The Compositional and Functional Attributes of Commercial Flours from Tropical Fruits (Breadfruit and Banana)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition Analysis
2.2. pH
2.3. Water and Oil Holding Capacity
2.4. Bulk Density
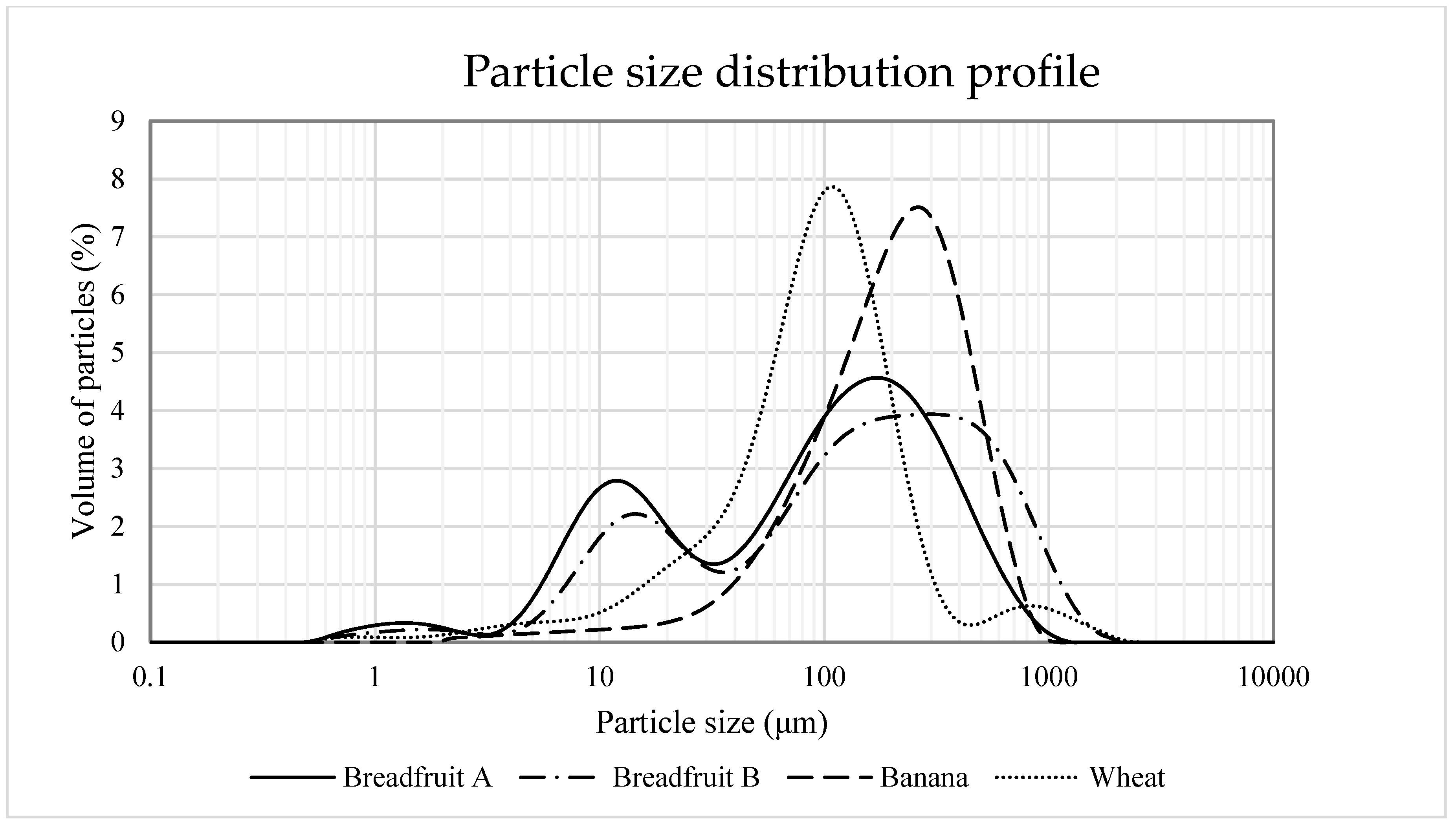
2.5. Particle Size Distribution
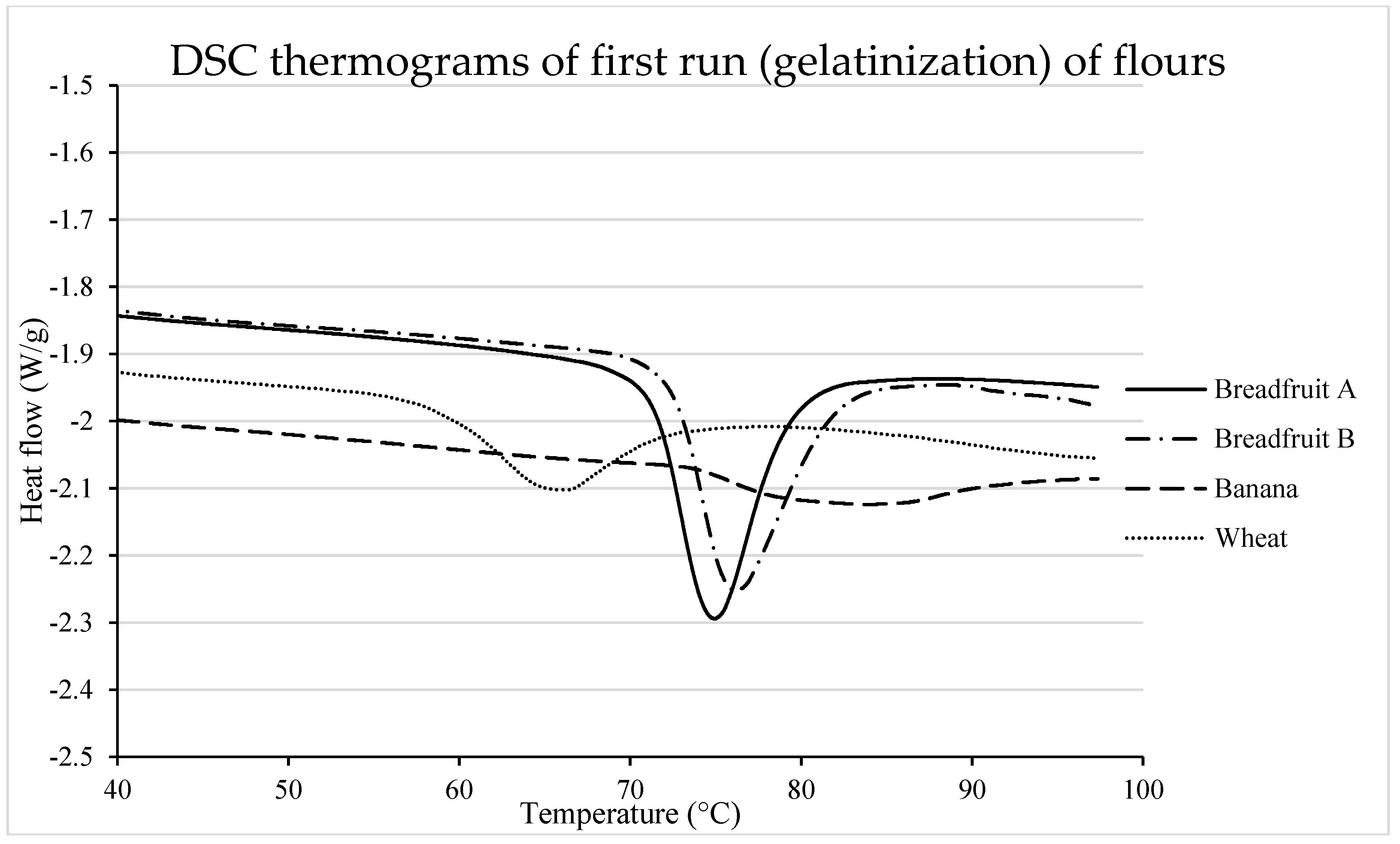
2.6. Differential Scanning Calorimetry (DSC)
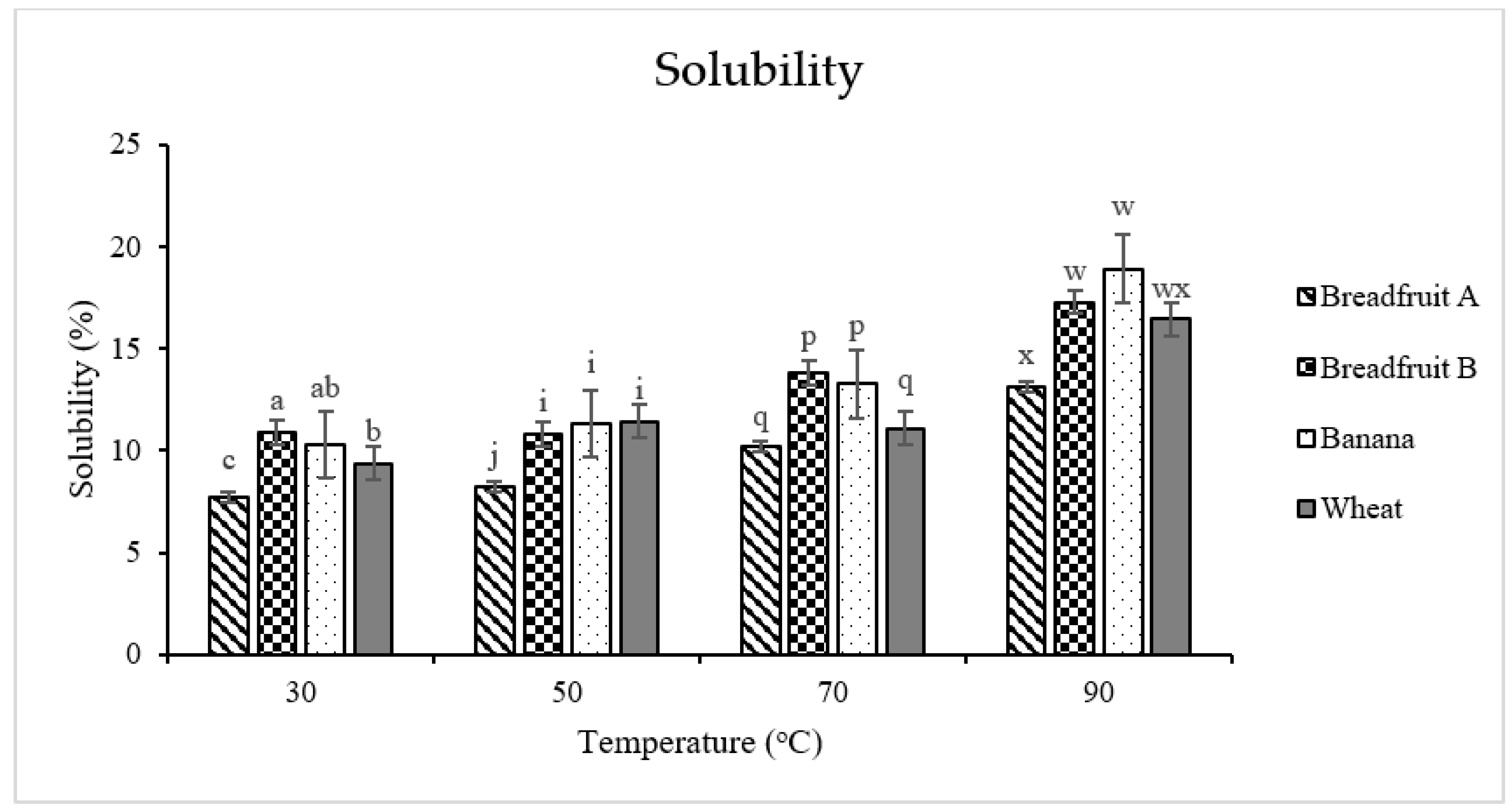
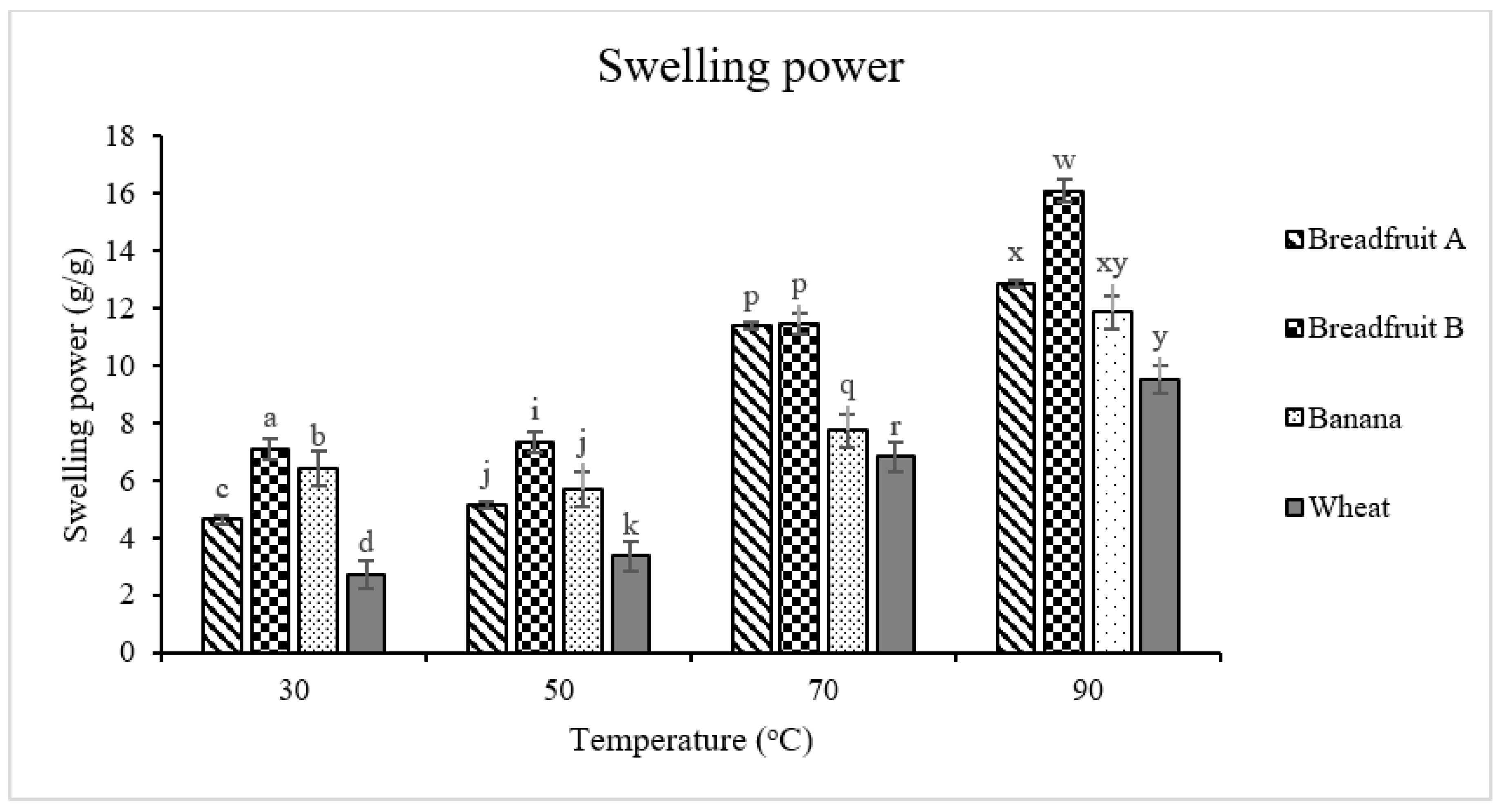
2.7. Solubility and Swelling Power
2.8. Pasting Properties
2.9. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. Functional Properties
3.2.1. pH, Water Holding Capacity, Oil Holding Capacity, Particle Size, and Bulk Density
3.2.2. Starch Gelatinization
3.2.3. Solubility and Swelling Power
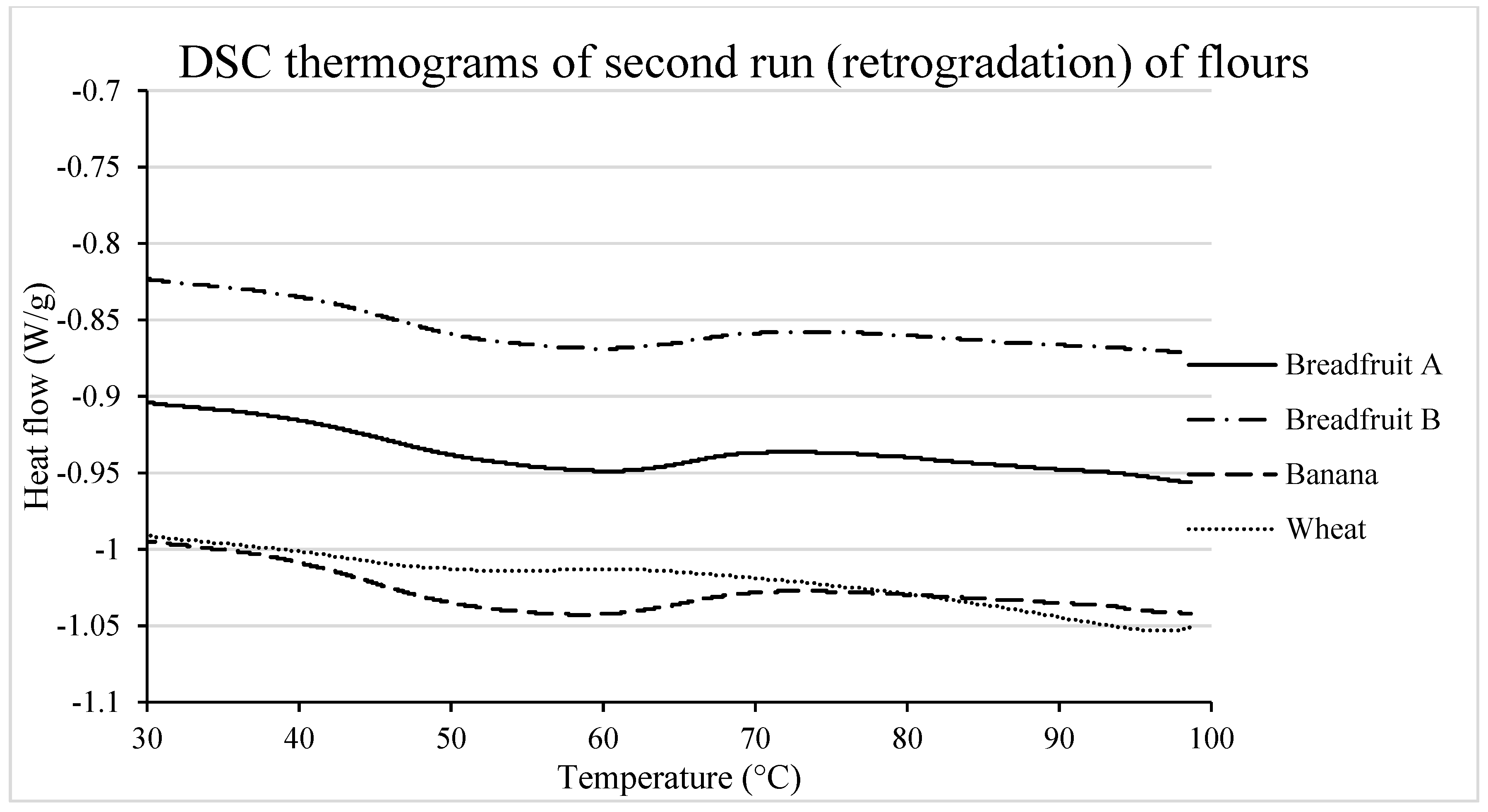
3.2.4. Re-association of Starch Molecules During Retrogradation
