Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat
Abstract
:1. Introduction
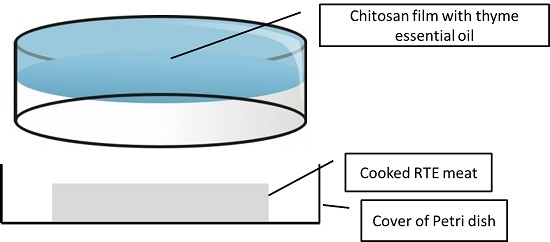
2. Materials and Methods
3. Results and Discussion
3.1. Essential Oil Composition
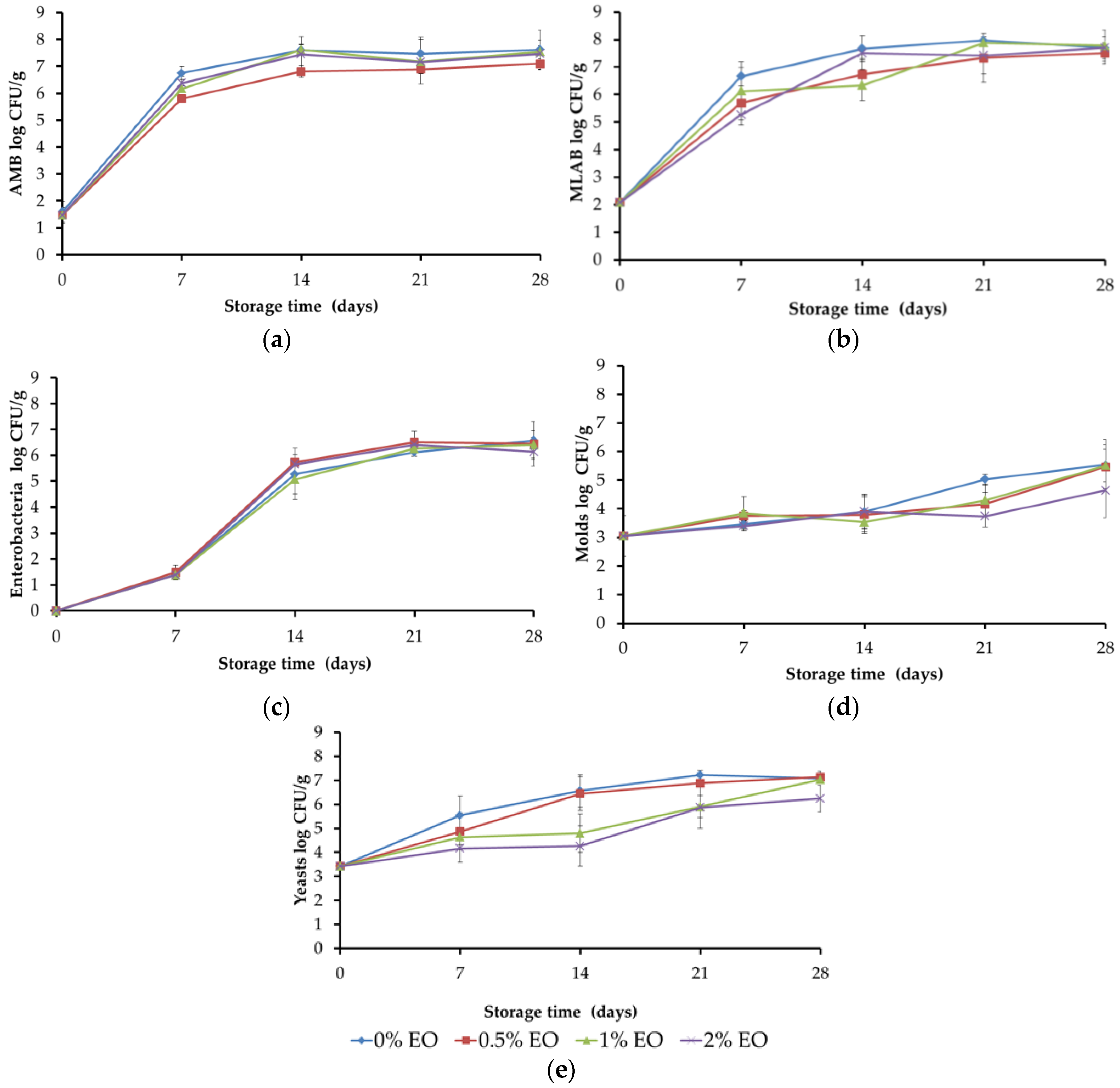
3.2. Evolution of Microbial Populations in Packaged RTE Meat during Storage
3.3. Evolution of the pH of RTE Meat during Storage in Active Packaging
3.4. Evolution of Color Parameters of Packaged RTE Meat during Storage
3.5. Sensory Charactaristics of Packaged RTE Meat
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigós, M.d.C.; Jiménez, A. Active edible films: Current state and future trends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Perdones, A.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Giatrakou, V.; Ntzimani, A.; Savvaidis, I.N. Combined chitosan-thyme treatments with modified atmosphere packaging on a ready-to-cook poultry product. J. Food Prot. 2010, 73, 663–669. [Google Scholar] [PubMed]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Barber, X.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. Effect of chitosan edible films added with thymus moroderi and thymus piperella essential oil on shelf-life of cooked cured ham. J. Food Sci. Technol. 2015, 52, 6493–6501. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with thymus moroderi or thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J. Food Sci. 2011, 76, R164–R177. [Google Scholar] [CrossRef] [PubMed]
- Telci, I.; Demirtas, I.; Sahin, A. Variation in plant properties and essential oil composition of sweet fennel (Foeniculum vulgare mill.) fruits during stages of maturity. Ind. Crops Prod. 2009, 30, 126–130. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Mohamady, M.; Fernández-López, J.; ElRazik, K.A.; Omer, E.; Pérez-Alvarez, J.; Sendra, E. In vitro antioxidant and antibacterial activities of essentials oils obtained from egyptian aromatic plants. Food Control 2011, 22, 1715–1722. [Google Scholar] [CrossRef]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical composition, antioxidant and antimicrobial activity of essential oils from organic fennel, parsley, and lavender from Spain. Foods 2016, 5. [Google Scholar] [CrossRef]
- Jay, J.M. Microbiological analysis: total bacterial count. In Encyclopedia of Meat Sciences; Elsevier: Oxford, UK, 2004; pp. 768–773. [Google Scholar]
- Fernández-Ginés, J.M.; Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Alvarez, J.A. Effect of storage conditions on quality characteristics of bologna sausages made with citrus fiber. J. Food Sci. 2003, 68, 710–715. [Google Scholar] [CrossRef]
- Torras, J.; Grau, M.D.; López, J.F.; De Las Heras, F.X.C. Analysis of essential oils from chemotypes of thymus vulgaris in Catalonia. J. Sci. Food Agric. 2007, 87, 2327–2333. [Google Scholar] [CrossRef]
