Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of Heating Profiles
Abstract
1. Introduction
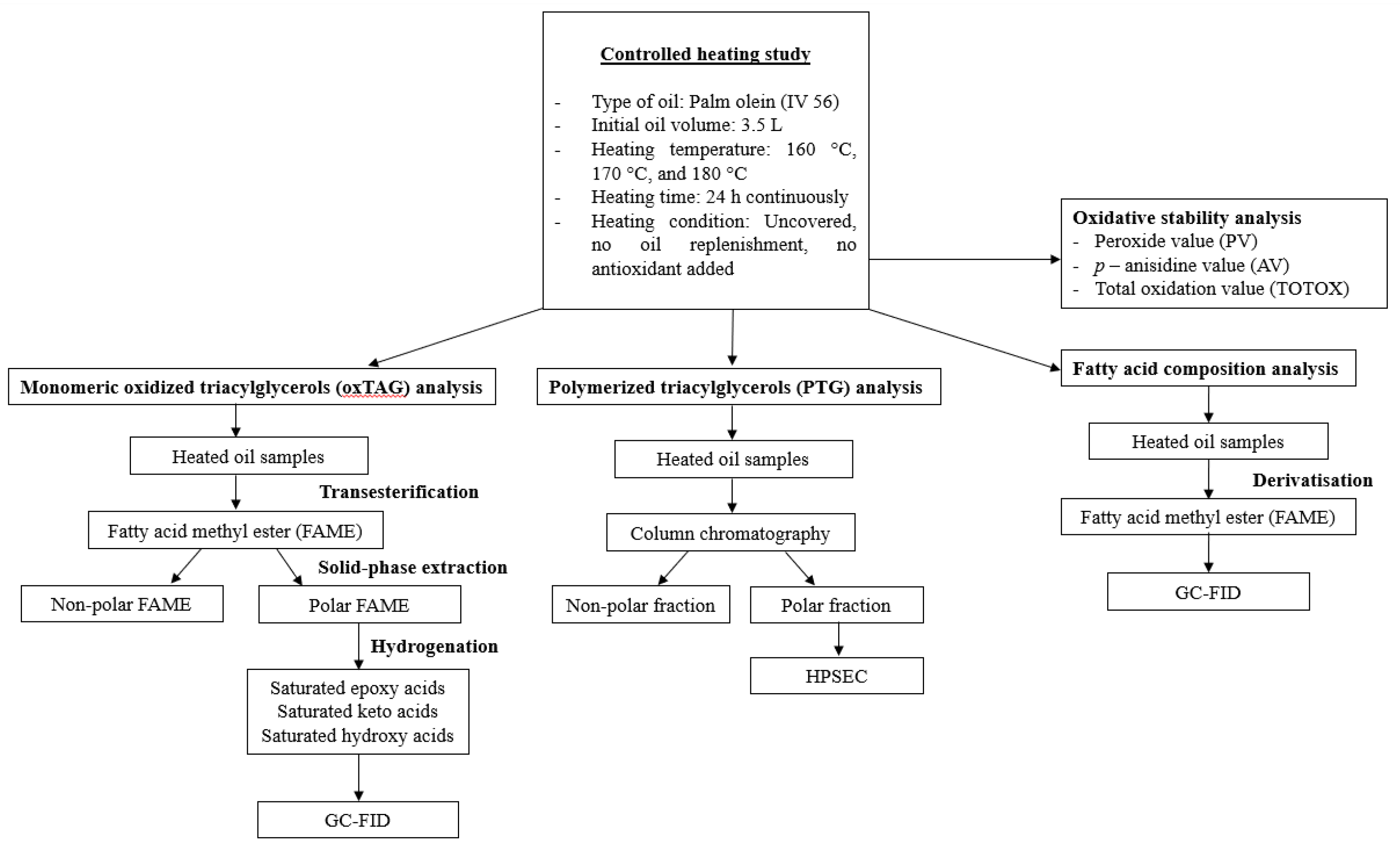
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Samples
2.4. Determination of Epoxy, Keto, and Hydroxy Fatty Acids
2.4.1. Transesterification
2.4.2. Isolation of Polar FAMEs
2.4.3. Hydrogenation
2.4.4. Gas Chromatography
2.5. Determination of Polymerized Triacylglycerols
2.5.1. Isolation of Total Polar Compound
2.5.2. High-Performance Size-Exclusion Chromatography (HPSEC)
2.6. Determination of Oxidative Stability
Peroxide Value (PV), p-Anisidine Value (AV), and Total Oxidation (TOTOX) Value
2.7. Determination of Fatty Acid Composition
2.7.1. Derivatization
2.7.2. Gas Chromatography
2.8. Statistical Analysis
3. Results and Discussion
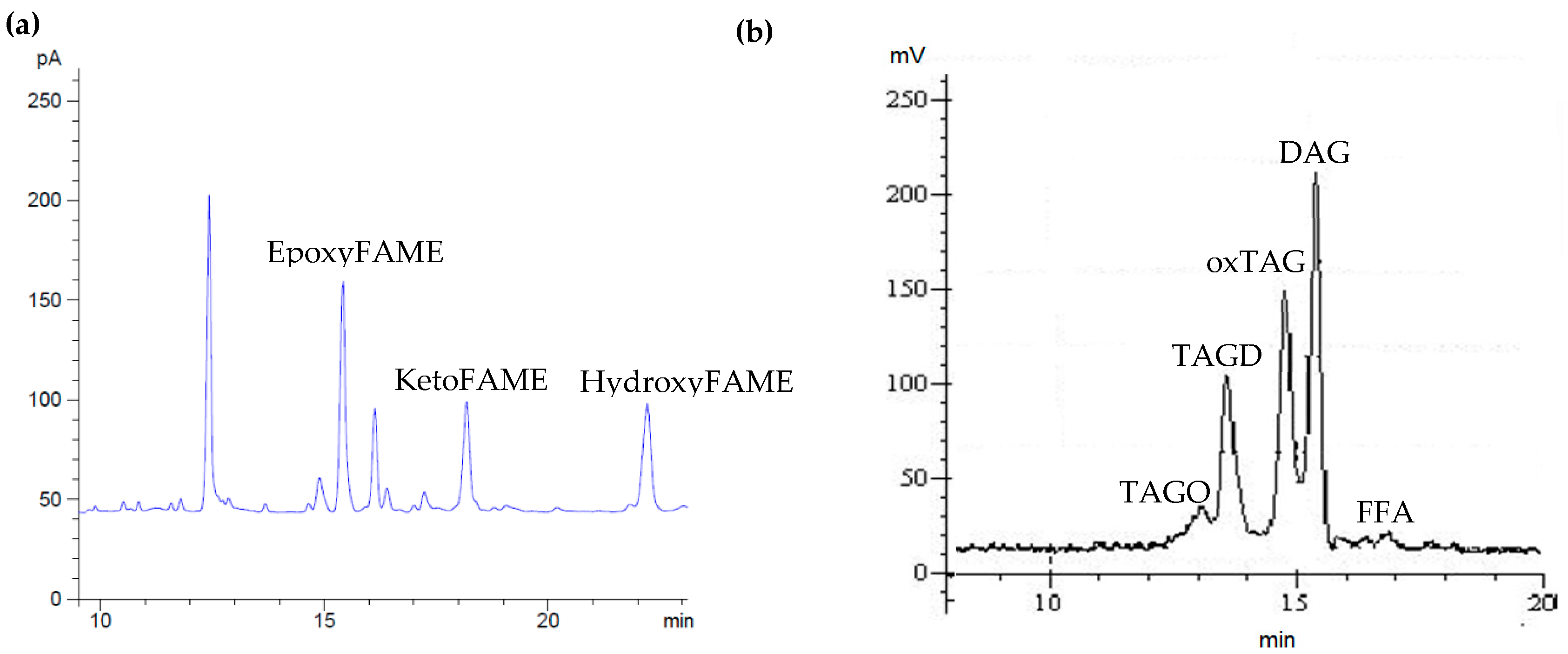
3.1. Effect of Temperature and Heating Time on the Formation of Monomeric Oxidized Triacylglycerols
3.2. Effect of Temperature and Heating Time on the Total Polar Content (TPC)
3.3. Effect of Temperature and Heating Time on the Formation of Polymerized Triacylglycerols
3.4. Effect of Temperature and Heating Time on the Oxidative Stability of RBDPO
3.5. Effect of Temperature and Heating Time on the Fatty Acid Composition of RBDPO
3.6. Correlation among Analytical Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feng, H.X.; Sam, R.; Jiang, L.Z.; Li, Y.; Cao, W.M. High-performance size-exclusion chromatography studies on the formation and distribution of polar compounds in camellia seed oil during heating. J. Zhejiang Univ. Sci. B 2016, 17, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Marmesat, S.; Márquez-Ruiz, G.; Dobarganes, M.C. Formation of short-chain glycerol-bound oxidation products and oxidised monomeric triacylglycerols during deep-frying and occurrence in used frying fats. Eur. J. Lipid Sci. Technol. 2004, 106, 728–735. [Google Scholar] [CrossRef]
- Berdeaux, O.; Dutta, P.C.; Dobarganes, M.C.; Sébédio, J.L. Analytical Methods for Quantification of Modified Fatty Acids and Sterols Formed as a Result of Processing. Food Anal. Method 2008, 2, 30–40. [Google Scholar] [CrossRef]
- Brühl, L.; Weisshaar, R.; Matthäus, B. Epoxy fatty acids in used frying fats and oils, edible oils and chocolate and their formation in oils during heating. Eur. J. Lipid Sci. Technol. 2016, 118, 425–434. [Google Scholar] [CrossRef]
