Towards By-Product Utilisation of Pea Hulls: Isolation and Quantification of Galacturonic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
3. Results and Discussion
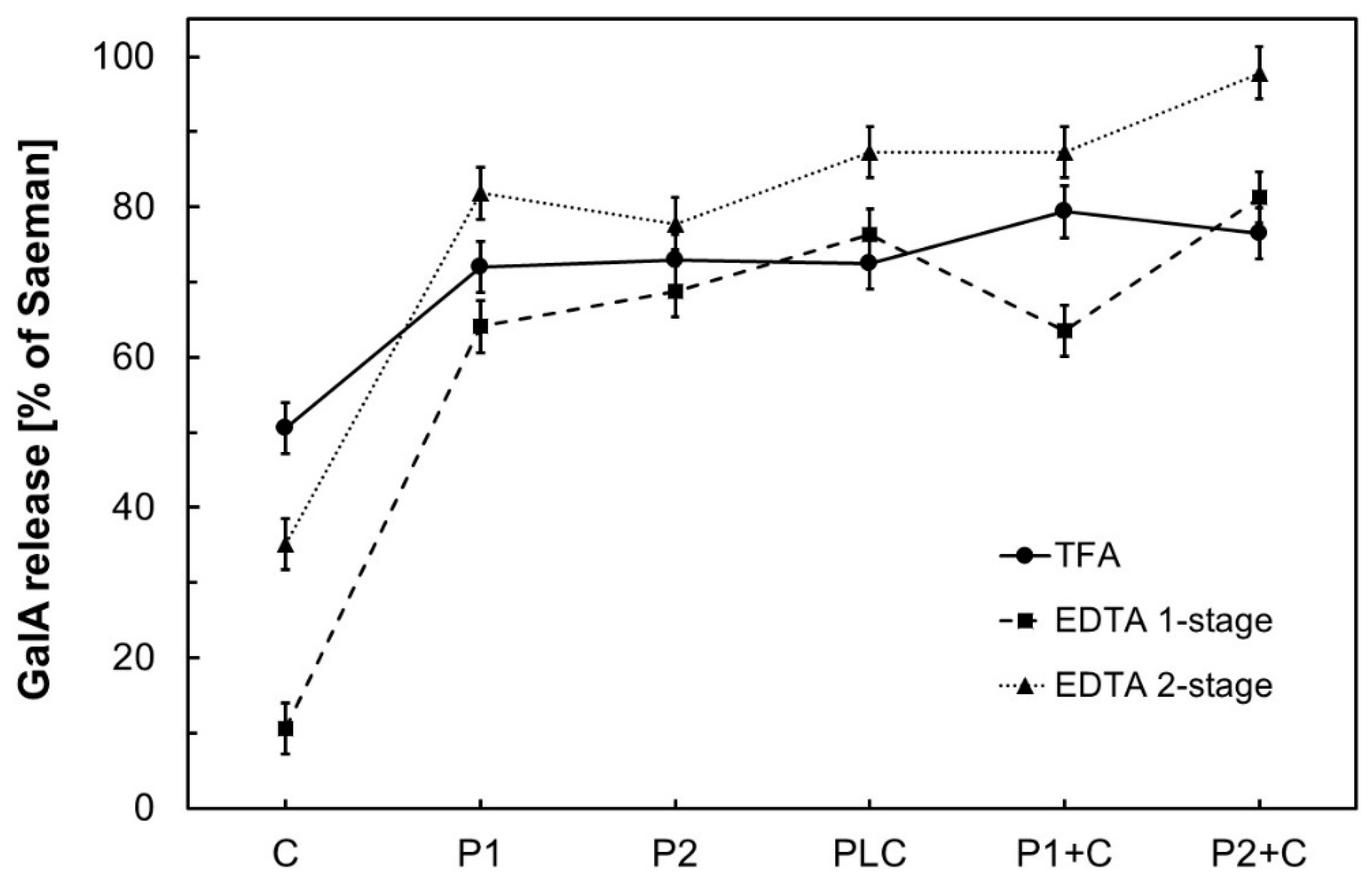
3.1. Comparison of Galacturonic Acid (GalA) Release
3.2. GalA Content of Different Pea Hull Varieties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzpatrick, K. Legumes and whole grains: On trend. Prepared Foods (BNP Media) 2012, 16, 62–72. [Google Scholar]
- Food and Agriculture Organisation of the United Nations—FAO Statistics of Pea Production. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 6 December 2017).
