Controlling Blown Pack Spoilage Using Anti-Microbial Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Treatment
2.3. Coatings Preparations and Packaging of Beef
2.3.1. Preparation of Film Forming Solutions and Coatings
2.3.2. Vacuum Packaging of Beef
2.4. Inoculation of Beef Samples and Monitoring for Blown Pack Spoilage
2.4.1. Preparation of Blown Pack Spoilage C. estertheticum
2.4.2. Preparation of Spore Inocula
2.4.3. Preparation of Meat Samples, C. estertheticum Inoculation and Packing
2.5. Monitoring Vacuum Packs
2.6. Statistical Analysis
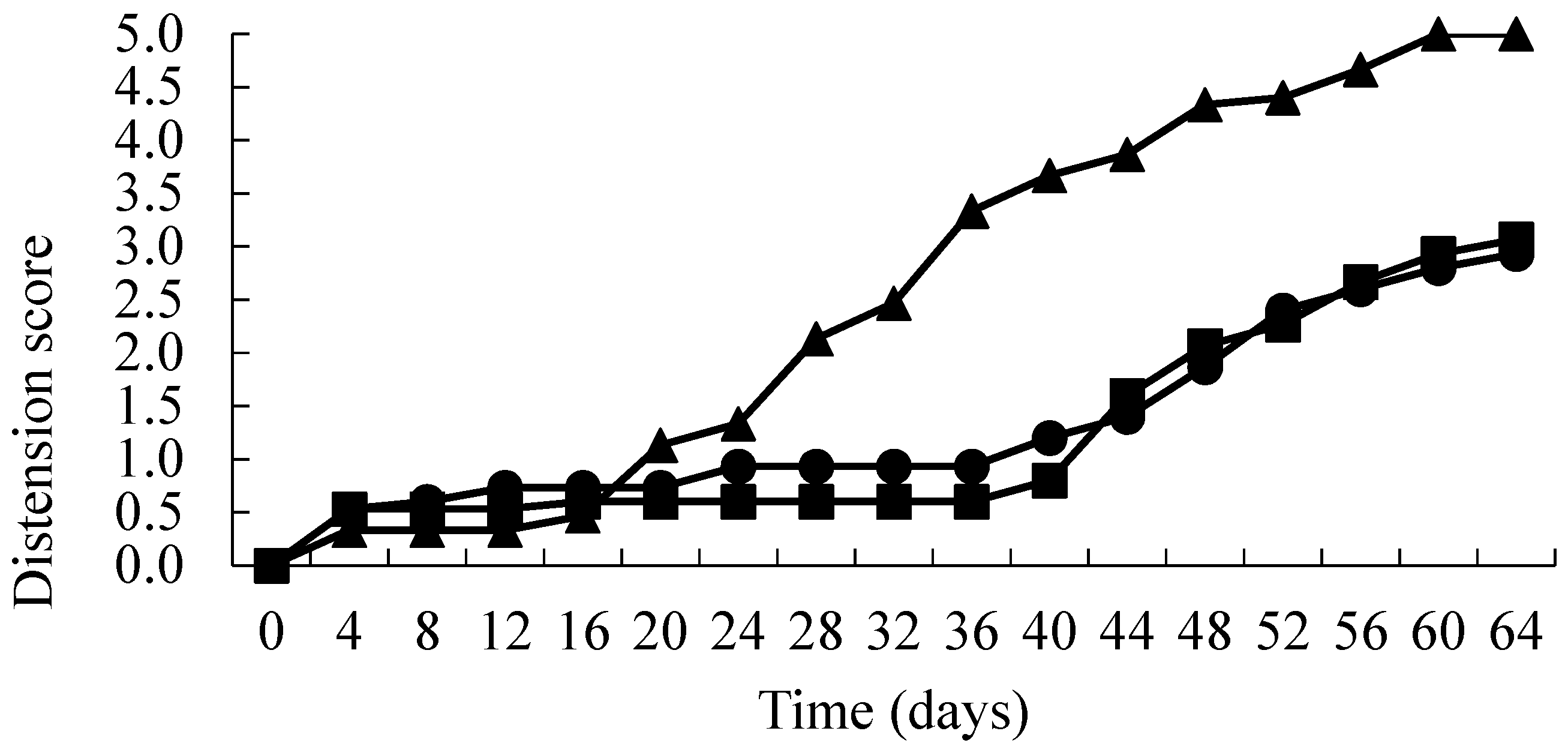
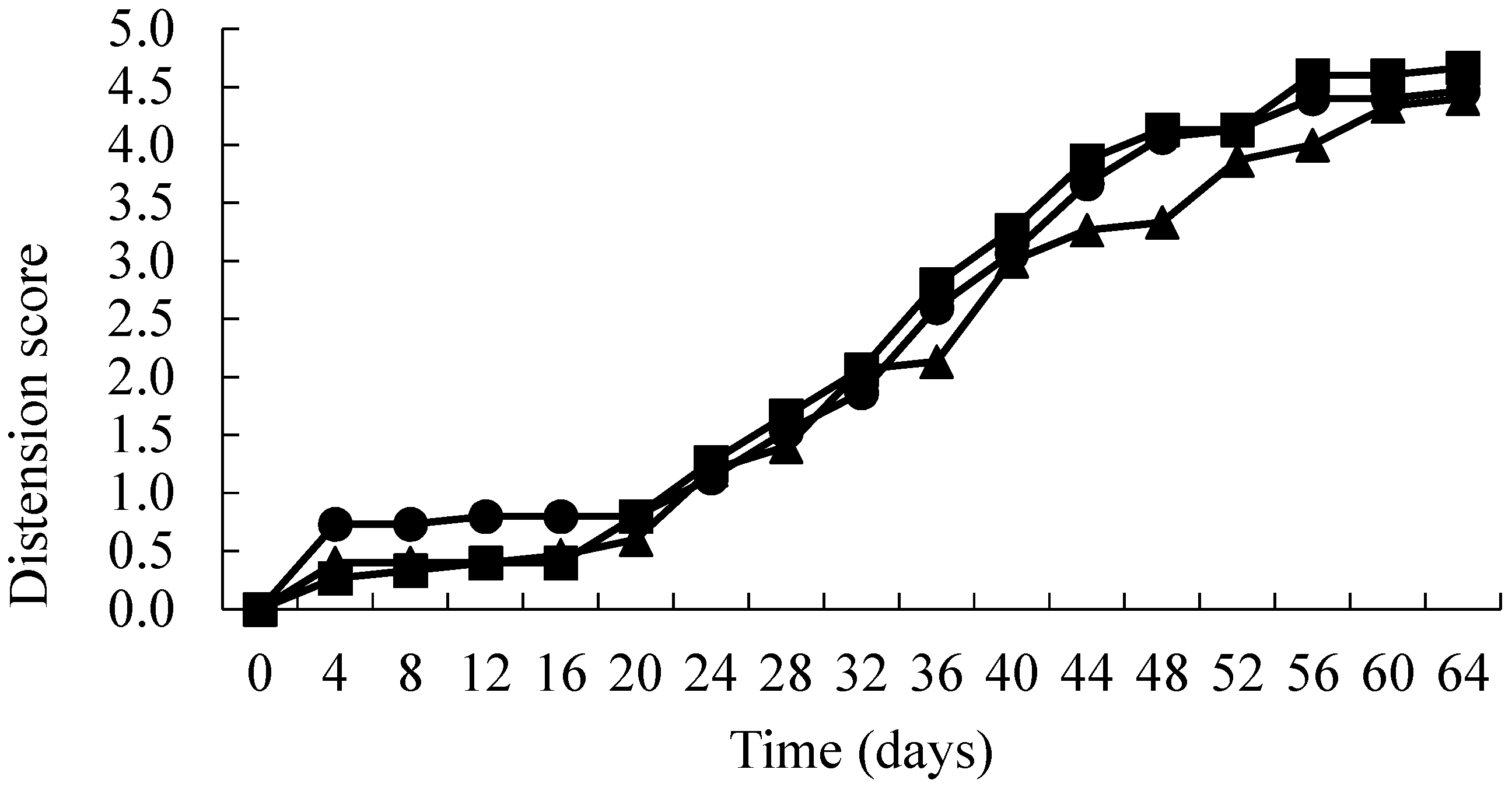
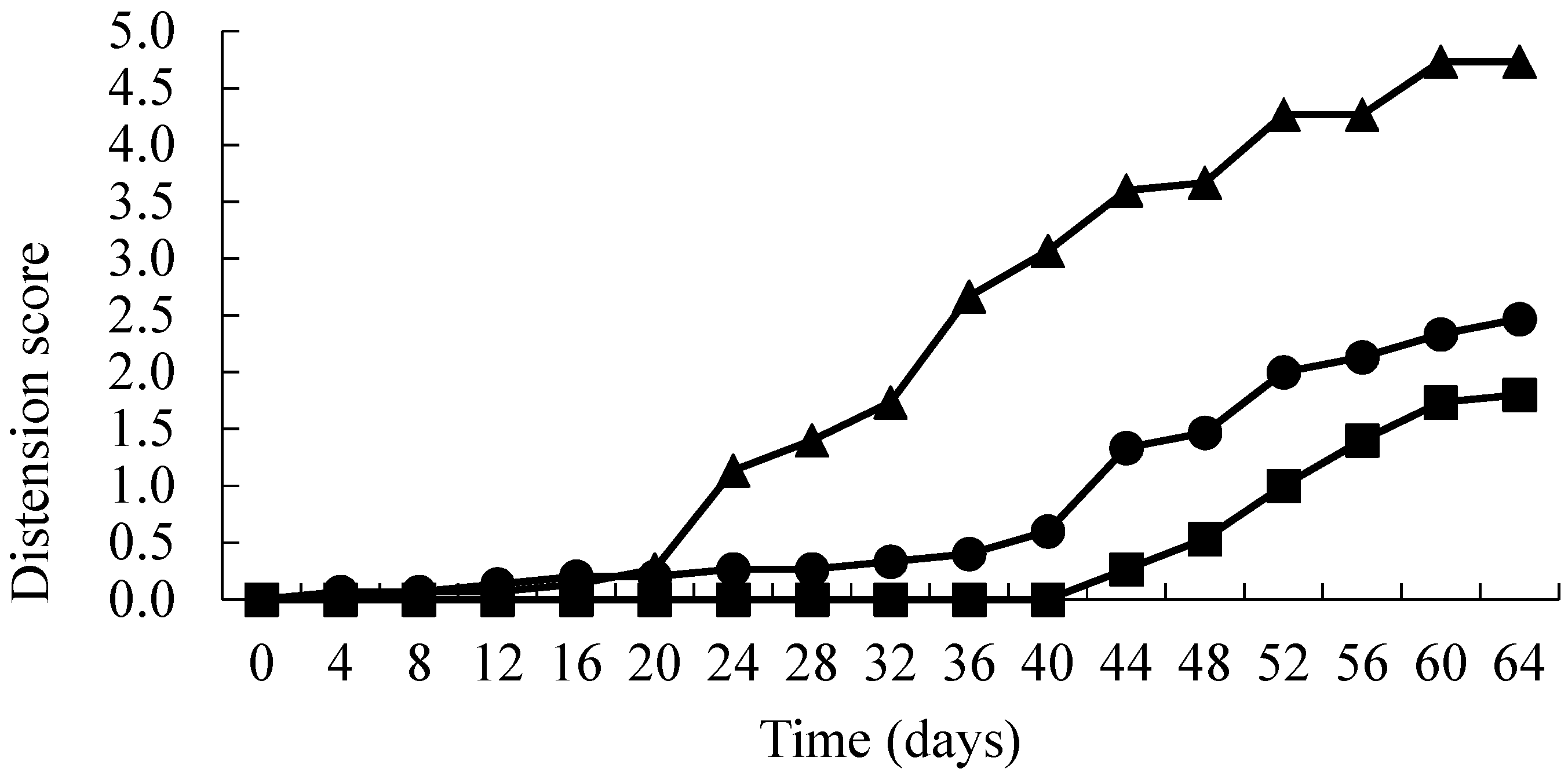
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author contributions
Conflicts of interest
References
- Bolton, D.J.; Carroll, J.; Walsh, D. A four-year survey of blown pack spoilage Clostridium estertheticum and Clostridium gasigenes on beef primal cuts. Lett. Appl. Microbiol. 2015, 61, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Broda, D.M.; Saul, D.J.; Lawson, P.A.; Bell, R.G.; Musgrave, D.R. Clostridium gasigenes sp. nov., a psychrophile causing spoilage of vacuum-packed meat. Int. J. Syst. Evol. Microbiol. 2000, 1, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Adam, K.H.; Flint, S.H.; Brightwell, G. Psychrophilic and psychrotrophic clostridia: Sporulation and germination processes and their role in the spoilage of chilled, vacuum-packaged beef, lamb and venison. Int. J. Food Sci. Technol. 2010, 45, 1539–1544. [Google Scholar] [CrossRef]
