 | 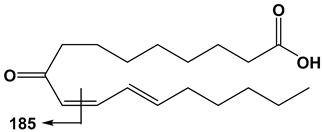 |
| 18:3 | 13-OxoODE | 293 | 113 | 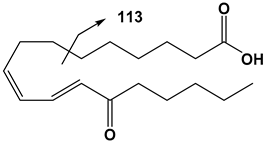 |
| 20:4 | AA | 303 | 259 | 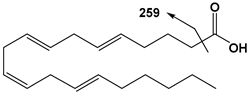 |
| 20:4 | 15-HETE | 319 | 175 | 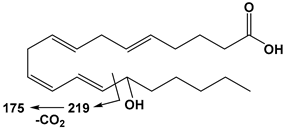 |
| 20:4 | PGF2α | 353 | 193 | 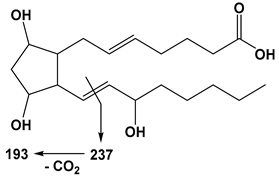 |
| 20:4 | 5-OxoETE | 317 | 203 | 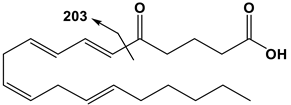 |
| 20:4 | 12-OxoETE | 317 | 153 | 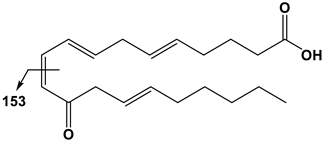 |
| 20:4 | 15-OxoETE | 317 | 113 | 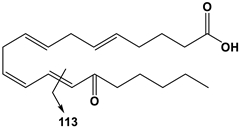 |
| Volatile Compound | LRI | m/z | Treatments | |||
|---|---|---|---|---|---|---|
| FC1 | F1 | F2 | F3 | |||
| Glycidol | 506 | 44 | 8.39 ± 0.52 b | 5.04 ± 1.02 b | 14.08 ± 1.54 a | 14.89 ± 1.70 a |
| Methanethiol | 509 | 48 | 0.41 ± 0.06 b | 0.93 ± 0.06 b | 8.39 ± 1.42 a | 6.80 ± 1.23 a |
| Pentane | 522 | 42 | 8.28 ± 0.26 a | 2.39 ± 1.05 b | 4.06 ± 2.53 ab | 7.93 ± 1.78 a |
| Acetone | 533 | 58 | 137.24 ± 38.23 | 112.18 ± 14.35 | 113.24 ± 30.45 | 164.53 ± 37.41 |
| Dimethyl sulfide | 535 | 62 | 3.74 ± 0.10 a | 3.50 ± 0.11 a | 1.34 ± 0.13 b | 3.13 ± 0.21 a |
| Carbon disulfide | 538 | 76 | 24.85 ± 0.31 b | 39.13 ± 3.73 a | 14.80 ± 3.96 c | 44.22 ± 2.22 a |
| Propanal, 2-methyl- | 562 | 72 | 1.30 ± 0.07 c | 1.36 ± 0.02 c | 4.82 ± 0.78 b | 5.85 ± 0.30 a |
| 1-Propanol | 577 | 59 | 1.30 ± 0.24 bc | 1.74 ± 0.04 b | 3.16 ± 0.25 a | 0.92 ± 0.31 c |
| 2,3-Butanedione | 593 | 86 | 108.44 ± 3.27 a | 70.70 ± 2.12 c | 54.22 ± 1.25 d | 81.52 ± 1.24 b |
| 2-Butanone | 599 | 72 | 10.34 ± 1.10 b | 9.64 ± 3.16 b | 32.66 ± 6.58 a | 35.05 ± 8.01 a |
| Hexane, 2,2-dimethyl- | 665 | 57 | 34.93 ± 0.77 | 34.11 ± 1.14 | 27.10 ± 9.37 | 31.50 ± 1.68 |
| Formic acid, 2-propenyl ester | 682 | 57 | 1.90 ± 0.59 b | 1.97 ± 0.21 b | 1.87 ± 0.49 b | 4.61 ± 1.00 a |
| Heptane | 685 | 71 | 0.72 ± 0.10 b | 1.06 ± 0.01 b | 1.85 ± 0.06 a | 1.90 ± 0.19 a |