- Ali-Khan, S. Seed hull content in field pea. Can. J. Plant. Sci. 1993, 73, 611–613. [Google Scholar] [CrossRef] [Green Version]
- Kosson, R.; Czuchajowska, Z.; Pomeranz, Y. Smooth and wrinkled peas. 2. Distribution of protein, lipid, and fatty acids in seed and milling fractions. J. Agric. Food Chem. 1994, 42, 96–99. [Google Scholar] [CrossRef]
- Le Goff, A.; Renard, C.M.G.C.; Bonnin, E.; Thibault, J.-F. Extraction, purification and chemical characterisation of xylogalacturonans from pea hulls. Carbohyd. Polym. 2001, 45, 325–334. [Google Scholar] [CrossRef]
- Weightman, R.; Renard, C.M.G.C.; Thibault, J.-F. Structure and properties of the polysaccharides from pea hulls. Part 1: Chemical extraction and fractionation of the polysaccharides. Carbohyd. Polym. 1994, 24, 139–148. [Google Scholar] [CrossRef]
- Leterme, P.; Théwis, A.; Leeuwen, P.V.; Monmart, T.; Huisman, J. Chemical composition of pea fibre isolates and their effect on the endogenous amino acid flow at the ileum of the pig. J. Sci. Food Agric. 1996, 72, 127–134. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Baciu, I.-E.; Jördening, H.-J. Kinetics of galacturonic acid release from sugar-beet pulp. Enzyme Microb. Tech. 2004, 34, 505–512. [Google Scholar] [CrossRef]
- Arnous, A.; Meyer, A.S. Comparison of methods for compositional characterization of grape (Vitis vinifera L.) and apple (Malus domestica) skins. Food Bioprod. Process. 2008, 86, 79–86. [Google Scholar] [CrossRef]
- Happi Emaga, T.; Rabetafika, N.; Blecker, C.S.; Paquot, M. Kinetics of the hydrolysis of polysaccharide galacturonic acid and neutral sugars chains from flaxseed mucilage. Biotechnol. Agron. Soc. Environ. 2012, 16, 139–147. [Google Scholar]
- Manns, D.; Deutschle, A.L.; Saake, B.; Meyer, A.S. Methodology for quantitative determination of the carbohydrate composition of brown seaweeds (Laminariaceae). RSC Adv. 2014, 4, 25736–25746. [Google Scholar] [CrossRef] [Green Version]
- Pettolino, F.A.; Walsh, C.; Fincher, G.B.; Bacic, A. Determining the polysaccharide composition of plant cell walls. Nat. protoc. 2012, 7, 1590–1607. [Google Scholar] [CrossRef] [PubMed]
- Panouillé, M.; Thibault, J.-F.; Bonnin, E. Cellulase and protease preparations can extract pectins from various plant byproducts. J. Agric. Food Chem. 2006, 54, 8926–8935. [Google Scholar] [CrossRef] [PubMed]
- Garna, H.; Mabon, N.; Wathelet, B.; Paquot, M. New method for a two-step hydrolysis and chromatographic analysis of pectin neutral sugar chains. J. Agric. Food Chem. 2004, 52, 4652–4659. [Google Scholar] [CrossRef] [PubMed]
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic enzyme preparations as effective alternative to acid in pectin extraction. Food Hydrocoll. 2015, 44, 156–161. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzynska-Janiszewska, A.; Stodolak, B. Development of complete hydrolysis of pectins from apple pomace. Food Chem. 2015, 172, 675–680. [Google Scholar] [CrossRef]
- Vetter, S. Aspekte der Wasserwechselwirkunkgen in dispersen Obst- und Gemüse-Modellsystemen. Dissertation, Technische Universität Berlin, 28.11.2003. Available online: https://depositonce.tu-berlin.de/bitstream/11303/935/1/Dokument_45.pdf (accessed on 1 November 2018).