- Dobarganes, C.; Marquez-Ruiz, G. Oxidized fats in foods. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gertz, C.; Aladedunye, F.; Matthäus, B. Oxidation and structural decomposition of fats and oils at elevated temperatures. Eur. J. Lipid Sci. Technol. 2014, 116, 1457–1466. [Google Scholar] [CrossRef]
- Marmesat, S.; Velasco, J.; Dobarganes, M.C. Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J. Chromatogr. A 2008, 1211, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Brühl, L. Fatty acid alterations in oils and fats during heating and frying. Eur. J. Lipid Sci. Technol. 2014, 116, 707–715. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Nie, S.; Xie, M.; Chen, F.; Luo, P.G. Formation of trans fatty acids during the frying of chicken fillet in corn oil. Int. J. Food Sci. Nutr. 2014, 65, 306–310. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, D.; Xia, H.; Wang, F.; Yang, X.; Pan, D.; Wang, S.; Yang, L.; Lu, H.; Shu, G.; et al. A comparative study of the effects of palm olein, cocoa butter and extra virgin olive oil on lipid profile, including low-density lipoprotein subfractions in young healthy Chinese people. Int. J. Food Sci. Nutr. 2019, 70, 355–366. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices, 6th ed.; American Oil Chemists’ Society (Method Cd 8b-90): Champaign, IL, USA, 2013. [Google Scholar]
- AOCS. Official Methods and Recommended Practices, 6th ed.; American Oil Chemists’ Society (Method Cd 18-90): Champaign, IL, USA, 2013. [Google Scholar]
- Márquez-Ruiz, G.; Dobarganes, C. Short-chain fatty acid formation during thermoxidation and frying. J. Agric. Food Chem. 1996, 70, 120–126. [Google Scholar] [CrossRef]
- Velasco, J.; Berdeaux, O.; Márquez-Ruiz, G.; Dobarganes, M.C. Sensitive and accurate quantitation of monoepoxy fatty acids in thermoxidized oils by gas–liquid chromatography. J. Chromatogr. A 2002, 982, 145–152. [Google Scholar] [CrossRef]
- Bansal, G.; Zhou, W.B.; Barlow, P.J.; Lo, H.L.; Neo, F.L. Performance of palm olein in repeated deep frying and controlled heating processes. Food Chem. 2010, 121, 338–347. [Google Scholar] [CrossRef]
- Baltacıoğlu, C. Effect of different frying methods on the total trans fatty acid content and oxidative stability of oils. J. Am. Oil Chem. Soc. 2017, 94, 923–934. [Google Scholar] [CrossRef]
