In Situ Raman Analysis of CO2—Assisted Drying of Fruit-Slices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fruits and Computation of the Water Mass Ratio
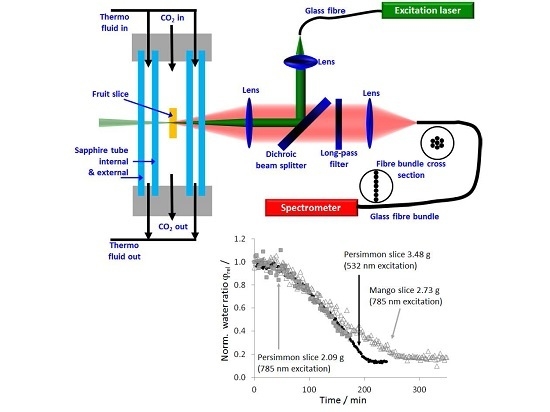
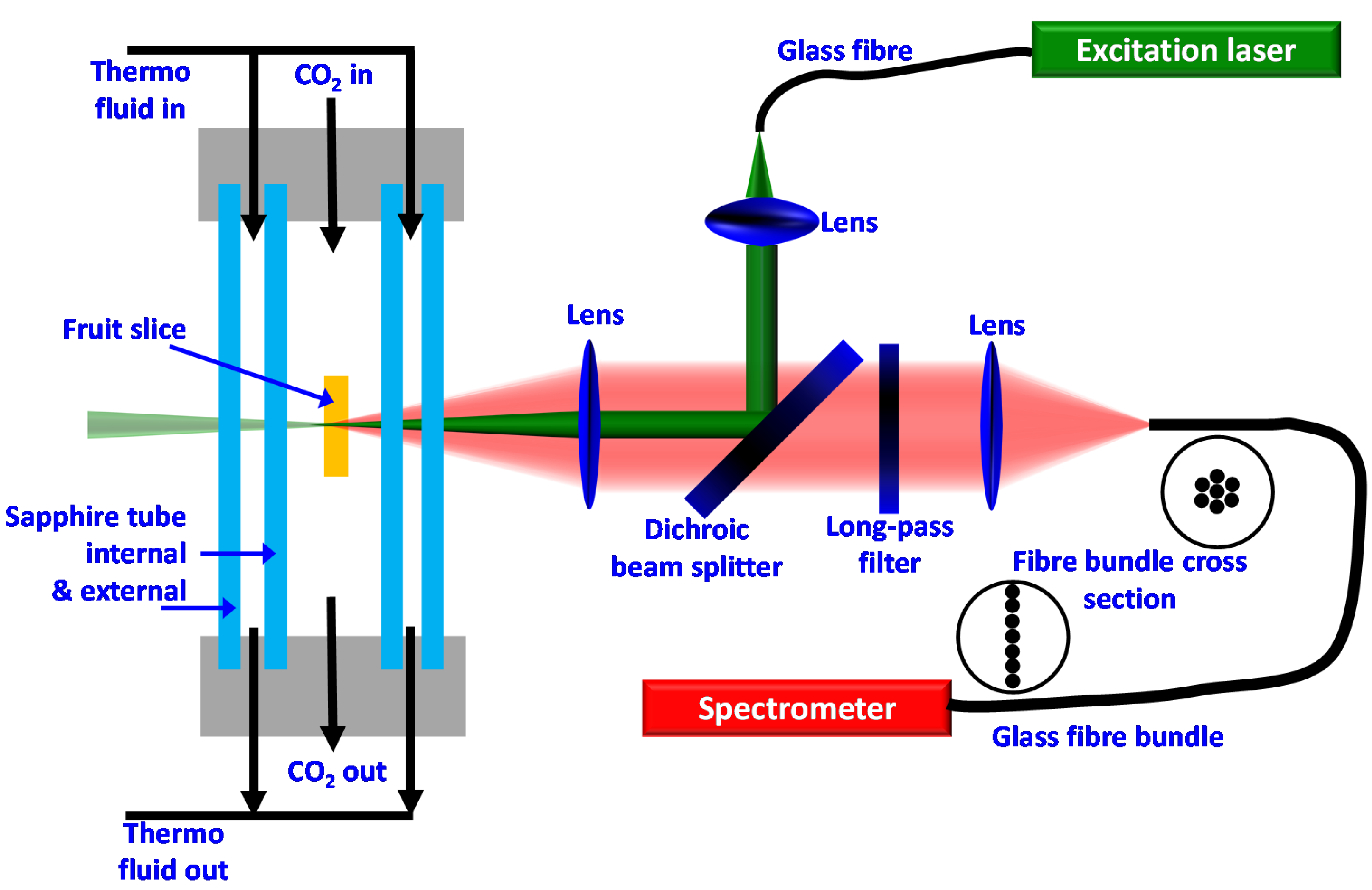
2.2. Experimental CO2-Drying and Raman Setup
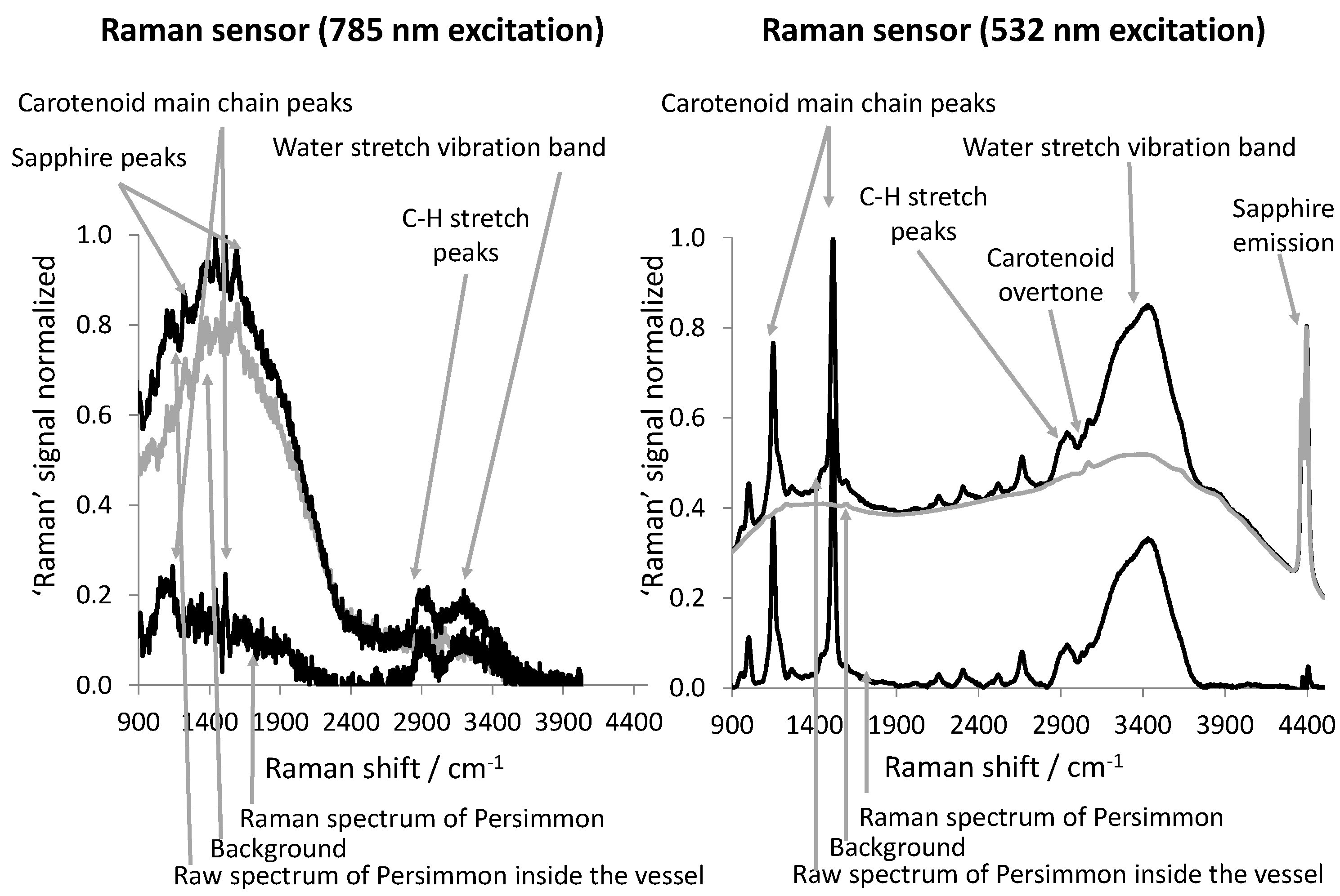
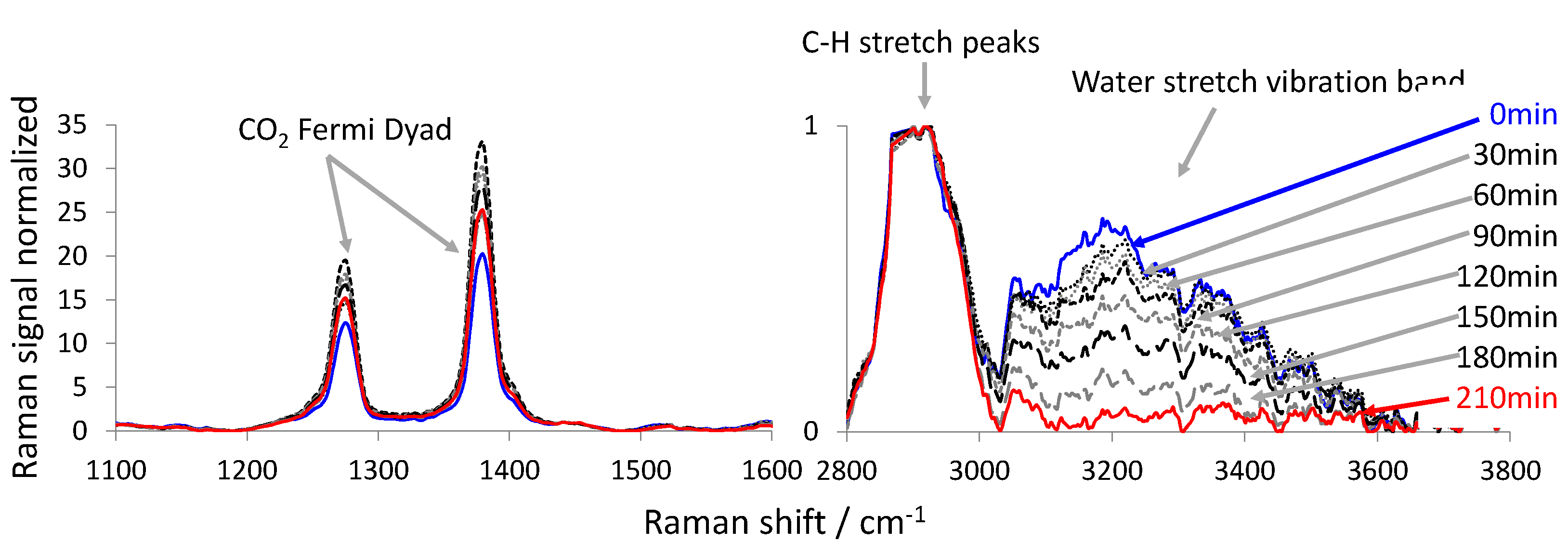
2.3. Evaluation of the Raman Spectra
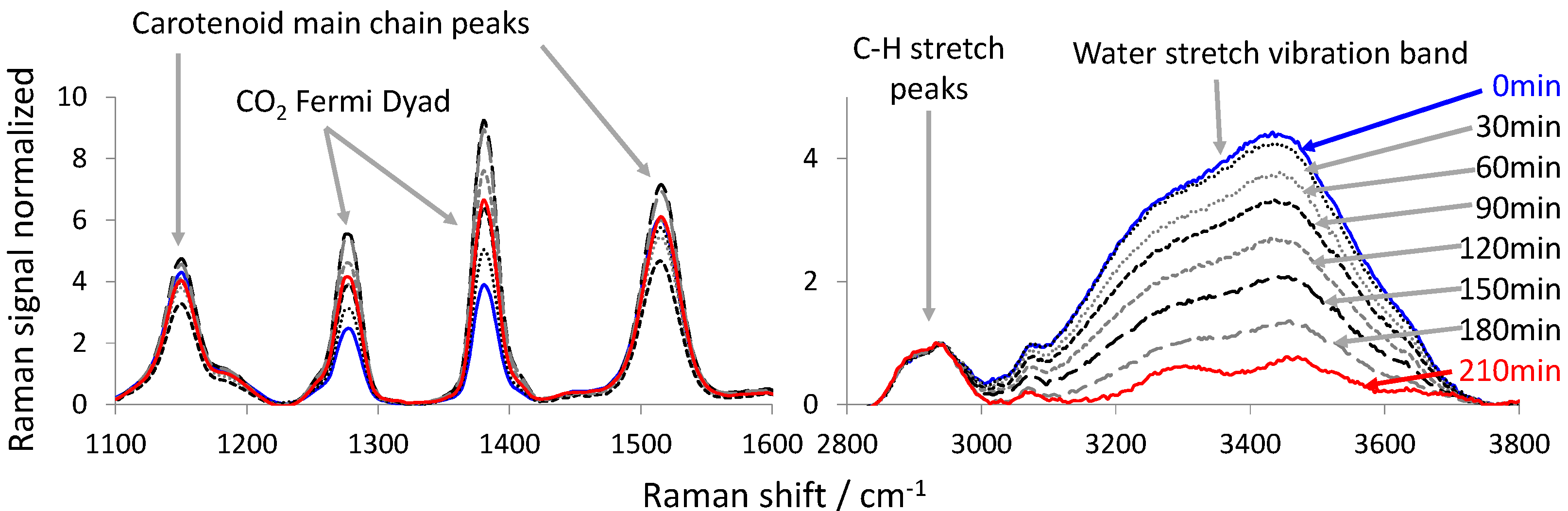
- the intensity of the water stretch vibration band as the integral of the Raman spectra between 3110 and 3800 cm−1;
- the intensity of the carbon-hydrogen (C-H) vibration band of the fruit tissue as the integral of the Raman spectrum between 2800 and 3110 cm−1.
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, R.P.; Heldman, D.R. Introduction to Food Engineering; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Slade, L.; Levine, H. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. Nutr. 1991, 30, 115–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Mujumdar, A.S. Drying Technologies in Food Processing; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Suvarnakuta, P.; Devahastin, S.; Mujumdar, A.S. Drying kinetics and β-Carotene degradation in carrot undergoing different drying processes. J. Food Sci. 2005, 70, s520–s526. [Google Scholar] [CrossRef]
- Polydera, A.; Stoforos, N.; Taoukis, P. Quality degradation kinetics of pasteurised and high pressure processed fresh Navel orange juice: Nutritional parameters and shelf life. Innov. Food Sci. Emerg. Technol. 2005, 6, 1–9. [Google Scholar] [CrossRef]
- Brown, Z.; Fryer, P.; Norton, I.; Bakalis, S.; Bridson, R. Drying of foods using supercritical carbon dioxide - Investigations with carrot. Innov. Food Sci. Emerg. Technol. 2008, 9, 280–289. [Google Scholar] [CrossRef]
