Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Onion Sample Preparation
2.3. PEF Treatment
2.4. Headspace SPME-GC-MS
2.5. Statistical Analysis
3. Results and Discussion
3.1. Identification and Characterisation of Volatile Compounds
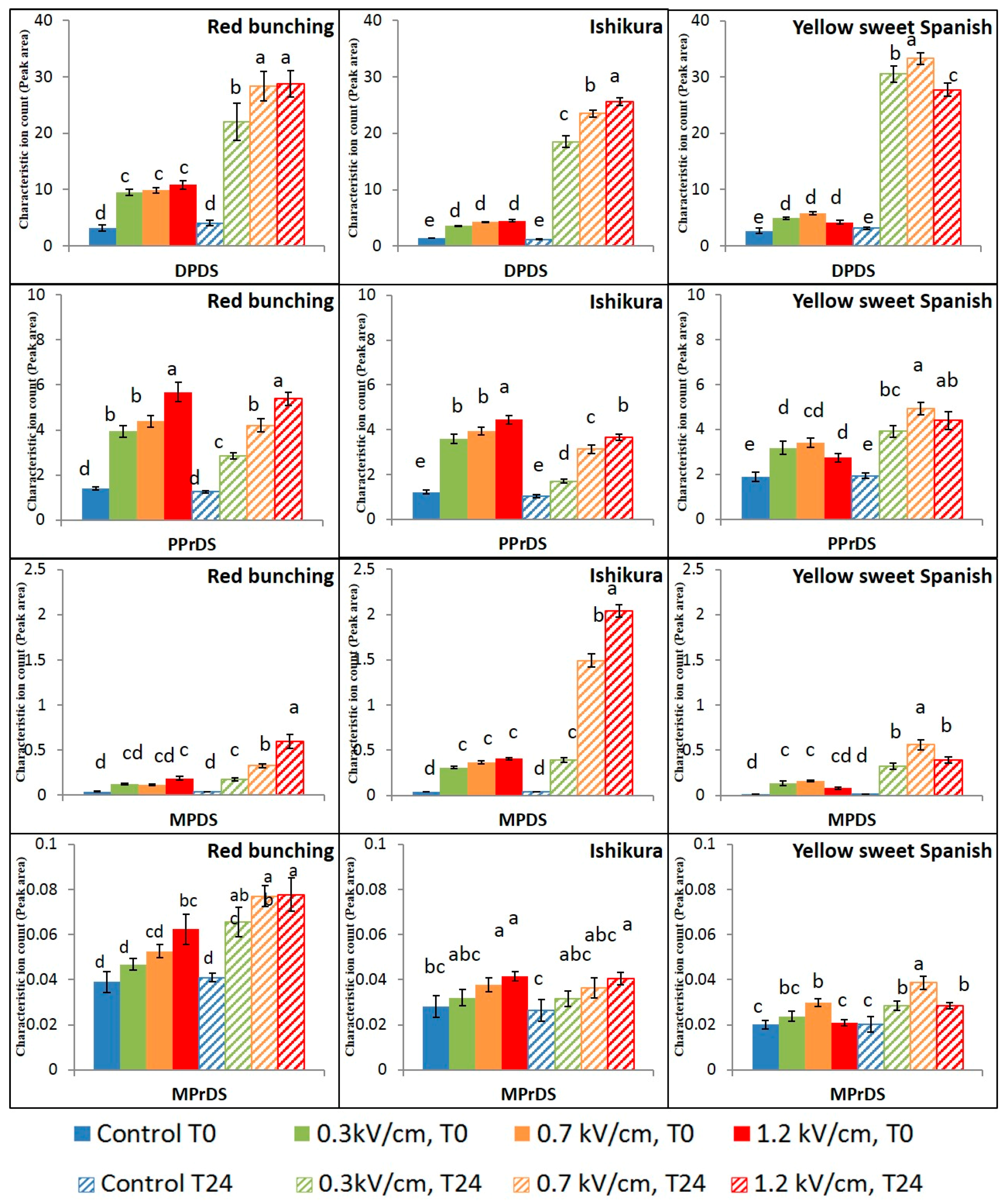
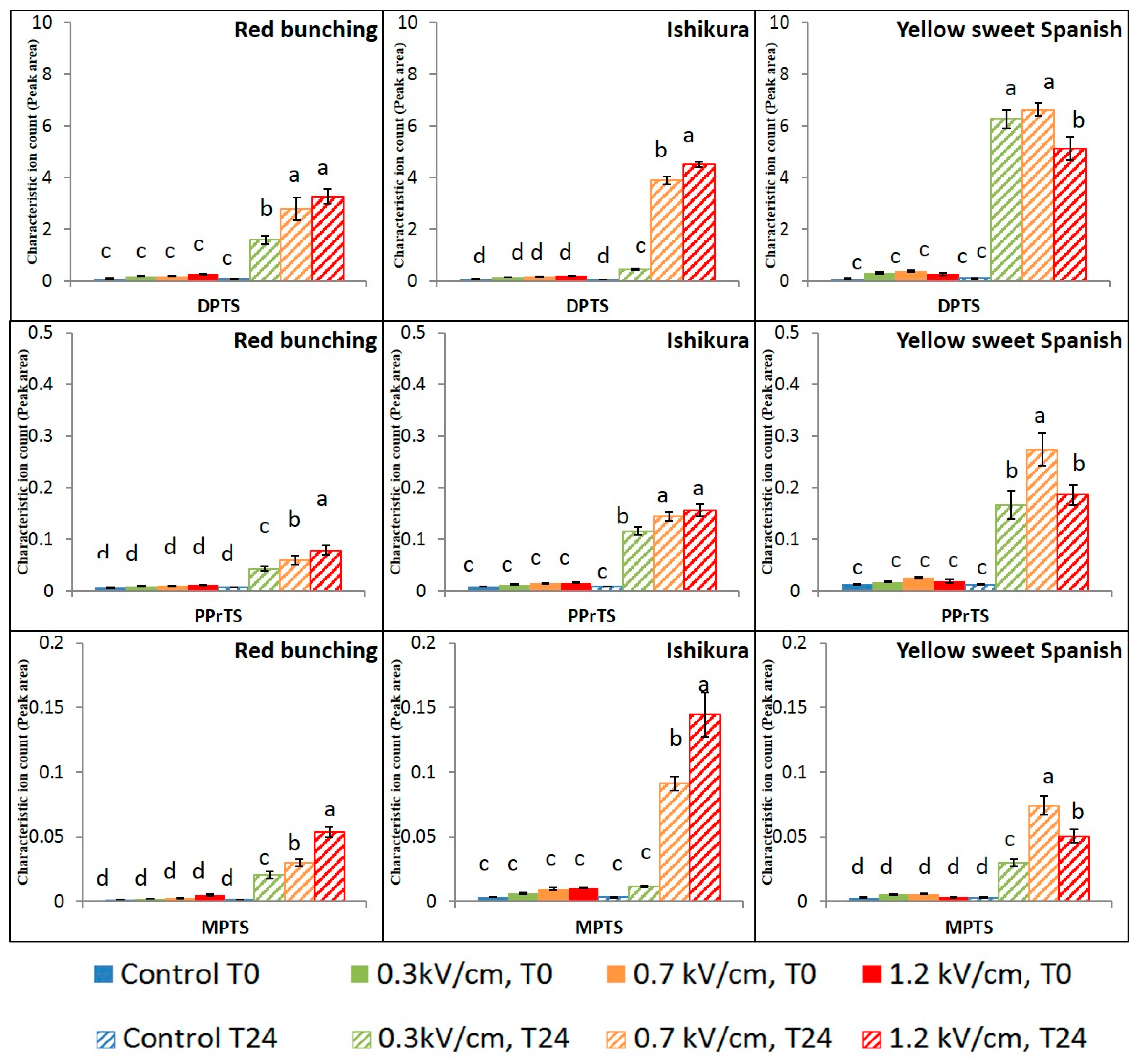
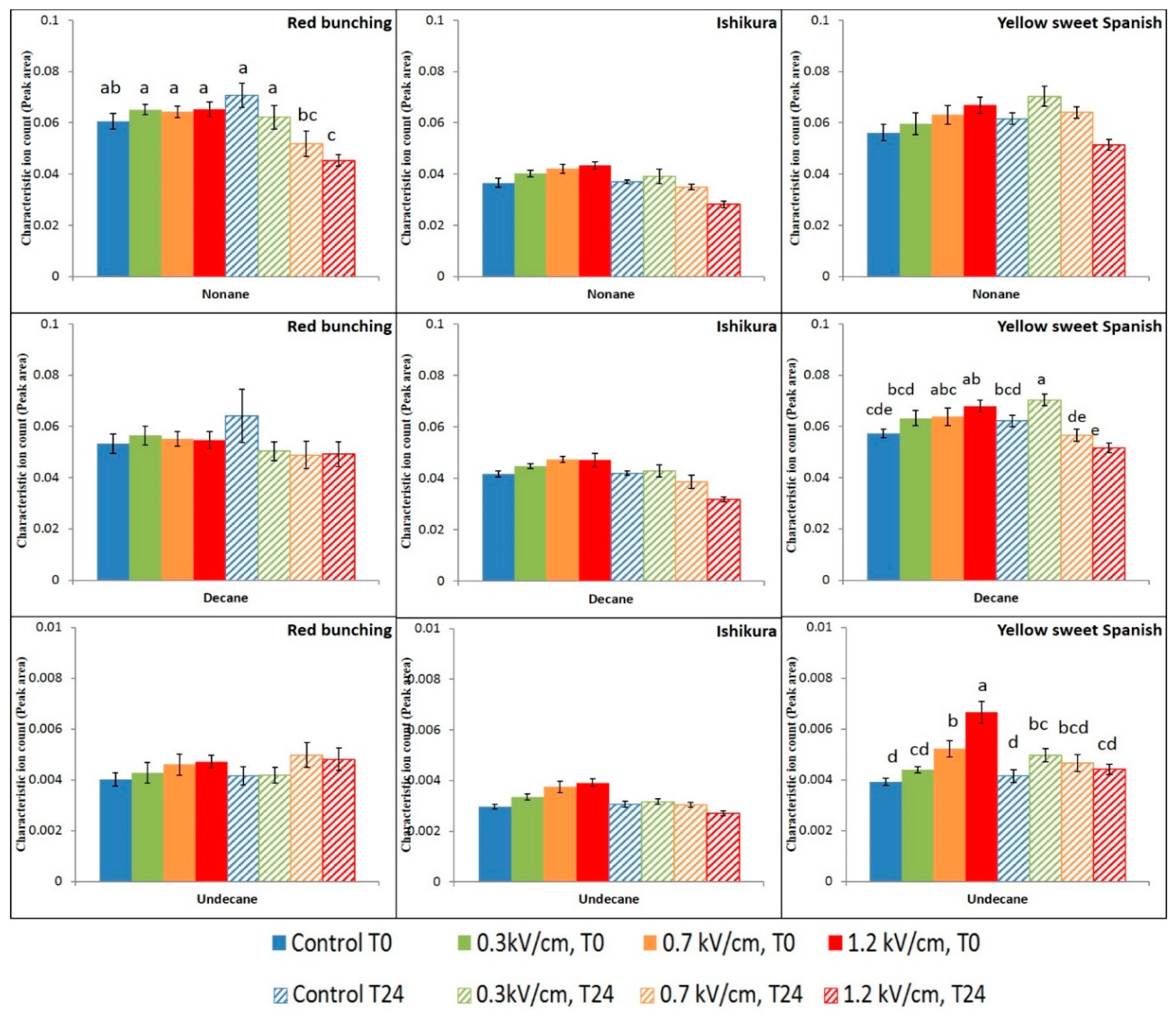
3.2. Evaluation of PEF Treatment Intensities and Incubation Time on the Volatile Compounds
3.3. Effect of PEF Processing Intensities on the Volatile Concentrations
3.4. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doevenspeck, H. Influencing cells and cell walls by electrostatic impulses. Meat Ind. 1961, 13, 968–987. [Google Scholar]
- Raso, J.; Heinz, V. Pulsed Electric Fields Technology for the Food Industry, 1st ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Raso, J.; Barbosa-Cánovas, G.V. Nonthermal preservation of foods using combined processing techniques. Crit. Rev. Food Sci. Nutr. 2003, 43, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.; Ade-Omowaye, B.I.; Heinz, V. Nutritional improvement of plant foods by non-thermal processing. Proc. Nutr. Soc. 2002, 61, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lebovka, N.I.; Praporscic, I.; Vorobiev, E. Effect of moderate thermal and pulsed electric field treatments on textural properties of carrots, potatoes and apples. Innov. Food Sci. Emerg. Technol. 2004, 5, 9–16. [Google Scholar] [CrossRef]