- Marquez-Ruiz, G.; Holgado, F.; Garcia-Martinez, M.C.; Dobarganes, M.C. A direct and fast method to monitor lipid oxidation progress in model fatty acid methyl esters by high-performance size-exclusion chromatography. J. Chromatogr. A 2007, 1165, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Salta, F.N.; Chiou, A.; Andrikopoulos, N.K. Formation and distribution of oxidized fatty acids during deep- and pan-frying of potatoes. Eur. J. Lipid Sci. Technol. 2007, 109, 1111–1123. [Google Scholar] [CrossRef]
- Li, C.M.; Kimura, F.; Endo, Y.; Maruyama, C.; Fujimoto, K. Deterioration of diacylglycerol- and triacylglycerol-rich oils during frying of potatoes. Eur. J. Lipid Sci. Technol. 2005, 107, 173–179. [Google Scholar] [CrossRef]
- Márquez-Ruiz, G.; Martín-Polvillo, M.; Dobarganes, M. Quantitation of oxidized triglyceride monomers and dimers as an useful measurement for early and advanced stages of oxidation. Grasas Aceites 1996, 47, 48–53. [Google Scholar] [CrossRef]
- Aladedunye, F.A.; Przybylski, R. Degradation and Nutritional Quality Changes of Oil during Frying. J. Am. Oil Chem. Soc. 2008, 86, 149–156. [Google Scholar] [CrossRef]
- Abidi, S.L.; Rennick, K.A. Determination of nonvolatile components in polar fractions of rice bran oils. J. Am. Oil Chem. Soc. 2003, 80, 1057–1062. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. F 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Aladedunye, F.A. Curbing thermo-oxidative degradation of frying oils: Current knowledge and challenges. Eur. J. Lipid Sci. Technol. 2015, 117, 1867–1881. [Google Scholar] [CrossRef]
- Arslan, F.N.; Şapçı, A.N.; Duru, F.; Kara, H. A study on monitoring of frying performance and oxidative stability of cottonseed and palm oil blends in comparison with original oils. Int. J. Food Prop. 2017, 20, 704–717. [Google Scholar] [CrossRef]
- Enríquez-Fernández, B.E.; Álvarez de la Cadena y Yañez, L.; Sosa-Morales, M.E. Comparison of the stability of palm olein and a palm olein/canola oil blend during deep-fat frying of chicken nuggets and French fries. Int. J. Food Sci. Technol. 2011, 46, 1231–1237. [Google Scholar] [CrossRef]
- Abbas, A.; Hadi, B.; Abd, L.; Hidayu, O.; Nik, M. Effect of microwave heating with different exposure times on the degradation of corn oil. Int. Food Res. J. 2016, 23, 842–848. [Google Scholar]
- Sebastian, A.; Ghazani, S.M.; Marangoni, A.G. Quality and safety of frying oils used in restaurants. Food Res. Int. 2014, 64, 420–423. [Google Scholar] [CrossRef]