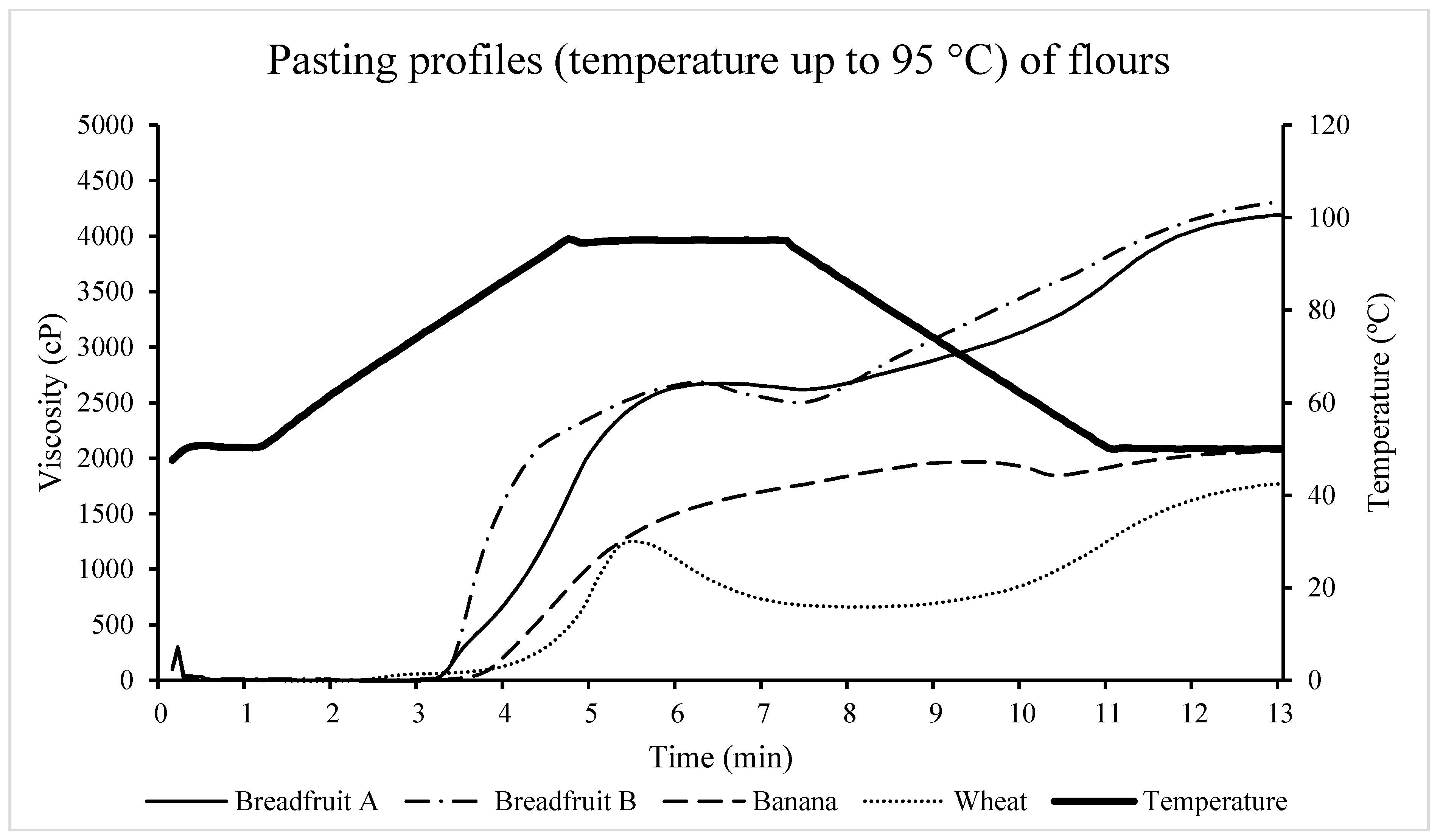
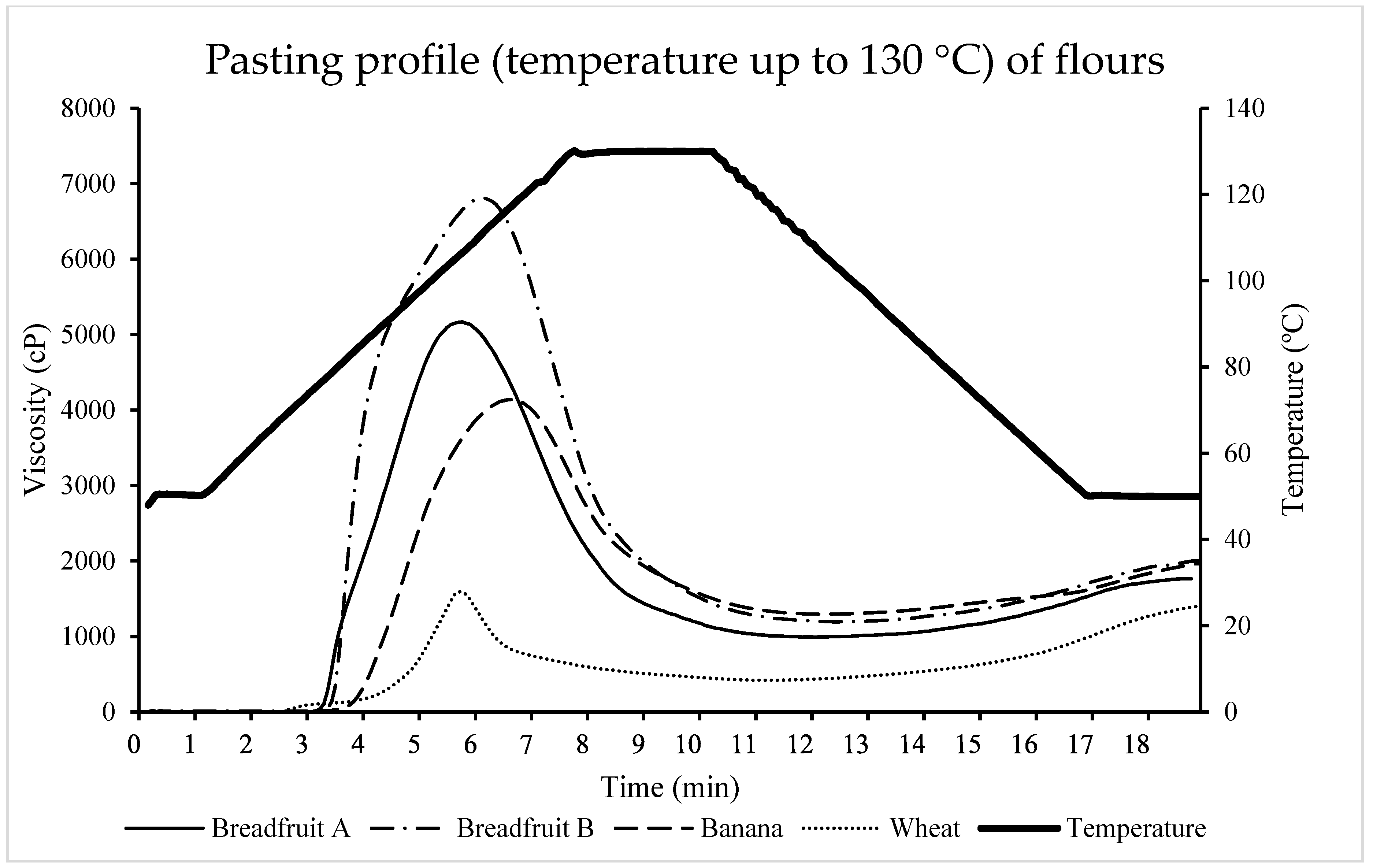
3.2.5. Pasting Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swinkels, J.J.M. Composition and Properties of Commercial Native Starches. Starch-Stärke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.P.; Ragone, D.; Tavana, N.G.; Bernotas, D.W.; Murch, S.J. Beyond the Bounty: Breadfruit (Artocarpus altilis) for food security and novel foods in the 21st century. Ethnobot. Res. Appl. 2011, 9, 129–149. [Google Scholar] [CrossRef]
- Arias, P.; Dankers, C.; Liu, P.; Pilkauskas, P. The World Banana Economy, 1985–2002; Food and Agriculture Organization: Rome, Italy, 2003. [Google Scholar]
- Jones, A.M.P.; Ragone, D.; Aiona, K.; Lane, W.A.; Murch, S.J. Nutritional and morphological diversity of breadfruit (Artocarpus, Moraceae): Identification of elite cultivars for food security. J. Food Compos. Anal. 2011, 24, 1091–1102. [Google Scholar] [CrossRef]