- Bazhal, M.I.; Lebovka, N.I.; Vorobiev, E. Optimisation of pulsed electric field strength for electroplasmolysis of vegetable tissues. Biosystems Eng. 2003, 86, 339–345. [Google Scholar] [CrossRef]
- Asavasanti, S.; Ersus, S.; Ristenpart, W.; Stroeve, P.; Barrett, D.M. Critical electric field strengths of onion tissues treated by pulsed electric fields. J. Food Sci. 2010, 75, E433–E443. [Google Scholar] [CrossRef] [PubMed]
- Fincan, M.; DeVito, F.; Dejmek, P. Pulsed electric field treatment for solid–liquid extraction of red beetroot pigment. J. Food Eng. 2004, 64, 381–388. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Shynkaryk, N.V.; Vorobiev, E. Pulsed electric field enhanced drying of potato tissue. J. Food Eng. 2007, 78, 606–613. [Google Scholar] [CrossRef]
- Janositz, A.; Semrau, J.; Knorr, D. Impact of PEF treatment on quality parameters of white asparagus (Asparagus officinalis L.). Innov. Food Sci. Emerg. Technol. 2011, 12, 269–274. [Google Scholar] [CrossRef]
- Knorr, D.; Angersbach, A. Impact of high-intensity electric field pulses on plant membrane permeabilization. Trends Food Sci. Technol. 1998, 9, 185–191. [Google Scholar] [CrossRef]