- Karimi, S.; Wawire, M.; Mathooko, F.M. Impact of frying practices and frying conditions on the quality and safety of frying oils used by street vendors and restaurants in Nairobi, Kenya. J. Food Compos. Anal. 2017, 62, 239–244. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, Y.; Cao, P.; Liu, Y. Effects of frying oils’ fatty acids profile on the formation of polar lipids components and their retention in French fries over deep-frying process. Food Chem. 2017, 237, 98–105. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.M.; Calvo, M.V.; Fontecha, J.; Alonso, L. Alterations in the Fatty Acid Composition in Infant Formulas and ω3-PUFA Enriched UHT Milk during Storage. Foods 2019, 8, 163. [Google Scholar] [CrossRef]
- Marangoni, F.; Galli, C.; Ghiselli, A.; Lercker, G.; La Vecchia, C.; Maffeis, C.; Agostoni, C.; Ballardini, D.; Brignoli, O.; Faggiano, P.; et al. Palm oil and human health. Meeting report of NFI: Nutrition Foundation of Italy symposium. Int. J. Food Sci. Nutr. 2017, 68, 643–655. [Google Scholar] [CrossRef] [PubMed]
| Heating Temperature (°C) | Heating Time (h) | EpoxyFAMEs | KetoFAMEs | HydroxyFAMEs | Total |
|---|---|---|---|---|---|
| Fresh oil | 0 | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 160 | 4 | 0.05 ± 0.00 ab | 0.05 ± 0.01 ab | 0.13 ± 0.01 bc | 0.23 ± 0.05 ab |
| 8 | 0.11 ± 0.01 abc | 0.08 ± 0.02 ab | 0.19 ± 0.02 bcd | 0.39 ± 0.06 bcdef | |
| 12 | 0.17 ± 0.02 cde | 0.12 ± 0.03 abc | 0.21 ± 0.07 bcde | 0.51 ± 0.05 bcdefg | |
| 16 | 0.27 ± 0.01 ef | 0.20 ± 0.03 bcde | 0.29 ± 0.02 def | 0.75 ± 0.05 fghi | |
| 20 | 0.36 ± 0.03 fgh | 0.21 ± 0.06 bcde | 0.27 ± 0.04 def | 0.83 ± 0.07 ghi | |
| 24 | 0.42 ± 0.07 gh | 0.21 ± 0.08 bcde | 0.25 ± 0.08 cdef | 0.88 ± 0.11 hi | |
| 170 | 4 | 0.13 ± 0.01bcd | 0.05 ± 0.01ab | 0.13 ± 0.01bc | 0.31 ± 0.05abcd |
| 8 | 0.18 ± 0.03 cde | 0.11 ± 0.03 ab | 0.18 ± 0.03 bcd | 0.46 ± 0.04 bcdef | |
| 12 | 0.24 ± 0.03 def | 0.18 ± 0.02 bcd | 0.23 ± 0.02 bcdef | 0.66 ± 0.03 defgh | |
| 16 | 0.32 ± 0.10 fg | 0.28 ± 0.13 cdef | 0.29 ± 0.08 def | 0.90 ± 0.02 hi | |
| 20 | 0.31 ± 0.09 fg | 0.29 ± 0.10 def | 0.28 ± 0.07 def | 0.88 ± 0.01 hi | |
| 24 | 0.36 ± 0.06 fgh | 0.35 ± 0.08 ef | 0.32 ± 0.05 ef | 1.03 ± 0.02 i | |
| 180 | 4 | 0.09 ± 0.00 abc | 0.08 ± 0.01 ab | 0.10 ± 0.00 ab | 0.27 ± 0.01 abc |
| 8 | 0.17 ± 0.02 cde | 0.08 ± 0.03 ab | 0.10 ± 0.02 ab | 0.36 ± 0.05 abcde | |
| 12 | 0.27 ± 0.02 ef | 0.17 ± 0.06 bcd | 0.17 ± 0.04 bcd | 0.61 ± 0.06 cdefgh | |