| Acetic acid | 704 | 60 | 10.31 ± 0.37 c | 31.86 ± 9.63 b | 39.78 ± 6.43 ab | 46.79 ± 35.52 a |
| 1-Butanol | 718 | 56 | 1.86 ± 0.27 b | 2.57 ± 0.51 ab | 4.41 ± 0.97 a | 3.11 ± 0.75 ab |
| Pentanal | 739 | 58 | 15.71 ± 0.63 c | 11.24 ± 0.70 c | 29.86 ± 3.25 b | 44.44 ± 4.05 a |
| Disulfide, dimethyl | 793 | 94 | 1.09 ± 0.32 b | 13.22 ± 6.32 b | 4.98 ± 2.27 b | 95.68 ± 6.9 a |
| Acetoin | 799 | 88 | 145.28 ± 4.40 b | 204.05 ± 22.72 a | 171.95 ± 1.26 ab | 203.77 ± 12.49 a |
| Propanoic acid, 2-hydroxy-2-methyl-, ethyl ester | 830 | 59 | 3.46 ± 0.16 b | 2.47 ± 0.12 c | 1.00 ± 0.23 d | 4.13 ± 0.13 a |
| Octane | 835 | 85 | 3.42 ± 0.39 b | 3.98 ± 0.33 b | 7.35 ± 1.76 a | 6.17 ± 0.42 a |
| Propanoic acid | 837 | 74 | 1.02 ± 0.35 b | 2.68 ± 0.76 b | 5.03 ± 1.66 a | 2.82 ± 0.94 b |
| 1-Pentanol | 860 | 55 | 40.35 ± 0.92 a | 11.08 ± 0.75 c | 9.57 ± 0.28 c | 14.48 ± 0.42 b |
| Prenol | 869 | 71 | 2.54 ± 0.54 b | 4.53 ± 0.30 a | 0.89 ± 0.03 c | 1.30 ± 0.65 bc |
| Hexanal | 878 | 56 | 135.79 ± 5.78 d | 183.42 ± 3.42 c | 299.82 ± 25.45 a | 238.40 ± 2.87 b |
| Propanoic acid, 2-methyl- | 901 | 73 | 0.64 ± 0.2 b | 1.99 ± 0.44 a | 2.77 ± 0.20 a | 1.83 ± 0.48 a |
| 2,3-Butanediol | 924 | 45 | 1.85 ± 0.24 c | 10.39 ± 2.17a | 3.42 ± 0.47 bc | 6.02 ± 0.71 b |
| 2,3-Butanediol, [R-(R*,R*)]- | 932 | 45 | 14.61 ± 7.04 c | 107.30 ± 15.60 a | 69.60 ± 2.26 b | 65.07 ± 2.17 b |
| Butanoic acid | 933 | 60 | 101.64 ± 5.86 c | 377.09 ± 7.29 b | 447.78 ± 58.78 a | 352.37 ± 10.48 b |
| Pentane, 2,2-dimethyl- | 950 | 57 | 0.40 ± 0.18 | 0.36 ± 0.03 | 0.64 ± 0.18 | 0.54 ± 0.03 |
| 2-Heptanone | 983 | 58 | 3.35 ± 0.15 b | 3.45 ± 0.27 b | 4.16 ± 0.18 a | 3.88 ± 0.16 ab |
| Heptanal | 990 | 70 | 8.69 ± 0.71 c | 12.75 ± 1.84 b | 14.78 ± 1.46 b | 18.42 ± 0.14 a |
| .alpha.-Phellandrene | 994 | 93 | 2.69 ± 0.05 b | 2.78 ± 0.12 b | 3.62 ± 0.13 a | 3.82 ± 0.02 a |
| Pentanoic acid | 1021 | 60 | 2.91 ± 0.59 | 4.32 ± 0.77 | 4.31 ± 0.50 | 4.14 ± 1.03 |