- Cavill, L.; Renteria-Monterrubio, A.L.; Helps, C.R.; Corry, J.E.L. Detection of cold-tolerant clostridia other than Clostridium estertheticum in raw vacuum-packed chill-stored meat. Food Microbiol. 2011, 28, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Dainty, R.H.; Edwards, R.A.; Hibbard, C.M. Spoilage of vacuum-packed beef by a clostridium sp. J. Sci. Food Agric. 1989, 49, 473–486. [Google Scholar] [CrossRef]
- Moschonas, G.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. The effect of storage temperature and inoculum level on the time of onset of “blown pack” spoilage. J. Appl. Microbiol. 2010, 108, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gill, C.O.; Balamurugan, S. Enumeration of Clostridium estertheticum spores in samples from meat plant conveyors and silage stacks by conventional and real time PCR procedures. Int. J. Food Saf. 2010, 12, 115–121. [Google Scholar]
- Moschonas, G.; Bolton, D.J.; Sheridan, J.J.; McDowell, D.A. The effect of heat shrink treatment and storage temperature on the time of onset of “blown pack” spoilage. Meat Sci. 2011, 87, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.; Cutter, C.; Willett, J. Incorporation of bacteriocin in plastic retains activity and inhibits surface growth of bacteria on meat. Food Microbiol. 1999, 16, 229–235. [Google Scholar] [CrossRef]
- Seydim, A.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Cooksey, K. Antimicrobial food packaging materials. Addit. Polym. 2001, 8, 6–10. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Clarke, D.; Molinaro, S.; Tyuftin, A.; Bolton, D.; Fanning, S.; Kerry, J.P. Incorporation of commercially-derived antimicrobials into gelatin-based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control 2016, 64, 202–211. [Google Scholar] [CrossRef]