- Salido, S.; Altarejos, J.; Nogueras, M.; Sánchez, A.; Luque, P. Chemical composition and seasonal variations of rosemary oil from Southern Spain. J. Essent. Oil Res. 2003, 15, 10–14. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical composition and in vitro antibacterial properties of essential oils of four thymus species from organic growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- De Oliveira, K.Á.R.; de Sousa, J.P.; da Costa Medeiros, J.A.; de Figueiredo, R.C.B.Q.; Magnani, M.; de Siqueira Júnior, J.P.; de Souza, E.L. Synergistic inhibition of bacteria associated with minimally processed vegetables in mixed culture by carvacrol and 1,8-cineole. Food Control 2015, 47, 334–339. [Google Scholar] [CrossRef]
- Emiroǧlu, Z.K.; Yemiş, G.P.; Coşkun, B.K.; Candoǧan, K. Antimicrobial activity of soy edible films incorporated with thyme and oregano essential oils on fresh ground beef patties. Meat Sci. 2010, 86, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Q.; Zhang, L.L.; Zong, K.; Wang, A.M.; Yu, X.F. Effects of spices essential oils on the spoilage-related microbiota in chilled pork stored in antimicrobial pack. Food Sci. Technol. Res. 2012, 18, 695–704. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Giatrakou, V.; Ntzimani, A.; Savvaidis, I.N. Effect of chitosan and thyme oil on a ready to cook chicken product. Food Microbiol. 2010, 27, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast spoilage of foods and beverages. In The Yeasts; Elsevier Science: San Diego, CA, USA, 2011; Volume 1, pp. 53–63. [Google Scholar]
- Medeiros, E.A.A.; Soares, N.d.F.F.; Polito, T.d.O.S.; Sousa, M.M.d.; Silva, D.F.P. Sachês antimicrobianos em pós-colheita de manga. Rev. Bras. Frutic. 2011, 33, 363–370. [Google Scholar] [CrossRef]
- Han, J.H.; Patel, D.; Kim, J.E.; Min, S.C. Retardation of listeria monocytogenes growth in mozzarella cheese using antimicrobial sachets containing rosemary oil and thyme oil. J. Food Sci. 2014, 79, E2272–E2278. [Google Scholar] [CrossRef]
- Oral, N.; Vatansever, L.; Sezer, C.; Aydin, B.; Guven, A.; Gulmez, M.; Baser, K.H.; Kurkcuoglu, M. Effect of absorbent pads containing oregano essential oil on the shelf life extension of overwrap packed chicken drumsticks stored at four degrees celsius. Poult. Sci. 2009, 88, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Kodal Coşkun, B.; Çalikoǧlu, E.; Karagöz Emiroǧlu, Z.; Candoǧan, K. Antioxidant active packaging with soy edible films and oregano or thyme essential oils for oxidative stability of ground beef patties. J. Food Qual. 2014, 37, 203–212. [Google Scholar] [CrossRef]