| Dimethyl trisulfide | 1052 | 126 | 0.06 ± 0.04 c | 0.84 ± 0.29 b | 0.49 ± 0.20b | 10.19 ± 3.59a |
| Furan, 2-pentyl- | 1055 | 81 | 12.19 ± 0.30 d | 17.36 ± 0.80 c | 41.45 ± 5.14 a | 26.19 ± 1.01 b |
| Benzaldehyde | 1063 | 106 | 20.80 ± 1.14 b | 14.98 ± 1.32 b | 49.22 ± 2.72 a | 53.63 ± 6.10 a |
| 1-Octen-3-ol | 1069 | 57 | 11.33 ± 0.66 c | 13.87 ± 0.63 b | 22.84 ± 1.68 a | 14.42 ± 0.58 b |
| 5-Hepten-2-one, 6-methyl- | 1074 | 108 | 2.48 ± 0.11 a | 1.84 ± 0.18 b | 2.21 ± 0.10 a | 1.79 ± 0.08 b |
| Octanal | 1085 | 84 | 4.97 ± 0.32 c | 6.27 ± 1.45 bc | 8.84 ± 0.85 ab | 8.92 ± 0.12 a |
| Undecane, 3,6-dimethyl- | 1087 | 57 | 14.29 ± 2.23 b | 5.39 ± 0.27 c | 5.82 ± 0.64 c | 49.82 ± 1.31 a |
| Dodecane, 2,6,10-trimethyl- | 1098 | 57 | 11.79 ± 1.24 b | 4.12 ± 0.39 c | 3.80 ± 0.28 c | 39.75 ± 3.40 a |
| Hexanoic acid | 1102 | 60 | 14.99 ± 0.58 b | 31.96 ± 4.88 a | 25.57 ± 3.12 a | 25.40 ± 1.98 a |
| 2-Ethyl-1-hexanol | 1113 | 57 | 3.00 ± 0.16 c | 3.71 ± 0.11 b | 5.23 ± 0.06 a | 3.55 ± 0.20 b |
| Butane, 2,2-dimethyl- | 1133 | 57 | 0.53 ± 0.01 d | 0.66 ± 0.02 c | 0.87 ± 0.12 b | 1.21 ± 0.03 a |
| 1-Octanol | 1147 | 55 | 0.95 ± 0.25 | 0.72 ± 0.01 | 0.99 ± 0.16 | 0.98 ± 0.18 |
| 1-Hexen-3-one | 1152 | 70 | 1.53 ± 0.17 b | 0.73 ± 0.03 c | 0.53 ± 0.11 c | 4.85 ± 0.47 a |
| Nonanal | 1169 | 56 | 7.84 ± 0.38 | 12.20 ± 4.35 | 12.71 ± 1.49 | 11.90 ± 0.34 |
| Undecane, 4,4-dimethyl- | 1174 | 85 | 1.91 ± 0.17 b | 0.75 ± 0.07 c | 0.55 ± 0.03 c | 4.37 ± 0.33 a |
| Undecane, 3-methyl- | 1189 | 57 | 2.08 ± 0.01 b | 1.53 ± 0.09 c | 1.17 ± 0.08 d | 4.50 ± 0.12 a |
| Dodecane | 1210 | 71 | 3.76 ± 0.15 b | 3.05 ± 0.11 b | 3.54 ± 0.17 b | 5.67 ± 0.50 a |
| Decane, 5-ethyl-5-methyl- | 1226 | 71 | 2.46 ± 0.20 b | 0.73 ± 0.08 c | 0.41 ± 0.06 c | 4.87 ± 0.10 a |
| 2,2,5-Trimethylhexan-4-one | 1236 | 57 | 0.55 ± 0.08 b | 0.14 ± 0.04 c | 0.12 ± 0.03 c | 0.85 ± 0.12 a |
| Undecane, 5-ethyl- | 1241 | 57 | 1.73 ± 0.24 a | 0.39 ± 0.04 b | 0.23 ± 0.10 b | 2.36 ± 0.70 a |