- Meterc, D.; Petermann, M.; Weidner, E. Drying of aqueous green tea extracts using a supercritical fluid spray process. J. Supercrit. Fluids 2008, 45, 253–259. [Google Scholar] [CrossRef]
- Khalloufi, S.; Almeida-Rivera, C.; Bongers, P. Supercritical-CO2 drying of foodstuffs in packed beds: Experimental validation of a mathematical model and sensitive analysis. J. Food Eng. 2010, 96, 141–150. [Google Scholar] [CrossRef]
- Bušić, A.; Vojvodić, A.; Komes, D.; Akkermans, C.; Belščak-Cvitanović, A.; Stolk, M.; Hofland, G. Comparative evaluation of CO2 drying as an alternative drying technique of basil (Ocimum basilicum L.)—The effect on bioactive and sensory properties. Food Res. Int. 2014, 64, 34–42. [Google Scholar] [CrossRef]
- Medina-Gonzalez, Y.; Camy, S.; Condoret, J.-S. Cellulosic materials as biopolymers and supercritical CO2 as a green process: Chemistry and applications. Int. J. Sustain. Eng. 2012, 5, 47–65. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Franich, R.A.; Gallagher, S.; Kroese, H. Dewatering green sapwood using carbon dioxide cycled between supercritical fluid and gas phase. J. Supercrit. Fluids 2014, 89, 113–118. [Google Scholar] [CrossRef]
- Bourdoux, S.; Li, D.; Rajkovic, A.; Devlieghere, F.; Uyttendaele, M. Performance of Drying Technologies to Ensure Microbial Safety of Dried Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1056–1066. [Google Scholar] [CrossRef]
- Ferrentino, G.; Spilimbergo, S. High pressure carbon dioxide pasteurization of solid foods: Current knowledge and future outlooks. Trends Food Sci. Technol. 2011, 22, 427–441. [Google Scholar] [CrossRef]
- Spilimbergo, S.; Komes, D.; Vojvodic, A.; Levaj, B.; Ferrentino, G. High pressure carbon dioxide pasteurization of fresh-cut carrot. J. Supercrit. Fluids 2013, 79, 92–100. [Google Scholar] [CrossRef]