- Angersbach, A.; Heinz, V.; Knorr, D. Effects of pulsed electric fields on cell membranes in real food systems. Innov. Food Sci. Emerg. Technol. 2000, 1, 135–149. [Google Scholar] [CrossRef]
- Ben Ammar, J.; Lanoisellé, J.L.; Lebovka, N.I.; Van Hecke, E.; Vorobiev, E. Impact of a pulsed electric field on damage of plant tissues: effects of cell size and tissue electrical conductivity. J. Food Sci. 2011, 76, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Faridnia, F.; Burritt, D.J.; Bremer, P.J.; Oey, I. Innovative approach to determine the effect of pulsed electric fields on the microstructure of whole potato tubers: Use of cell viability, microscopic images and ionic leakage measurements. Food Res. Int. 2015, 77, 556–564. [Google Scholar] [CrossRef]
- Liu, T.; Dodds, E.; Leong, S.Y.; Eyres, G.T.; Burritt, D.J.; Oey, I. Effect of pulsed electric fields on the structure and frying quality of “kumara” sweet potato tubers. Innov. Food Sci. Emerg. Technol. 2017, 39, 197–208. [Google Scholar] [CrossRef]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions-A global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Selby, C.; Galpin, I.J.; Collin, H.A. Comparison of the onion plant (Allium cepa) and onion tissue culture. I. Alliinase activity and flavour precursor compounds. New Phytol. 1979, 83, 351–359. [Google Scholar] [CrossRef]