| 16 | 0.33 ± 0.02 fg | 0.19 ± 0.08 bcde | 0.19 ± 0.06 bcd | 0.71 ± 0.08 efghi | |
| 20 | 0.46 ± 0.06 h | 0.31 ± 0.06 def | 0.28 ± 0.04 def | 1.05 ± 0.16 i | |
| 24 | 0.73 ± 0.05 i | 0.42 ± 0.12 f | 0.35 ± 0.09 f | 1.50 ± 0.25 j |
| Heating Temperature (°C) | Heating Time (h) | Polar Fraction Content (%, g 100 g−1) | Polar Fraction Composition (%, g 100 g−1) | ||||
|---|---|---|---|---|---|---|---|
| TAG Oligomers | TAG Dimers | Oxidized TAG Monomers | Diacylglycerols | Free Fatty Acids | |||
| Fresh oil | 0 | 8.38 ± 0.53 a | 0.00 ± 0.00 a | 0.27 ± 0.05 a | 1.21 ± 0.05 a | 6.54 ± 0.59 a | 0.35 ± 0.11 c |
| 160 | 4 | 10.85 ± 0.42 ab | 0.25 ± 0.06 a | 1.67 ± 0.17 ab | 2.49 ± 0.28 ab | 6.17 ± 0.65 a | 0.27 ± 0.04 bc |
| 8 | 13.47 ± 0.45 bc | 0.75 ± 0.04 ab | 2.73 ± 0.19 bcd | 3.53 ± 0.17 bcd | 6.23 ± 0.22 a | 0.24 ± 0.04 bc | |
| 12 | 16.00 ± 1.31 cd | 1.34 ± 0.15 abcd | 3.65 ± 0.33 de | 4.91 ± 0.33 de | 5.91 ± 0.79 a | 0.19 ± 0.03 abc | |
| 16 | 19.15 ± 0.28 de | 1.98 ± 0.06 bcde | 4.80 ± 0.11 ef | 6.05 ± 0.25 ef | 6.15 ± 0.15 a | 0.18 ± 0.07 abc | |
| 20 | 22.11 ± 0.41 efg | 2.76 ± 0.22 defg | 5.75 ± 0.11 fgh | 7.43 ± 0.18 fg | 6.08 ± 0.15 a | 0.09 ± 0.10 ab | |
| 24 | 25.05 ± 0.30 fghi | 3.64 ± 0.30 fg | 6.63 ± 0.06 ghi | 8.61 ± 0.24 gh | 6.05 ± 0.27 a | 0.13 ± 0.15 abc | |
| 170 | 4 | 12.04 ± 0.57 abc | 0.42 ± 0.24 a | 1.98 ± 0.36 abc | 3.10 ± 0.24 abcd | 6.27 ± 0.47 a | 0.25 ± 0.05 bc |
| 8 | 15.95 ± 1.20 cd | 1.25 ± 0.31 abc | 3.43 ± 0.59 cde | 4.80 ± 0.44 cde | 6.24 ± 0.38 a | 0.22 ± 0.15 abc | |
| 12 | 19.95 ± 2.09 de | 2.04 ± 0.47 bcde | 4.81 ± 0.79 ef | 6.68 ± 1.04 efg | 6.23 ± 0.31 a | 0.19 ± 0.07 abc | |
| 16 | 23.36 ± 2.56 efgh | 2.84 ± 0.64 efg | 5.99 ± 1.07 fgh | 8.02 ± 1.09 fgh | 6.37 ± 0.09 a | 0.14 ± 0.11 abc | |
| 20 | 27.46 ± 3.33 hij | 4.19 ± 1.02 gh | 7.09 ± 1.21 hi | 9.90 ± 1.25 hi | 6.21 ± 0.11 a | 0.06 ± 0.12 ab | |
| 24 | 30.35 ± 3.21 jk | 5.40 ± 0.86 hi | 7.89 ± 1.08 ij | 10.96 ± 1.42 i | 6.10 ± 0.17 a | 0.00 ± 0.00 a | |
| 180 | 4 | 12.14 ± 1.22 abc | 0.46 ± 0.20 a | 2.42 ± 0.53 bcd | 2.86 ± 0.52 abc | 6.19 ± 0.12 a | 0.21 ± 0.09 abc |
| 8 | 15.92 ± 1.68 cd | 1.44 ± 0.09 abcde | 3.84 ± 0.69 de | 4.84 ± 0.68 cde | 5.68 ± 0.68 a | 0.13 ± 0.02 abc | |
| 12 | 21.19 ± 1.96 ef | 2.68 ± 0.60 cdef | 5.45 ± 0.71 fg | 6.81 ± 0.96 efg | 6.16 ± 0.38 a | 0.09 ± 0.10 ab | |
| 16 | 25.87 ± 2.37 ghij | 3.84 ± 0.79 fg | 6.95 ± 0.58 ghi | 8.65 ± 1.37 gh | 6.19 ± 0.38 a | 0.24 ± 0.06 bc | |