- Agterof, W.G.M.; Bhatia, R.; Hofland, G.W. Dehydration Method; Feyecon Development & Implementation B.V.: Weesp, The Netherlands, 2012. [Google Scholar]
- Kazarian, S.G. Applications of FTIR Spectroscopy to Supercritical Fluid Drying, Extraction and Impregnation. Appl. Spectrosc. Rev. 1997, 32, 301–348. [Google Scholar] [CrossRef]
- Ferrentino, G.; Schuster, J.; Braeuer, A.; Spilimbergo, S. In situ Raman quantification of the dissolution kinetics of carbon dioxide in liquid solutions during a dense phase and ultrasound treatment for the inactivation of Saccharomyces cerevisiae. J. Supercrit. Fluids 2016, 111, 104–111. [Google Scholar] [CrossRef]
- Crimaldi, J.P. The effect of photobleaching and velocity fluctuations on single-point LIF measurements. Exp. Fluids 1997, 23, 325–330. [Google Scholar] [CrossRef]
- Gebrekidan, M.T.; Knipfer, C.; Stelzle, F.; Popp, J.; Will, S.; Braeuer, A. A shifted-excitation Raman difference spectroscopy (SERDS) evaluation strategy for the efficient isolation of Raman spectra from extreme fluorescence interference. J. Raman Spectrosc. 2016, 47, 198–209. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Gupta, R.B.; Clarke, M.J.; Johnston, K.P.; Poliakoff, M. How is hydrogen-bonding influenced by solvent density? The spectroscopic study and modeling of the interaction between a proton donor and acceptor from the gas phase to supercritical fluid states. J. Am. Chem. Soc. 1993, 115, 11099–11109. [Google Scholar] [CrossRef]