- Bacon, J.R.; Moates, G.K.; Ng, A.; Rhodes, M.J.; Smith, A.C.; Waldron, K.W. Quantitative analysis of flavour precursors and pyruvate levels in different tissues and cultivars of onion (Allium cepa). Food Chem. 1999, 64, 257–261. [Google Scholar] [CrossRef]
- Block, E. The chemistry of garlic and onions. Sci. Am. 1985, 252, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mondy, N.; Duplat, D.; Christides, J.P.; Arnault, I.; Auger, J. Aroma analysis of fresh and preserved onions and leek by dual solid-phase microextraction–liquid extraction and gas chromatography–mass spectrometry. J. Chromatogr. A 2002, 963, 89–93. [Google Scholar] [CrossRef]
- Iranshahi, M. A review of volatile sulfur-containing compounds from terrestrial plants: biosynthesis, distribution and analytical methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007, 30, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Lee, J.H.; Choi, S.H.; Lee, J.S.; Ohnisi-Kameyama, M.; Kozukue, N.; Levin, C.E.; Friedman, M. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J. Agric. Food Chem. 2008, 56, 8541–8548. [Google Scholar] [CrossRef] [PubMed]
- Block, E. The organosulfur chemistry of the genus Allium-implications for the organic chemistry of sulfur. Angew. Chem. Intern. Ed. 1992, 31, 1135–1178. [Google Scholar] [CrossRef]
- Block, E.; Putman, D.; Zhao, S.H. Allium chemistry: GC-MS analysis of thiosulfinates and related compounds from onion, leek, scallion, shallot, chive, and Chinese chive. J. Agric. Food Chem. 1992, 40, 2431–2438. [Google Scholar] [CrossRef]
- Schmidt, N.E.; Santiago, L.M.; Eason, H.D.; Dafford, K.A.; Grooms, C.A.; Link, T.E.; Manning, D.T.; Cooper, S.D.; Keith, R.C.; Chance, W.O.; et al. Rapid extraction method of quantitating the lachrymatory factor of onion using gas chromatography. J. Agric. Food Chem. 1996, 44, 2690–2693. [Google Scholar] [CrossRef]
- Eady, C.C.; Kamoi, T.; Kato, M.; Porter, N.G.; Davis, S.; Shaw, M.; Kamoi, A.; Imai, S. Silencing onion lachrymatory factor synthase causes a significant change in the sulfur secondary metabolite profile. Plant Physiol. 2008, 147, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.C.; Ho, C.T. Volatile constituents of the distilled oils of welsh onions (Allium fistulosum L. variety maichuon) and scallions (Allium fistulosum L. variety caespitosum). J. Agric. Food Chem. 1992, 40, 111–117. [Google Scholar] [CrossRef]