- FAO. Breadfruit and Breadnut Orchard Establishment and Management; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Huang, S.; Mejía Vasquez, S.M.; Murch, S.J.; Bohrer, B.M. Cooking loss, texture properties, and color of comminuted beef prepared with breadfruit (Artocarpus altilis) flour. Meat Muscle Biol. 2019, 3, 231–243. [Google Scholar] [CrossRef]
- Ovando-Martinez, M.; Sáyago-Ayerdi, S.; Agama-Acevedo, E.; Goñi, I.; Bello-Pérez, L.A. Unripe banana flour as an ingredient to increase the undigestible carbohydrates of pasta. Food Chem. 2009, 113, 121–126. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, L.; Zhang, Y.H.; Qin, L.Q. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Br. J. Nutr. 2011, 106, 1649–1654. [Google Scholar] [CrossRef]
- Turi, C.E.; Liu, Y.; Ragone, D.; Murch, S.J. Breadfruit (Artocarpus altilis and hybrids): A traditional crop with the potential to prevent hunger and mitigate diabetes in Oceania. Trends Food Sci. Technol. 2015, 45, 264–272. [Google Scholar] [CrossRef]
- Hettiaratchi, U.P.K.; Ekanayake, S.; Welihinda, J. Chemical compositions and glycemic responses to banana varieties. Int. J. Food Sci. Nutr. 2011, 62, 307–309. [Google Scholar] [CrossRef]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990. [Google Scholar] [CrossRef]
- Roman, L.; Gomez, M.; Hamaker, B.R.; Martinez, M.M. Banana starch and molecular shear fragmentation dramatically increase structurally driven slowly digestible starch in fully gelatinized bread crumb. Food Chem. 2019, 274, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Segundo, C.; Román, L.; Gómez, M.; Martínez, M.M. Mechanically fractionated flour isolated from green bananas (M. cavendishii var. nanica) as a tool to increase the dietary fiber and phytochemical bioactivity of layer and sponge cakes. Food Chem. 2017, 219, 240–248. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, Official Method 925.10 Moisture in Flour by Air Oven Method, 20th ed.; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis, Official Method 920.85 Fat in Flour by Ether Extract Method, 20th ed.; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis, Official Method 992.15 Protein in Foods by Combustion Method, 20th ed.; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis, Official Method 923.03 Ash in Flour by Ashing Method, 20th ed.; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2016. [Google Scholar]
- AOAC. Official Methods of Analysis, Official Method 996.11 Total Starch in Foods by Amyloglucosidase-Alpha-Amylase Method, 20th ed.; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2016. [Google Scholar]
- Menezes, E.W.; Tadini, C.C.; Tribess, T.B.; Zuleta, A.; Binaghi, J.; Pak, N.; Vera, G.; Dan, M.C.T.; Bertolini, A.C.; Cordenunsi, B.R.; et al. Chemical Composition and Nutritional Value of Unripe Banana Flour (Musa acuminata, var. Nanicão). Plant Foods Hum. Nutr. 2011, 66, 231–237. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). Food Energy-Methods of Analysis and Conversion Factors; FAO Food and Nutrition Paper 77; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 9251050147. [Google Scholar]
- Buchholz, A.C.; Schoeller, D.A. Is a calorie a calorie? Am. J. Clin. Nutr. 2004, 79, 899S–906S. [Google Scholar] [CrossRef] [PubMed]
- Perten Instruments. Assessing Ingredient Performance Using the RVA 4800. Available online: https://www.perten.com/Publications/Articles/Assessing-ingredient-performance-using-the-RVA-4800/ (accessed on 21 August 2019).