- Hankel, R.F.; Rojas, P.E.; Cano-Sarabia, M.; Sala, S.; Veciana, J.; Braeuer, A.; Ventosa, N. Surfactant-free CO2-based microemulsion-like systems. Chem. Commun. 2014, 50, 8215–8218. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.J.; Siegler, P.; Guenther, A.; Wirth, K.-E.; Braeuer, A.S. Simultaneous analysis of the dispersed liquid and the bulk gas phase of water sprays using Raman spectroscopy. Appl. Spectrosc. 2016, 70, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of Lycopene and β-Carotene Content in Tomato Fruits and Related Products: Comparison of FT-Raman, ATR-IR, and NIR Spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.; Vandenabeele, P.; Edwards, H.M.; de Oliveira, L.C. NIR-FT-Raman spectroscopic analytical characterization of the fruits, seeds, and phytotherapeutic oils from rosehips. Anal. Bioanal. Chem. 2008, 392, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, F. Pump-Superkontinuum-Probe-Spektroskopie von Carotinoiden in Organischen Lösungsmitteln. Ph.D. Thesis, Georg-August-Universitaet Goettingen, Göttingen, Germany, 2011. [Google Scholar]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Kopczynski, M.; Ehlers, F.; Lenzer, T.; Oum, K. Evidence for an Intramolecular Charge Transfer State in 12′-Apo-β-caroten-12′-al and 8′-Apo-β-caroten-8′-al: Influence of Solvent Polarity and Temperature. J. Phys. Chem. A 2007, 111, 5370–5381. [Google Scholar] [CrossRef] [PubMed]
- Lenzer, T.; Ehlers, F.; Scholz, M.; Oswald, R.; Oum, K. Assignment of carotene S* state features to the vibrationally hot ground electronic state. Phys. Chem. Chem. Phys. 2010, 12, 8832–8839. [Google Scholar] [CrossRef] [PubMed]
- Golibrzuch, K.; Ehlers, F.; Scholz, M.; Oswald, R.; Lenzer, T.; Oum, K.; Kim, H.; Koo, S. Ultrafast excited state dynamics and spectroscopy of 13,13′-diphenyl-b-carotene. Phys. Chem. Chem. Phys. 2011, 13, 6340–6351. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.F.; Tavender, S.M.; Dixon, N.M.; Herman, H.; Williams, K.P.J.; Maddams, W.F. Raman Spectrum of beta-Carotene Using Laser Lines from Green (514.5 nm) to Near-Infrared (1064 nm): Implications for the Characterization of Conjugated Polyenes. Appl. Spectrosc. 1999, 53, 86–91. [Google Scholar] [CrossRef]
- Petry, R.; Schmitt, M.; Popp, J. Raman Spectroscopy—A Prospective Tool in the Life Sciences. ChemPhysChem 2003, 4, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Popp, J. Raman spectroscopy at the beginning of the twenty-first century. J. Raman Spectrosc. 2006, 37, 20–28. [Google Scholar] [CrossRef]
- Castiglioni, C.; Navarrete, J.T.L.; Zerbi, G.; Gussoni, M. A simple interpretation of the vibrational spectra of undoped. doped and photoexcited polyacetylene: Amplitude mode theory in the GF formalism. Solid State Commun. 1988, 65, 625–630. [Google Scholar] [CrossRef]
- Hanson, R.C.; Bachman, K. Raman studies of pressure tuning of the fermi resonance in solid CO2. Chem. Phys. Lett. 1980, 73, 338–342. [Google Scholar] [CrossRef]
- Quiño, J.; Ruehl, M.; Klima, T.; Ruiz, F.; Will, S.; Braeuer, A. Supercritical drying of aerogel: In situ analysis of concentration profiles inside the gel and derivation of the effective binary diffusion coefficient using Raman spectroscopy. J. Supercrit. Fluids 2016, 108, 1–12. [Google Scholar] [CrossRef]
- Stamenic, M.; Zizovic, I.; Eggers, R.; Jaeger, P.; Heinrich, H.; Rój, E.; Ivanovic, J.; Skala, D. Swelling of plant material in supercritical carbon dioxide. J. Supercrit. Fluids 2010, 52, 125–133. [Google Scholar] [CrossRef]
- Mercer, D.G. A comparison of the kinetics of mango drying in open-air, solar, and forced-air dryers. Afr. J. Food Agric. Nutr. Dev. 2012, 12, 6835. [Google Scholar]