- Fernández-López, J.; Yelo, A.; Sayas-Barberá, E.; Sendra, E.; Navarro, C.; Pérez-Alvarez, J.A. Shelf life of ostrich (Struthio camelus) liver stored under different packaging conditions. J. Food Prot. 2006, 69, 1920–1927. [Google Scholar] [PubMed]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT Food Sci. Technol. 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Pires, C.; Ramos, C.; Teixeira, B.; Batista, I.; Nunes, M.L.; Marques, A. Hake proteins edible films incorporated with essential oils: Physical, mechanical, antioxidant and antibacterial properties. Food Hydrocoll. 2013, 30, 224–231. [Google Scholar] [CrossRef]
- Chi, S.; Zivanovic, S.; Penfield, M.P. Application of chitosan films enriched with oregano essential oil on bologna—Active compounds and sensory attributes. Food Sci. Technol. Int. 2006, 12, 111–117. [Google Scholar] [CrossRef]
| Composition | Id. 1 | KI | Thymus vulgaris (%) |
|---|---|---|---|
| α-Pinene | KI, W | 937 | 1.12 |
| β-Pinene | KI, W | 948 | 2.85 |
| Camphene | KI, W | 970 | 4.78 |
| Sabinene | KI, W | 993 | 1.94 |
| β-Pinene | KI, W | 1000 | 2.76 |
| Myrcene | KI, W | 1003 | 3.27 |
| 1-Octen-3-ol | KI, W | 1023 | 0.78 |
| α-Terpinene | KI, W | 1035 | 0.87 |
| Limonene | KI, W | 1046 | 3.43 |
| β-Cymene | KI, W | 1051 | 3.74 |
| 1,8-Cineole | KI, W | 1062 | 12.30 |
| γ-Terpinene | KI, W | 1074 | 2.26 |
| Terpinolene | KI, W | 1103 | 0.45 |
| trans-Sabinene hydrate | KI, W | 1118 | 1.30 |
| Linalool | KI, W | 1142 | 1.90 |
| cis-Sabinene hydrate | KI, W | 1154 | 0.87 |
| Terpineol | KI, W | 1174 | 0.31 |
| trans-Pinocarveol | KI, W | 1199 | 1.36 |
| Verbenol | KI, W | 1207 | 1.32 |
| Camphor | KI, W | 1215 | 11.23 |
| Terpinen-4-ol | KI, W | 1228 | 5.50 |
| Borneol | KI, W | 1238 | 8.87 |
| α-Terpineol | KI, W | 1250 | 5.83 |
| Myrtenol | KI, W | 1260 | 0.23 |
| cis-Carveol | KI, W | 1263 | 0.25 |
| Citronellal | KI, W | 1279 | 0.17 |
| Linalyl acetate | KI, W | 1284 | 0.38 |
| Verbenone | KI, W | 1294 | 0.23 |
| Bornyl acetate | KI, W | 1331 | 2.38 |
| α-Terpenyl acetate | KI, W | 1388 | 0.93 |
| β-Bourbonene | KI, W | 1416 | 0.63 |
| Bornyl acetate | KI, W | 1421 | 1.91 |
| α-Gurjunene | KI, W | 1436 | 0.13 |
| trans-Caryophyllene | KI, W | 1460 | 3.98 |
| Alloaromadendrene | KI, W | 1497 | 2.03 |
| Germacrene-D | KI, W | 1522 | 0.57 |
| α-Murolene | KI, W | 1525 | 0.73 |
| Bicyclogermacrene | KI, W | 1537 | 1.65 |
| δ-Cadinene | KI, W | 1545 | 2.74 |
| Hedycariol | KI, W | 1616 | 1.12 |
| Variables | A = Thyme EO Concentration 1 | B = Storage Time (Days) 1 | A × B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 0.5% | 1% | 2% | F-Value | 0 | 7 | 14 | 21 | 28 | F-Value | F-Value | |
| AMB | a | a | a | a | 1.568NS | a | b | c | c | d | 1882.396 ** | 2.619 * |