- Ersus, S.; Oztop, M.H.; McCarthy, M.J.; Barrett, D.M. Disintegration efficiency of pulsed electric field induced effects on onion (Allium cepa L.) tissues as a function of pulse protocol and determination of cell integrity by H-NMR relaxometry. J. Food Sci. 2010, 75, E444–E452. [Google Scholar] [CrossRef] [PubMed]
- Maskooki, A.; Eshtiaghi, M.N. Impact of pulsed electric field on cell disintegration and mass transfer in sugar beet. Food Bioprod. Process. 2012, 90, 377–384. [Google Scholar] [CrossRef]
- Villière, A.; Le Roy, S.; Fillonneau, C.; Guillet, F.; Falquerho, H.; Boussely, S.; Prost, C. Evaluation of aroma profile differences between sué, sautéed, and pan-fried onions using an innovative olfactometric approach. Flavour 2015, 4, 24–43. [Google Scholar] [CrossRef]
- Tsuge, K.; Kataoka, M.; Seto, Y. Determination of S-methyl-, S-propyl-, and S-propenyl-l-cysteine sulfoxides by gas chromatography-mass spectrometry after tert-butyldimethylsilylation. J. Agric. Food Chem. 2002, 50, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.E.; Randle, W.M. Changes in the S-alk(en)yl cysteine sulfoxides and their biosynthetic intermediates during onion storage. J. Am. Soc. Hortic. Sci. 1999, 124, 177–183. [Google Scholar]
- Randle, W.M. Onion Flavor Chemistry and Factors Influencing Flavor Intensity. In Spices; American Chemical Society: Athens, Greece, GA, USA, 2007; pp. 41–52. [Google Scholar]