- Lund, B.M.; Graham, A.F.; George, S.M.; Brown, D. The combined effect of incubation temperature, pH and sorbic acid on the probability of growth of nonproteolytic type B Clostridium botulinum. J. Appl. Bacteriol. 1990, 89, 481–492. [Google Scholar] [CrossRef]
- Boerema, J.A.; Broda, D.M.; Penney, N.; Brightwell, G. Influence of peroxyacetic acid-based carcass rinse on the onset of “blown pack” spoilage in artificially inoculated vacuum-packed chilled beef. J. Food Prot. 2007, 70, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Lawson, P.; Dainty, R.H.; Kristiansen, N.; Berg, J.; Collins, M.D. Characterization of a psychrotrophic Clostridium causing spoilage in vacuum-packed cooked pork: Description of Clostridium algidicarnis sp. nov. Lett. Appl. Microbiol. 1994, 19, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gill, C.O.; Balamurugan, S. Effects of temperature and pH on the growth of bacteria isolated from blown packs of vacuum-packaged beef. J. Food Prot. 2009, 72, 2380–2385. [Google Scholar] [CrossRef]
- Silva, A.R.; Paulo, E.N.; Sant’ana, A.S.; Chaves, R.D.; Massaguer, P.R. Involvement of Clostridium gasigenes and Clostridium algidicarnis in ‘blown pack’ spoilage of Brazilian vacuum-packed beef. Int. J. Food Microbiol. 2011, 148, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H. Antimicrobial food packaging. Food Technol. 2000, 54, 56–65. [Google Scholar]