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Losonczi, A.; Csiszár, E.; Szakács, G.; Bezúr, L. Role of the EDTA chelating agent in bioscouring of cotton. Text. Res. J. 2005, 75, 411–417. [Google Scholar] [CrossRef]
- Kurin-Csörgei, K.; Epstein, I.R.; Orbán, M. Systematic design of chemical oscillators using complexation and precipitation equilibria. Nature 2005, 433, 139–142. [Google Scholar] [CrossRef]
- Yang, S.; Hou, Y.; Hu, D. On pH and Ca2+ oscillations monitored by pH electrode and Ca-ISE in bromate-sulfite-ferrocyanide system introduced Ca-EDTA. B. Kor. Chem. Soc. 2015, 36, 237–243. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H.; Dongowski, G. Thermal analysis of chemically and mechanically modified pectins. Food Hydrocoll. 2007, 21, 1101–1112. [Google Scholar] [CrossRef]
- Rosenbohm, C.; Lundt, I.; Christensen, T.; Young, N. Chemically methylated and reduced pectins: Preparation, characterisation by 1H NMR spectroscopy, enzymatic degradation, and gelling properties. Carbohyd. Res. 2003, 338, 637–649. [Google Scholar] [CrossRef]
- Kim, Y.; Williams, M.A.; Luzio, G.A.; Cameron, R.G. Introduction and characterization of charged functional domains into an esterified pectic homogalacturonan by a citrus pectin methylesterase and comparison of its modes of action to other pectin methylesterase isozymes. Food Hydrocoll. 2017, 69, 422–431. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Reichert, R. Quantitative isolation and estimation of cell wall material from dehulled pea (Pisum sativum) flours and concentrates. Cereal Chem. 1981, 58, 266–270. [Google Scholar]
- Weightman, R.; Renard, C.M.G.C.; Gallant, D.; Thibault, J.-F. Structure and properties of the polysaccharides from pea hulls—II. Modification of the composition and physico-chemical properties of pea hulls by chemical extraction of the constituent polysaccharides. Carbohyd. Polym. 1995, 26, 121–128. [Google Scholar] [CrossRef]
| Activity | P1 Pectinex Ultra SPL (Novozymes) | P2 Vegazym M (Erbslöh) | PLC Ultrazym AFP-L (Novozymes) | C Celluclast 1.5L (Novozymes) |
|---|---|---|---|---|
| Polygalacturonase 3.2.1.15 | X | X | X | |
| Pectin lyase 4.2.2.10 | X | |||
| Cellulase 3.2.1.4 | X | X |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14,215.75 | 17 | 836.22 | 103.32 | <0.0001 * |
| A (digestion method) | 1757.36 | 2 | 878.68 | 108.57 | <0.0001 * |
| B (type of enzyme) | 10,878.32 | 5 | 2175.66 | 268.82 | <0.0001 * |
| AB | 1580.07 | 10 | 158.01 | 19.52 | <0.0001 * |
| Pure error | 145.68 | 18 | 8.09 | ||
| Corr. total | 14,361.43 | 35 |
| GalA Content (%) | |
|---|---|
| Salamanca | 11.1 ± 0.1 |
| Rocket | 11.0 ± 0.3 |
| Starter | 10.9 ± 0.2 |
| Navarro | 11.0 ± 0.3 |
| James | 11.5 ± 0.2 |
| Gregor | 11.7 ± 0.2 |
| Commercial samples (n = 14) | 9.7 ± 0.1–12.4 ± 0.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutöhrlein, F.; Drusch, S.; Schalow, S. Towards By-Product Utilisation of Pea Hulls: Isolation and Quantification of Galacturonic Acid. Foods 2018, 7, 203. https://doi.org/10.3390/foods7120203
Gutöhrlein F, Drusch S, Schalow S. Towards By-Product Utilisation of Pea Hulls: Isolation and Quantification of Galacturonic Acid. Foods. 2018; 7(12):203. https://doi.org/10.3390/foods7120203
Chicago/Turabian StyleGutöhrlein, Friederike, Stephan Drusch, and Sebastian Schalow. 2018. "Towards By-Product Utilisation of Pea Hulls: Isolation and Quantification of Galacturonic Acid" Foods 7, no. 12: 203. https://doi.org/10.3390/foods7120203
APA StyleGutöhrlein, F., Drusch, S., & Schalow, S. (2018). Towards By-Product Utilisation of Pea Hulls: Isolation and Quantification of Galacturonic Acid. Foods, 7(12), 203. https://doi.org/10.3390/foods7120203