- Griffin, J.S.; Mills, D.H.; Cleary, M.; Nelson, R.; Manno, V.P.; Hodes, M. Continuous extraction rate measurements during supercritical CO2 drying of silica alcogel. J. Supercrit. Fluids 2014, 94, 38–47. [Google Scholar] [CrossRef]
- Pudney, P.D.A.; Gambelli, L.; Gidley, M.J. Confocal Raman Microspectroscopic Study of the Molecular Status of Carotenoids in Tomato Fruits and Foods. Appl. Spectrosc. 2011, 65, 127–135. [Google Scholar] [CrossRef]

| Persimmon Slice 1 | Persimmon Slice 2 | Mango Slice | ||
|---|---|---|---|---|
| Before drying | ()/g | 3.48 | 2.09 | 2.73 |
| Norm. water ratio | 1 | 1 | 1 | |
| After CO2 drying | ()/g | 0.74 | 0.61 | 0.44 |
| Norm. water ratio | 0.077 | 0.124 | 0.026 | |
| After thermal drying | /g | 0.51 | 0.40 | 0.38 |
| Norm. water ratio | 0 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braeuer, A.S.; Schuster, J.J.; Gebrekidan, M.T.; Bahr, L.; Michelino, F.; Zambon, A.; Spilimbergo, S. In Situ Raman Analysis of CO2—Assisted Drying of Fruit-Slices. Foods 2017, 6, 37. https://doi.org/10.3390/foods6050037
Braeuer AS, Schuster JJ, Gebrekidan MT, Bahr L, Michelino F, Zambon A, Spilimbergo S. In Situ Raman Analysis of CO2—Assisted Drying of Fruit-Slices. Foods. 2017; 6(5):37. https://doi.org/10.3390/foods6050037
Chicago/Turabian StyleBraeuer, Andreas Siegfried, Julian Jonathan Schuster, Medhanie Tesfay Gebrekidan, Leo Bahr, Filippo Michelino, Alessandro Zambon, and Sara Spilimbergo. 2017. "In Situ Raman Analysis of CO2—Assisted Drying of Fruit-Slices" Foods 6, no. 5: 37. https://doi.org/10.3390/foods6050037
APA StyleBraeuer, A. S., Schuster, J. J., Gebrekidan, M. T., Bahr, L., Michelino, F., Zambon, A., & Spilimbergo, S. (2017). In Situ Raman Analysis of CO2—Assisted Drying of Fruit-Slices. Foods, 6(5), 37. https://doi.org/10.3390/foods6050037