- Malhotra, B.; Keshwani, A.; Harsha, K. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, K. Utilization of antimicrobial packaging films for the inhibition of selected microorganisms. In Food Packaging: Testing Methods and Applications; ACS Publications: Washington, DC, USA, 2000; pp. 17–25. [Google Scholar]
- Natrajan, N.; Sheldon, B.W. Efficacy of Nisin-Coated polymer films to inactivate Salmonella Typhimurium on fresh broiler skin. J. Food Prot. 2000, 63, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.; Parushev, S.; Stamboliyska, B.; Tsvetkova, I.; Ninova, M.; Najdenski, H. Examination of growth inhibitory properties of synthetic chalcones for which antibacterial activity was predicted. Eur. J. Med. Chem. 2009, 44, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Alam, Z.; Feng, L.; Tsutsumi, L.S.; Sun, D.; Hurdle, J.G. Prospects for flavonoid and related phytochemicals as nature-inspired treatments for Clostridium difficile infection. J. Appl. Microbiol. 2014, 116, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in undersatdning the antibacterial properties of flavonoids. Int. J. Antimicrobiol. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2002, 82, 632–639. [Google Scholar] [CrossRef]
- Sun, C.Q.; O’ Conner, C.J.; Robertson, C.J. The antimicrobial properties of milk fat after partial hydrolysis of calf pregastric lipase. Chem. Biol. Interact. 2002, 40, 185–198. [Google Scholar] [CrossRef]