| 20 | 29.25 ± 2.12 ijk | 5.30 ± 0.80 hi | 7.82 ± 0.73 ij | 9.93 ± 0.89 hi | 6.20 ± 0.35 a | 0.00 ± 0.00 a | |
| 24 | 32.92 ± 1.96 k | 6.74 ± 1.27 i | 8.91 ± 0.43 j | 11.18 ± 0.95 i | 6.08 ± 0.55 a | 0.00 ± 0.00 a | |
| Heating Temperature (° C) | Heating Time (h) | Peroxide Value (meq O2/kg) | Anisidine Value | TOTOX Value |
|---|---|---|---|---|
| Fresh oil | 0 | 1.40 ± 0.22 a | 2.20 ± 0.64 a | 5.00 ± 0.96 a |
| 160 | 4 | 8.12 ± 1.89 bc | 38.87 ± 1.08 b | 55.10 ± 4.84 bc |
| 8 | 7.00 ± 1.48 bc | 53.82 ± 6.07 cde | 67.81 ± 5.40 cdef | |
| 12 | 8.12 ± 3.68 bc | 58.57 ± 5.44 cdef | 74.82 ± 3.01 defg | |
| 16 | 6.75 ± 1.19 bc | 66.53 ± 4.59 efg | 80.02 ± 5.52 fghi | |
| 20 | 6.87 ± 1.38 bc | 67.44 ± 5.72 efg | 81.18 ± 4.01 ghi | |
| 24 | 6.13 ± 1.97 b | 70.44 ± 7.65 fgh | 82.69 ± 6.63 ghi | |
| 170 | 4 | 8.75 ± 2.96 bc | 34.01 ± 3.88 b | 51.50 ± 4.29 b |
| 8 | 7.87 ± 1.89 bc | 47.79 ± 5.07 bcd | 63.52 ± 4.76 bcd | |
| 12 | 7.50 ± 0.58 bc | 64.32 ± 13.25 efg | 79.31 ± 12.18 fgh | |
| 16 | 7.87 ± 0.75 bc | 58.83 ± 6.75 def | 74.58 ± 7.45 defg | |
| 20 | 8.62 ± 0.74 bc | 68.83 ± 1.37 fg | 86.07 ± 1.67 ghi | |
| 24 | 7.25 ± 0.65 bc | 76.07 ± 8.87 gh | 90.56 ± 7.58 hij | |
| 180 | 4 | 10.10 ± 0.47 c | 44.64 ± 2.68 bc | 64.84 ± 3.07 cde |
| 8 | 9.74 ± 0.30 bc | 58.36 ± 2.99 cdef | 77.83 ± 3.08 efgh | |
| 12 | 9.61 ± 0.31 bc | 73.74 ± 0.68 gh | 92.96 ± 0.87 ij | |
| 16 | 8.36 ± 0.25 bc | 84.24 ± 0.74 hi | 100.95 ± 0.53 jk | |
| 20 | 7.99 ± 0.36 bc | 93.62 ± 2.43 i | 109.59 ± 2.45 k | |
| 24 | 7.49 ± 0.58 bc | 96.33 ± 2.48 i | 111.31 ± 3.07 k |
| Heating Temperature (°C) | Heating Time (h) | Fatty Acid Composition (Relative Percentages) | C8 (ppm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C12:0 | C14:0 | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | C18:2/C16:0 | |||
| Fresh oil | 0 | 0.24 ± 0.00 a | 1.53 ± 0.04c | 39.80 ± 0.01 a | 4.19 ± 0.00 a | 43.90 ± 0.05 b | 10.33 ± 0.01 cde | N.D. | 0.26 ± 0.00 i | 0.00 ± 0.00 a |
| 160 | 4 | 0.21 ± 0.02 a | 1.03 ± 0.07 ab | 41.43 ± 0.62 a | 7.69 ± 0.82 ab | 39.21 ± 1.42 ab | 10.43 ± 0.73 cde | N.D. | 0.25 ± 0.0 hi | 0.00 ± 0.00 a |
| 8 | 0.22 ± 0.01 a | 0.98 ± 0.05 ab | 41.40 ± 0.93 a | 9.92 ± 0.10 bc | 38.14 ± 1.53 ab | 9.26 ± 0.50 bcde | N.D. | 0.22 ± 0.01 fghi | 2.84 ± 0.50 ab | |
| 12 | 0.23 ± 0.01 a | 1.02 ± 0.05 ab | 41.88 ± 1.77 a | 9.82 ± 0.24 bc | 38.20 ± 1.31 ab | 8.76 ± 0.51 bcde | N.D. | 0.21 ± 0.02 defghi | 4.83 ± 0.43 abcd | |