| Tridecane | 1282 | 57 | 1.16 ± 0.06 c | 3.26 ± 0.03 b | 4.42 ± 0.61 a | 1.46 ± 0.17 c |
| 2,2,6,6-Tetramethylheptane | 1330 | 57 | 1.43 ± 0.15 a | 0.56 ± 0.04 c | 0.53 ± 0.05 c | 0.99 ± 0.10 b |
| Hexane, 3,3-dimethyl- | 1348 | 57 | 1.68 ± 0.24 a | 1.28 ± 0.13 ab | 1.44 ± 0.23 ab | 0.88 ± 0.10 b |
| Pentadecanal | 1548 | 82 | 0.56 ± 0.06 c | 0.73 ± 0.06 c | 1.97 ± 0.09 a | 1.11 ± 0.04 b |
| Free Fatty Acids | Treatments | |||
|---|---|---|---|---|
| FC1 | F1 | F2 | F3 | |
| C6:0 | 0.20 ± 0.13 a | 0.15 ± 0.02 a | 0.24 ± 0.05 a | 0.12 ± 0.07 a |
| C8:0 | 0.14 ± 0.27 ab | 0.13 ± 0.01 b | 0.18 ± 0.31 a | 0.16 ± 0.12 ab |
| C10:0 | 0.10 ± 0.02 a | 0.11 ± 0.02 a | 0.14 ± 0.03 a | 0.14 ± 0.01 a |
| C11:0 | 0.11 ± 0.01 a | 0.13 ± 0.02 a | 0.18 ± 0.01 a | 0.19 ± 0.01 a |
| C12:0 | 0.12 ± 0. 02 a | 0.11 ± 0.01 a | 0.11 ± 0.01 a | 0.12 ± 0.05 a |
| C13:0 | 0.08 ± 0.02 a | 0.08 ± 0.01 a | 0.09 ± 0.02 a | 0.09 ± 0.01 a |
| C14:0 | 1.12 ± 0.15 a | 0.97 ± 0.07 a | 1.22 ± 0.46 a | 1.25 ± 0.64 a |
| C14:1n-5 | 0.21 ± 0.01 a | 0.16 ± 0.02 a | 0.15 ± 0.05 a | 0.19 ± 0.06 a |
| C15:0 | 0.23 ± 0.02 a | 0.24 ± 0.03 a | 0.24 ± 0.02 a | 0.20 ± 0.06 a |
| C15:1n-5 | 2.43 ± 0.64 a | 2.65 ± 0.53 a | 3.78 ± 0.16 a | 3.83 ± 0.27 a |
| C16:0 | 23.43 ± 3.16 a | 22.25 ± 1.95 b | 22.24 ± 0.01 b | 24.14 ± 4.45 a |
| C16:1n-7 | 1.39 ± 0.22 a | 1.16 ± 0.08 a | 1.12 ± 0.02 a | 1.40 ± 0.39 a |
| C17:0 | 0.64 ± 0.08 ab | 0.74 ± 0.05 ab | 0.79 ± 0.08 a | 0.58 ± 0.16 b |
| C18:0 | 22.57 ± 2.81 ab | 23.76 ± 2.02 a | 24.43 ± 0.46 a | 20.80 ± 2.89 b |
| 9t-C18:1 | 0.33 ± 0.05 b | 0.67 ± 0.20 a | 0.58 ± 0.08 ab | 0.28 ± 0.07 b |
| 11t-C18:1 | 1.24 ± 0.14 a | 0.98 ± 0.28 a | 1.07 ± 0.02 a | 1.36 ± 0.32 a |
| C18:1n-9 | 24.19 ± 2.69 a | 26.76 ± 2.31 a | 26.09 ± 1.45 a | 24.85 ± 6.99 a |
| C18:1n-7 | 1.42 ± 0.21 b | 1.68 ± 0.13 a | 1.66 ± 0.02 a | 1.37 ± 0.26 b |
| 9t,11t-C18:2 | 0.22 ± 0.04 a | 0.16 ± 0.02 a | 0.18 ± 0.01 a | 0.23 ± 0.01 a |