- Yusuf, O.K.; Bewaji, C.O. Evaluation of essential oils composition of methanolic Allium sativum extract on Trypanosoma brucei infected rats. Res. Pharm. Biotech. 2011, 3, 17–21. [Google Scholar]
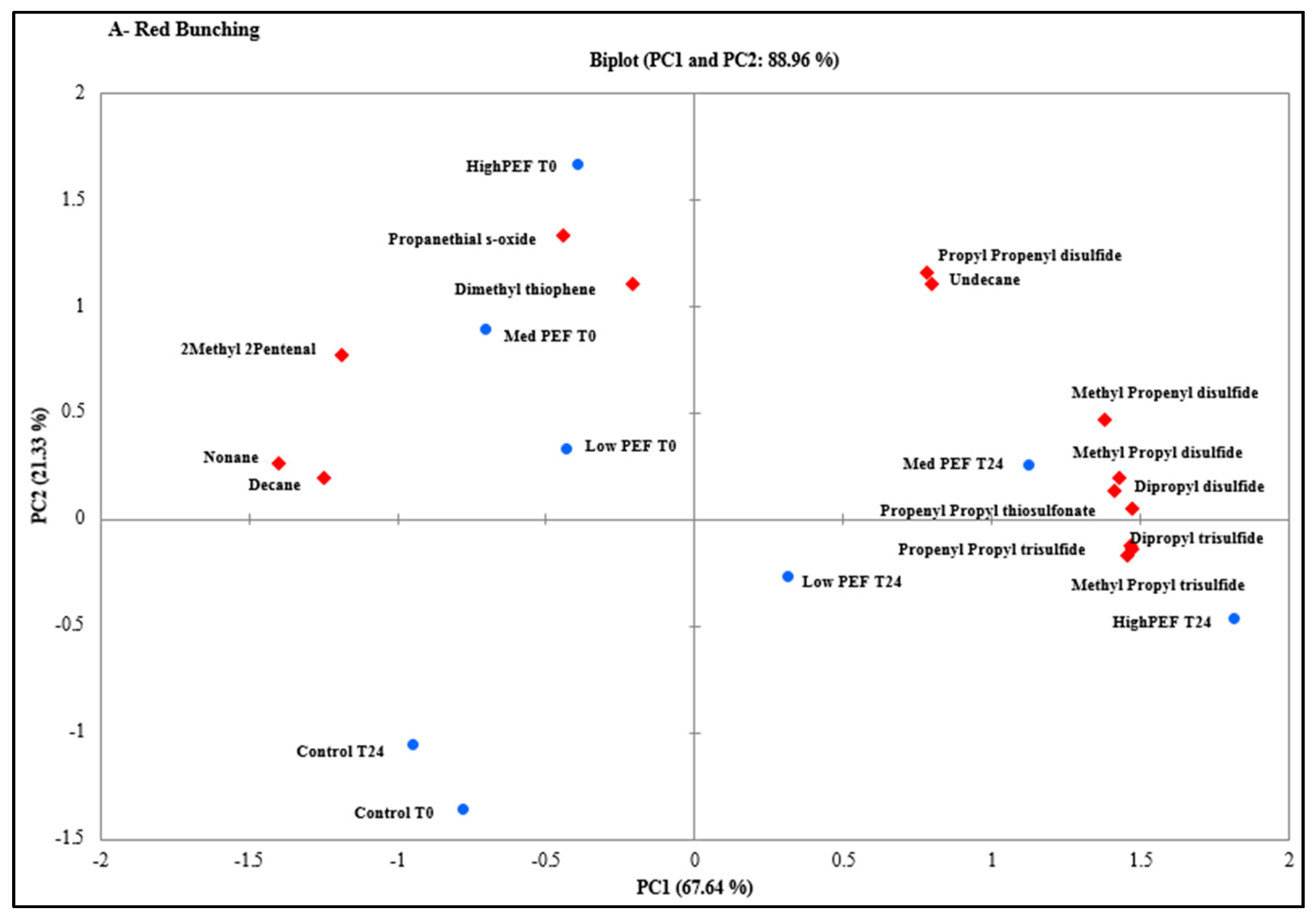
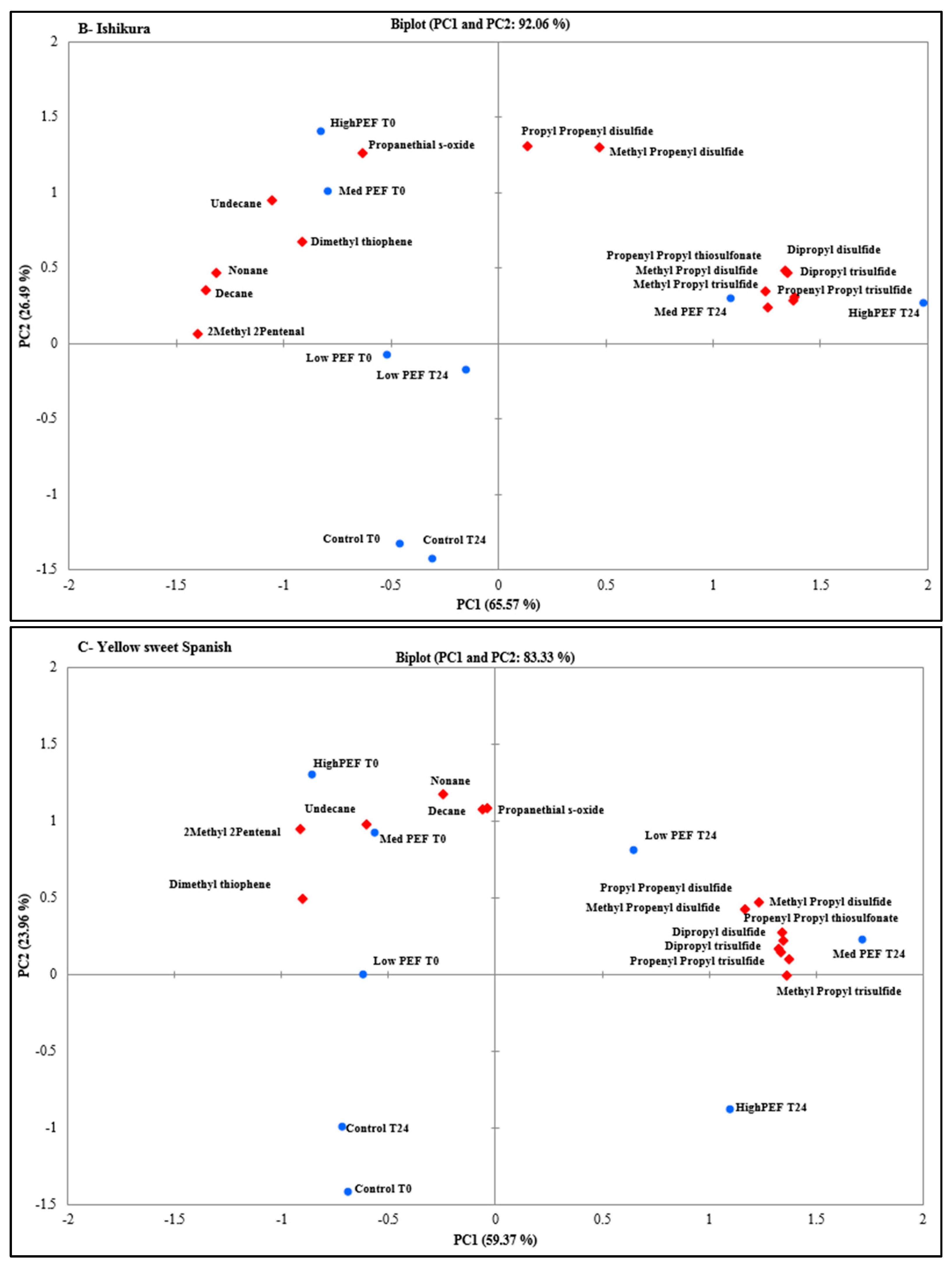
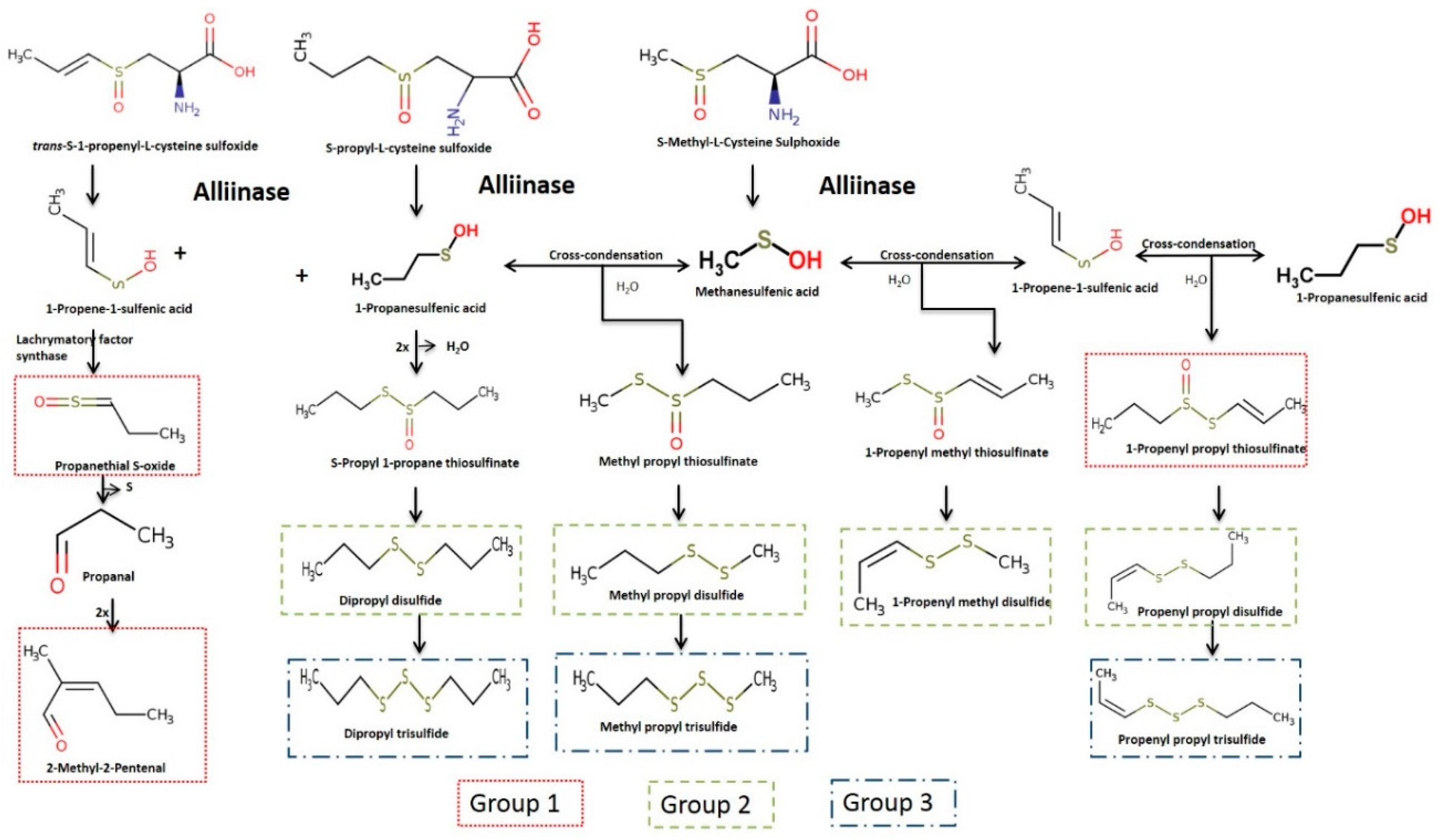
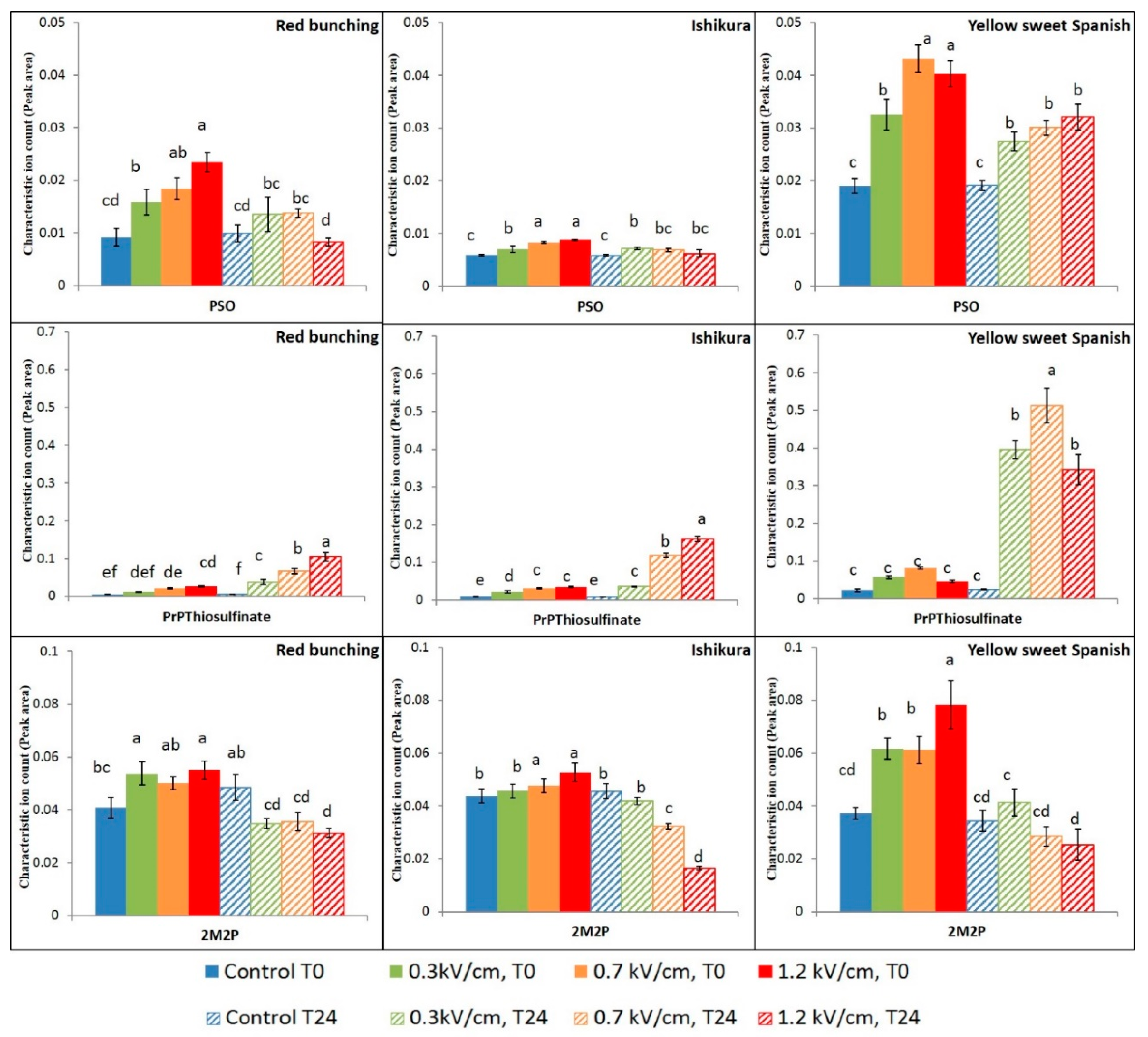
| CAS Number | Volatile Compounds (Abbreviations) | M.W | R.T a (min) | Calculated RI b | Lit RI c | E.I. d |
|---|---|---|---|---|---|---|
| 111-84-2 | Nonane | 128 | 6.9 | 895 | 900 | 128 |
| 67-56-1 | Methanol | 32 | 7.2 | 910 | 920 | 32 |
| 124-18-5 | Decane | 142 | 9.6 | 1007 | 1000 | 142 |
| 1120-21-4 | Undecane | 156 | 12.9 | 1112 | 1100 | 156 |
| 623-36-9 | 2-Methyl-2-pentenal (2M2P) | 98 | 15.5 | 1180 | 1160 | 98 |
| 2179-60-4 | Methyl propyl disulfide (MPDS) | 122 | 17.6 | 1257 | 1224 | 122/80 |
| 32157-29-2 | Propanethial S-oxide (PSO) | 90 | 17.7 | 1257 | - | 90 |
| 638-02-8 | Dimethyl thiophene (DMT) | 112 | 18.4 | 1286 | 1253 | 111 |
| 5905-47-5 | Methyl propenyl disulfide (MPrDS) | 120 | 19.4 | 1316 | 1327 | 120 |
| 629-19-6 | Dipropyl disulfide (DPDS) | 150 | 21.9 | 1400 | 1370 | 150/108 |
| 5905-46-4 | Propyl propenyl disulfide (PPrDS) | 148 | 23.4 | 1460 | 1428 | 148 |