| 16 | 0.23 ± 0.02 a | 1.10 ± 0.22 ab | 42.08 ± 3.41 a | 11.58 ± 0.87 c | 36.87 ± 3.07 ab | 8.02 ± 1.05 abcd | N.D. | 0.19 ± 0.04 bcdefg | 5.78 ± 0.32 bcd | |
| 20 | 0.23 ± 0.02 a | 1.02 ± 0.04 ab | 42.98 ± 0.81 a | 12.48 ± 0.74 c | 35.27 ± 1.14 ab | 7.89 ± 0.44 abc | N.D. | 0.18 ± 0.01 bcdef | 7.07 ± 0.56 bcd | |
| 24 | 0.23 ± 0.01 a | 1.08 ± 0.11 ab | 45.84 ± 4.31 a | 12.53 ± 0.96 c | 33.14 ± 3.92 a | 7.03 ± 0.25 ab | N.D. | 0.15 ± 0.02 abc | 9.69 ± 1.83 de | |
| 170 | 4 | 0.23 ± 0.02 a | 1.08 ± 0.10 ab | 42.46 ± 0.75 a | 5.18 ± 0.28 a | 40.35 ± 1.85 ab | 10.70 ± 1.00 e | N.D. | 0.25 ± 0.02 hi | 0.00 ± 0.00 a |
| 8 | 0.25 ± 0.03 a | 1.17 ± 0.17 abc | 43.65 ± 1.29 a | 5.33 ± 0.44 a | 39.02 ± 2.14 ab | 10.44 ± 1.75 de | N.D. | 0.24 ± 0.04 ghi | 3.93 ± 0.32 abc | |
| 12 | 0.24 ± 0.02 a | 1.20 ± 0.16 abc | 45.69 ± 1.97 a | 6.49 ± 0.24 ab | 36.95 ± 1.68 ab | 9.28 ± 0.16 bcde | N.D. | 0.20 ± 0.01 cdefgh | 5.27 ± 0.58 abcd | |
| 16 | 0.24 ± 0.03 a | 1.11 ± 0.07 ab | 45.24 2.38 a | 6.77 ± 2.60 ab | 38.08 ± 0.82 ab | 8.44 ± 0.69 abcde | N.D. | 0.19 ± 0.01 bcdefg | 13.49 ± 0.91 ef | |
| 20 | 0.26 ± 0.09 a | 1.16 ± 0.36 abc | 47.69 ± 13.55 a | 6.81 ± 3.55 ab | 36.71 ± 11.76 ab | 7.21 ± 1.52 ab | N.D. | 0.16 ± 0.03 abcd | 13.82 ± 1.51 ef | |
| 24 | 0.26 ± 0.04 a | 1.20 ± 0.15 abc | 49.09 ± 5.71 a | 7.32 ± 1.51 ab | 34.67 ± 5.12 ab | 7.29 ± 0.89 ab | N.D. | 0.15 ± 0.01 abc | 16.77 ± 0.79 fg | |
| 180 | 4 | 0.19 ± 0.05 a | 0.93 ± 0.13 a | 43.94 ± 5.40 a | 5.02 ± 1.50 a | 39.28 ± 5.89 ab | 10.56 ± 1.47 de | N.D. | 0.24 ± 0.00 ghi | 2.16 ± 0.29 ab |
| 8 | 0.24 ± 0.05 a | 1.11 ± 0.17 ab | 44.94 ± 4.20 a | 5.30 ± 3.16 a | 38.32 ± 3.89 ab | 9.94 ± 1.35 cde | N.D. | 0.22 ± 0.02 efghi | 5.05 ± 1.98 abcd | |
| 12 | 0.22 ± 0.01 a | 1.10 ± 0.05 ab | 46.58 ± 2.33 a | 5.52 ± 1.33 a | 37.57 ± 2.61 ab | 8.68 ± 0.81 bcde | N.D. | 0.19 ± 0.01 bcdefg | 9.00 ± 3.25 cde | |
| 16 | 0.23 ± 0.02 a | 1.15 ± 0.07 ab | 48.37 ± 2.77 a | 5.82 ± 0.43 a | 35.87 ± 3.32 ab | 8.14 ± 0.88 abcd | N.D. | 0.17 ± 0.01 abcde | 19.29 ± 6.12 g | |
| 20 | 0.22 ± 0.01 a | 1.17 ± 0.07 abc | 49.26 ± 2.60 a | 5.94 ± 1.01 a | 35.63 ± 2.11 ab | 7.18 ± 0.41 ab | N.D. | 0.15 ± 0.01 ab | 21.08 ± 2.15 g | |
| 24 | 0.24 ± 0.01 a | 1.34 ± 0.20bc | 50.50 ± 4.33 a | 6.12 ± 0.75 ab | 34.96 ± 1.86 ab | 6.11 ± 1.57 a | N.D. | 0.12 ± 0.04 a | 29.13 ± 3.87 h | |