| C18:2n-6 | 12.65 ± 1.30 a | 11.22 ± 0.66 a | 9.55 ± 0.32 b | 11.53 ± 1.60 a |
| C18:3n-6 | 0.19 ± 0.04 a | 0.11 ± 0.02 b | 0.12 ± 0.03 b | 0.18 ± 0.02 ab |
| C18:3n-3 | 1.23 ± 0.13 a | 0.61 ± 0.02 b | 0.60 ± 0.02 b | 1.15 ± 0.24 a |
| 9c,11t-C18:2 (CLA) | 0.39 ± 0.05 a | 0.31 ± 0.02 ab | 0.20 ± 0.04 b | 0.41 ± 0.13 a |
| C20:0 | 0.19 ± 0.03 a | 0.21 ± 0.02 a | 0.26 ± 0.02 a | 0.22 ± 0.01 a |
| C20:1n-9 | 0.12 ± 0.01 a | 0.17 ± 0.03 a | 0.18 ± 0.01 a | 0.18 ± 0.06 a |
| C20:2n-6 | 0.12 ± 0.01 a | 0.11 ± 0.02 a | 0.13 ± 0.01 a | 0.14 ± 0.02 a |
| C20:3n-6 | 0.76 ± 0.08 a | 0.77 ± 0.06 a | 0.76 ± 0.04 a | 0.73 ± 0.09 a |
| C20:4n-6 | 2.88 ± 0.39 a | 2.70 ± 0.12 a | 2.63 ± 0.11 a | 2.74 ± 0.22 a |
| C20:5n-3 | 0.85 ± 0.08 ab | 0.60 ± 0.01 c | 0.72 ± 0.03 bc | 0.96 ± 0.09 a |
| C22:6n-3 | 0.46 ± 0.10 a | 0.31 ± 0.05 a | 0.29 ± 0.11 a | 0.45 ± 0.18 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nachtigall, F.M.; Vidal, V.A.S.; Pyarasani, R.D.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.R.; Santos, L.S. Substitution Effects of NaCl by KCl and CaCl2 on Lipolysis of Salted Meat. Foods 2019, 8, 595. https://doi.org/10.3390/foods8120595
Nachtigall FM, Vidal VAS, Pyarasani RD, Domínguez R, Lorenzo JM, Pollonio MAR, Santos LS. Substitution Effects of NaCl by KCl and CaCl2 on Lipolysis of Salted Meat. Foods. 2019; 8(12):595. https://doi.org/10.3390/foods8120595
Chicago/Turabian StyleNachtigall, Fabiane M., Vitor A. S. Vidal, Radha D. Pyarasani, Rubén Domínguez, José M. Lorenzo, Marise A. R. Pollonio, and Leonardo S. Santos. 2019. "Substitution Effects of NaCl by KCl and CaCl2 on Lipolysis of Salted Meat" Foods 8, no. 12: 595. https://doi.org/10.3390/foods8120595
APA StyleNachtigall, F. M., Vidal, V. A. S., Pyarasani, R. D., Domínguez, R., Lorenzo, J. M., Pollonio, M. A. R., & Santos, L. S. (2019). Substitution Effects of NaCl by KCl and CaCl2 on Lipolysis of Salted Meat. Foods, 8(12), 595. https://doi.org/10.3390/foods8120595