| 17619-36-2 | Methyl propyl trisulfide (MPTS) | 154 | 25.3 | 1533 | 1516 | 154/112 |
| 2217-02-9 | Fenchyl alcohol (IS) e | 81 | 26 | 1593 | 1574 | 81 |
| 6028-61-1 | Dipropyl trisulfide (DPTS) | 182 | 27.7 | 1700 | 1662 | 182/75 |
| Propenyl propyl thiosulfinate (PrPthiosulfinate) | 180 | 28.6 | 1770 | - | 180 | |
| 33922-73-5 | Propyl propenyl trisulfide (PPrTS) | 180 | 29.2 | 1817 | - | 180/74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandakumar, R.; Eyres, G.T.; Burritt, D.J.; Kebede, B.; Leus, M.; Oey, I. Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum). Foods 2018, 7, 183. https://doi.org/10.3390/foods7110183
Nandakumar R, Eyres GT, Burritt DJ, Kebede B, Leus M, Oey I. Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum). Foods. 2018; 7(11):183. https://doi.org/10.3390/foods7110183
Chicago/Turabian StyleNandakumar, Rajkumar, Graham T. Eyres, David J. Burritt, Biniam Kebede, Michelle Leus, and Indrawati Oey. 2018. "Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum)" Foods 7, no. 11: 183. https://doi.org/10.3390/foods7110183
APA StyleNandakumar, R., Eyres, G. T., Burritt, D. J., Kebede, B., Leus, M., & Oey, I. (2018). Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum). Foods, 7(11), 183. https://doi.org/10.3390/foods7110183