- Annamalai, T.; Nair, M.K.M.; Marek, P.; Vasudevan, P.; Schreiber, D.; Knight, R.; Hoagland, T.; Venkitanarayanan, K. In vitro inactivation of enterohemorrhagic Escherichia coli O157:H7 in bovine rumen fluid by caprylic acid. J. Food Prot. 2004, 67, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K.M.; Vasudevan, P.; Hoagland, T.; Venkitanarayanan, K. Inactivation of Escherichia coli O157:H7 and Listeria monocytogenes in milk by caprylic acid and monocaprylin. Food Microbiol. 2004, 21, 611–616. [Google Scholar] [CrossRef]
- Juneja, V.K.; Thippareddi, H. Inhibitory effects of organic acid salts on growth of Clostridium perfringens from spore inocula during chilling of marinated ground turkey breast. Int. J. Food Microbiol. 2004, 93, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reid, R.; Bolton, D.; Tiuftin, A.A.; Kerry, J.P.; Fanning, S.; Whyte, P. Controlling Blown Pack Spoilage Using Anti-Microbial Packaging. Foods 2017, 6, 67. https://doi.org/10.3390/foods6080067
Reid R, Bolton D, Tiuftin AA, Kerry JP, Fanning S, Whyte P. Controlling Blown Pack Spoilage Using Anti-Microbial Packaging. Foods. 2017; 6(8):67. https://doi.org/10.3390/foods6080067
Chicago/Turabian StyleReid, Rachael, Declan Bolton, Andrey A. Tiuftin, Joe P. Kerry, Séamus Fanning, and Paul Whyte. 2017. "Controlling Blown Pack Spoilage Using Anti-Microbial Packaging" Foods 6, no. 8: 67. https://doi.org/10.3390/foods6080067
APA StyleReid, R., Bolton, D., Tiuftin, A. A., Kerry, J. P., Fanning, S., & Whyte, P. (2017). Controlling Blown Pack Spoilage Using Anti-Microbial Packaging. Foods, 6(8), 67. https://doi.org/10.3390/foods6080067