| TPC | TAGO | TAGD | OXTAGM | DAG | FFA | EPOXY | KETO | HYDROXY | PV | AV | TOTOX | C8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1.000 | - | - | - | - | - | - | - | - | - | - | - | - |
| TAGO | 0.976 | 1.000 | - | - | - | - | - | - | - | - | - | - | - |
| TAGD | 0.792 | 0.838 | 1.000 | - | - | - | - | - | - | - | - | - | - |
| OXTAGM | 0.845 | 0.876 | 0.950 | 1.000 | - | - | - | - | - | - | - | - | - |
| DAG | −0.900 | −0.935 | −0.969 | −0.980 | 1.000 | - | - | - | - | - | - | - | - |
| FFA | −0.786 | −0.815 | −0.878 | −0.874 | 0.874 | 1.000 | - | - | - | - | - | - | - |
| EPOXY | 0.886 | 0.893 | 0.703 | 0.714 | −0.793 | −0.710 | 1.000 | - | - | - | - | - | - |
| KETO | 0.816 | 0.811 | 0.651 | 0.690 | −0.739 | −0.661 | 0.865 | 1.000 | - | - | - | - | - |
| HYDROXY | 0.750 | 0.752 | 0.677 | 0.719 | −0.738 | −0.701 | 0.802 | 0.886 | 1.000 | - | - | - | - |
| PV | −0.472 | −0.485 | −0.634 | −0.658 | 0.610 | 0.676 | −0.440 | −0.409 | −0.588 | 1.000 | - | - | - |
| AV | 0.853 | 0.897 | 0.909 | 0.880 | −0.928 | −0.850 | 0.817 | 0.752 | 0.722 | −0.644 | 1.000 | - | - |
| TOTOX | 0.858 | 0.905 | 0.886 | 0.848 | −0.913 | −0.810 | 0.824 | 0.757 | 0.685 | −0.507 | 0.986 | 1.000 | - |
| C8 | 0.885 | 0.865 | 0.623 | 0.652 | −0.735 | −0.628 | 0.870 | 0.816 | 0.672 | −0.329 | 0.786 | 0.813 | 1.000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khor, Y.P.; Hew, K.S.; Abas, F.; Lai, O.M.; Cheong, L.Z.; Nehdi, I.A.; Sbihi, H.M.; Gewik, M.M.; Tan, C.P. Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of Heating Profiles. Foods 2019, 8, 475. https://doi.org/10.3390/foods8100475
Khor YP, Hew KS, Abas F, Lai OM, Cheong LZ, Nehdi IA, Sbihi HM, Gewik MM, Tan CP. Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of Heating Profiles. Foods. 2019; 8(10):475. https://doi.org/10.3390/foods8100475
Chicago/Turabian StyleKhor, Yih Phing, Khai Shin Hew, Faridah Abas, Oi Ming Lai, Ling Zhi Cheong, Imededdine Arbi Nehdi, Hassen Mohamed Sbihi, Mohamed Mossad Gewik, and Chin Ping Tan. 2019. "Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of Heating Profiles" Foods 8, no. 10: 475. https://doi.org/10.3390/foods8100475
APA StyleKhor, Y. P., Hew, K. S., Abas, F., Lai, O. M., Cheong, L. Z., Nehdi, I. A., Sbihi, H. M., Gewik, M. M., & Tan, C. P. (2019). Oxidation and Polymerization of Triacylglycerols: In-Depth Investigations towards the Impact of Heating Profiles. Foods, 8(10), 475. https://doi.org/10.3390/foods8100475