- Galliard, T.; Bowler, P. Morphology and composition of starch. In Starch: Properties and Potential; Galliard, T., Ed.; John Wiley and Sons: Brisbane, Australia, 1987; pp. 55–78. [Google Scholar]
- Nwokocha, L.M.; Williams, P.A. Comparative study of physicochemical properties of breadfruit (Artocarpus altilis) and white yam starches. Carbohydr. Polym. 2011, 85, 294–302. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; OrtÍz-Maldonado, F.; Villagómez-Mendez, J.; Toro-Vazquez, J.F. Effect of fatty acids on clarity of starch pastes. Starch-Stärke 1998, 50, 383–386. [Google Scholar] [CrossRef]
- Adepeju, A.B.; Gbadamosi, S.O.; Adeniran, A.H.; Omobuwajo, T.O. Functional and pasting characteristics of breadfruit (Artocarpus altilis) flours. Afr. J. Food Sci. 2011, 5, 529–535. [Google Scholar]
- Osundahunsi, O.F.; Fagbemi, T.N.; Kesselman, E.; Shimoni, E. Comparison of the physicochemical properties and pasting characteristics of flour and starch from red and white sweet potato cultivars. J. Agric. Food Chem. 2003, 51, 2232–2236. [Google Scholar] [CrossRef]
- Odoemelam, S.A. Chemical composition and functional properties of conophor nut (Tetracarpidium conophorum) flour. Int. J. Food Sci. Technol. 2003, 38, 729–734. [Google Scholar] [CrossRef]
- Berry, C.S.; I’anson, K.; Miles, M.J.; Morris, V.J.; Russell, P.L. Physical chemical characterisation of resistant starch from wheat. J. Cereal Sci. 1988, 8, 203–206. [Google Scholar] [CrossRef]
- Kerr, W.L.; Ward, C.D.W.; McWatter, K.H.; Resurreccion, A.V.A. Effect of milling and particle size on functionality and physicochemical properties of cowpea flour. Cereal Chem. 2000, 77, 213–219. [Google Scholar] [CrossRef]
- Ahmed, J.; Taher, A.; Mulla, M.Z.; Al-Hazza, A.; Luciano, G. Effect of sieve particle size on functional, thermal, rheological and pasting properties of Indian and Turkish lentil flour. J. Food. Eng. 2016, 186, 34–41. [Google Scholar] [CrossRef]
- Abebe, W.; Collar, C.; Ronda, F. Impact of variety type and particle size distribution on starch enzymatic hydrolysis and functional properties of tef flours. Carboydr. Polym. 2015, 115, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, R.O.; Odedeji, J.O. Functional properties of wheat and sweet potato flour blends. Pak. J. Nutr. 2010, 9, 535–538. [Google Scholar] [CrossRef]
- Fagbemi, T.N. Effect of blanching and ripening on functional properties of plantain (Musa aab) flour. Plant Foods Hum. Nutr. 1999, 54, 261–269. [Google Scholar] [CrossRef]
- Rincón, A.M.; Padilla, F.C. Physicochemical properties of Venezuelan breadfruit (Artocarpus altilis) starch. Arch. Latinoam. Nutr. 2004, 54, 449–456. [Google Scholar]
- Tester, R.F.; Karkalas, J. Swelling and gelatinization of oat starches. Cereal Chem. 1996, 73, 271–277. [Google Scholar]
- Klucinec, J.D.; Thompson, D.B. Amylose and Amylopectin Interact in Retrogradation of Dispersed High-Amylose Starches. Cereal Chem. J. 1999, 76, 282–291. [Google Scholar] [CrossRef]
- Martinez, M.M.; Li, C.; Okoniewska, M.; Mukherjee, I.; Vellucci, D.; Hamaker, B. Slowly digestible starch in fully gelatinized material is structurally driven by molecular size and A and B1 chain lengths. Carbohydr. Polym. 2018, 197, 531–539. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.-C.; Åman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Roman, L.; Campanella, O.; Martinez, M.M. Shear-induced molecular fragmentation decreases the bioaccessibility of fully gelatinized starch and its gelling capacity. Carbohydr. Polym. 2019, 215, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, X.; Chen, L.; Xie, F.; Li, L.; Huang, J. Effect of heat-moisture treatment on multi-scale structures and physicochemical properties of breadfruit starch. Carbohydr. Polym. 2017, 161, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, L.; Li, X.; Xie, F.; Liu, H.; Yu, L. Thermal and Rheological Properties of Breadfruit Starch. J. Food Sci. 2011, 76, E55–E61. [Google Scholar] [CrossRef] [PubMed]
| Items | Units | Breadfruit A 1 | Breadfruit B 2 | Banana 3 | Wheat 4 | RDL 5 |
|---|---|---|---|---|---|---|
| As is basis | ||||||
| Moisture | g/100 g | 12.60 | 6.47 | 8.18 | 12.20 | 0.1 |
| Carbohydrates | g/100 g | 80.13 | 83.98 | 83.24 | 72.45 | 0.1 |
| Starch | g/100 g | 64.14 | 62.68 | 70.83 | 60.40 | 0.1 |
| Protein | g/100 g | 3.42 | 4.67 | 3.98 | 12.74 | 0.10 |
| Fat | g/100 g | 0.85 | 2.34 | 1.78 | 1.51 | 0.10 |
| Ash | g/100 g | 3.00 | 2.54 | 2.82 | 1.10 | 0.1 |
| Energy | kJ/100 g | 1430.30 | 1571.81 | 1526.70 | 1482.60 | 1.0 |
| Calories | kcal/100 g | 341.85 | 375.67 | 364.89 | 354.35 | 1.0 |
| Dry matter basis | ||||||
| Carbohydrates | g/100 g | 91.68 | 89.79 | 90.66 | 82.52 | |
| Starch | g/100 g | 73.39 | 67.02 | 77.14 | 68.79 | |
| Protein | g/100 g | 3.91 | 4.99 | 4.33 | 14.51 | |
| Fat | g/100 g | 0.97 | 2.50 | 1.94 | 1.72 | |
| Ash | g/100 g | 3.43 | 2.72 | 3.07 | 1.25 | |
| Energy | kJ/100 g | 1636.50 | 1680.54 | 1662.71 | 1688.61 | |
| Calories | kcal/100 g | 391.13 | 401.66 | 397.40 | 403.59 |
| Breadfruit A 2 | Breadfruit B 3 | Banana 4 | Wheat 5 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| pH | 6.09 a | 5.67 c | 5.07 d | 5.84 b | 0.02 | <0.0001 |
| WHC 6, g/g | 2.04 c | 3.32 a | 3.08 b | 1.25 d | 0.02 | <0.0001 |
| OHC 7, g/g | 1.71 b | 2.15 a | 0.94 d | 1.10 c | 0.04 | <0.0001 |
| BD 8, g/cm3 | 0.70 c | 0.52 d | 0.94 a | 0.78 b | 0.01 | <0.0001 |
| D4,3 9, um | 138.73 b | 220.94 a | 222.89 a | 132.54 b | 10.75 | <0.001 |
| Breadfruit A 2 | Breadfruit B 3 | Banana 4 | Wheat 5 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| Gelatinization | ||||||
| To (°C) | 71.33 c | 72.45 b | 74.19 a | 59.21 d | 0.23 | <0.0001 |
| Tp (°C) | 75.06 c | 75.97 b | 82.94 a | 65.47 d | 0.14 | <0.0001 |
| Tc (°C) | 88.03 b | 88.39 b | 96.20 a | 77.49 c | 0.26 | <0.0001 |
| Tc–To (°C) | 16.70 bc | 15.94 c | 22.01 a | 18.28 b | 0.45 | <0.0001 |
| ΔH (W/g) | 12.98 a | 12.31 a | 3.56 c | 6.30 b | 0.25 | <0.0001 |
| Retrogradation | ||||||
| To (°C) | 40.67 | 40.73 | 38.97 | 42.69 | 0.99 | 0.15 |
| Tp (°C) | 57.77 a | 55.66 a | 55.51 a | 51.77 b | 0.50 | <0.001 |
| Tc (°C) | 72.96 b | 73.70 ab | 75.32 a | 60.48 c | 0.52 | <0.0001 |
| Tc–To (°C) | 32.29 a | 32.97 a | 36.35 a | 17.79 c | 0.95 | <0.0001 |
| ΔH (W/g) | 5.50 b | 5.74 b | 6.99 a | 0.74 c | 0.14 | <0.0001 |
| Breadfruit A 2 | Breadfruit B 3 | Banana 4 | Wheat 5 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| Peak viscosity 6, cP | 2678.33 a | 2687.33 a | 1701.00 b | 1258.67 c | 34.85 | <0.0001 |
| Trough Viscosity 7, cP | 2613.33 a | 2499.33 a | 1524.33 b | 658.00 c | 36.28 | <0.0001 |
| Breakdown 8, cP | 65.00 c | 188.00 b | 176.67 b | 600.67 a | 5.42 | <0.0001 |
| Final Viscosity 9, cP | 4188.67 a | 4310.00 a | 2063.33 b | 1768.00 c | 39.31 | <0.0001 |
| Setback 10, cP | 1575.33 b | 1810.67 a | 539.00 d | 1110.00 c | 22.53 | <0.0001 |
| Peak time 11, min | 6.60 b | 6.36 b | 7.00 a | 5.44 c | 0.08 | <0.0001 |
| Pasting temperature 12, °C | 76.80 c | 76.75 c | 82.40 b | 86.85 a | 0.14 | <0.0001 |
| Breadfruit A 2 | Breadfruit B 3 | Banana 4 | Wheat 5 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| Peak viscosity 6, cP | 5169.33 b | 6809.00 a | 4142.00 c | 1597.00 d | 76.95 | <0.0001 |
| Trough Viscosity 7, cP | 991.67 c | 1195.33 b | 1296.67 a | 418.33 d | 12.43 | <0.0001 |
| Breakdown 8, cP | 4177.67 b | 5613.67 a | 2845.33 c | 1178.67 d | 67.72 | <0.0001 |
| Final Viscosity 9, cP | 1766.00 b | 2000.33 a | 1962.00 a | 1396.00 c | 42.03 | 0.0001 |
| Setback 10, cP | 774.33 b | 805.00 ab | 665.33 b | 977.67 a | 37.30 | <0.01 |
| Peak time 11, min | 5.85 c | 6.11 b | 6.58 a | 5.71 d | 0.02 | <0.0001 |
| Pasting temperature 12, °C | 77.85 | 78.45 | 82.73 | 76.53 | 2.71 | 0.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Martinez, M.M.; Bohrer, B.M. The Compositional and Functional Attributes of Commercial Flours from Tropical Fruits (Breadfruit and Banana). Foods 2019, 8, 586. https://doi.org/10.3390/foods8110586
Huang S, Martinez MM, Bohrer BM. The Compositional and Functional Attributes of Commercial Flours from Tropical Fruits (Breadfruit and Banana). Foods. 2019; 8(11):586. https://doi.org/10.3390/foods8110586
Chicago/Turabian StyleHuang, Shiqi, Mario M. Martinez, and Benjamin M. Bohrer. 2019. "The Compositional and Functional Attributes of Commercial Flours from Tropical Fruits (Breadfruit and Banana)" Foods 8, no. 11: 586. https://doi.org/10.3390/foods8110586
APA StyleHuang, S., Martinez, M. M., & Bohrer, B. M. (2019). The Compositional and Functional Attributes of Commercial Flours from Tropical Fruits (Breadfruit and Banana). Foods, 8(11), 586. https://doi.org/10.3390/foods8110586