| MLAB | a | a | a | a | 1.269NS | a | b | c | d | d | 609.583 ** | 3.159 * |
| Enterobacteria | a | a | a | a | 1.137NS | a | d | c | d | d | 839.761 ** | 0.963NS |
| Molds | a | a | a | a | 1.480NS | a | ab | b | b | c | 17.121 ** | 0.310NS |
| Yeasts | b | b | a | a | 24.332 ** | a | b | b | c | c | 101.708 ** | 3.808 ** |
| pH | d | c | a | b | 77.826 ** | c | c | b | a | b | 151.656 ** | 10.402 ** |
| L * | a | a | a | a | 1.733NS | c | b | b | b | a | 65.752 ** | 9.410 ** |
| a * | a | a | b | b | 16.269 ** | ab | a | b | c | c | 25.657 ** | 3.426 ** |
| b * | a | ab | ab | b | 1.931 * | bc | bc | c | b | a | 30.362 ** | 5.169 ** |
| C | a | ab | bc | c | 10.065 ** | ab | ab | bc | c | a | 5.190 ** | 1.516NS |
| H | bc | c | a | ba | 8.391 * | c | d | cd | b | a | 53.273 ** | 7.262 ** |
| Delta E | b | ab | a | ab | 11.260 ** | a | b | b | b | c | 67.798 ** | 10.303 ** |
| pH | Days of Storage | ||||
|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 6.35 ± 0.01 | 6.31 ± 0.01 | 6.23 ± 0.04 | 6.19 ± 0.04 | 6.33 ± 0.08 |
| 0.5% EO | 6.35 ± 0.01 | 6.30 ± 0.02 | 6.14 ± 0.07 | 5.97 ± 0.04 | 6.26 ± 0.06 |
| 1% EO | 6.27 ± 0.01 | 6.26 ± 0.07 | 6.08 ± 0.04 | 5.82 ± 0.02 | 6.00 ± 0.01 |
| 2% EO | 6.27 ± 0.01 | 6.27 ± 0.01 | 6.15 ± 0.01 | 5.90 ± 0.08 | 6.04 ± 0.04 |
| Lightness | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 66.28 ± 1.71 | 62.18 ± 0.92 | 59.82 ± 1.90 | 60.98 ± 1.85 | 61.85 ± 1.65 |
| 0.5% EO | 64.94 ± 1.25 | 63.14 ± 1.91 | 63.53 ± 1.47 | 62.64 ± 0.90 | 59.47 ± 1.48 |
| 1% EO | 66.79 ± 1.82 | 64.70 ± 1.10 | 62.81 ± 1.26 | 63.97 ± 1.11 | 60.26 ± 1.65 |
| 2% EO | 66.10 ± 1.07 | 59.56 ± 1.36 | 61.88 ± 0.61 | 61.15 ± 0.45 | 61.66 ± 2.82 |
| a * (Redness/Greenness) | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 6.03 ± 0.40 | 5.79 ± 0.24 | 6.13 ± 0.35 | 6.73 ± 0.58 | 6.27 ± 0.75 |
| 0.5% EO | 6.28 ± 0.65 | 5.49 ± 0.53 | 5.41 ± 0.68 | 7.08 ± 0.47 | 6.91 ± 0.38 |
| 1% EO | 6.52 ± 0.23 | 6.03 ± 0.23 | 7.08 ± 0.80 | 6.89 ± 0.38 | 7.60 ± 0.48 |
| 2% EO | 6.45 ± 0.32 | 6.39 ± 0.75 | 6.54 ± 0.46 | 7.44 ± 0.74 | 7.52 ± 0.51 |
| b * (Yellowness/Blueness) | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 8.31 ± 0.30 | 8.27 ± 0.27 | 8.82 ± 0.78 | 7.67 ± 0.67 | 7.24 ± 0.25 |
| 0.5% EO | 8.27 ± 0.23 | 8.46 ± 0.45 | 9.09 ± 0.94 | 7.97 ± 0.41 | 6.91 ± 0.25 |
| 1% EO | 8.15 ± 0.42 | 8.55 ± 0.73 | 7.84 ± 0.21 | 8.68 ± 0.36 | 7.51 ± 0.38 |
| 2% EO | 8.57 ± 0.24 | 8.61 ± 0.03 | 8.64 ± 0.59 | 8.23 ± 0.27 | 7.70 ± 0.42 |
| C (Chroma) | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 10.28 ± 0.23 | 10.10 ± 0.23 | 10.48 ± 0.58 | 10.22 ± 0.88 | 9.93 ± 0.33 |
| 0.5% EO | 10.40 ± 0.33 | 10.10 ± 0.48 | 10.58 ± 0.95 | 10.63 ± 0.32 | 9.78 ± 0.22 |
| 1% EO | 10.41 ± 0.43 | 10.47 ± 0.63 | 10.52 ± 0.56 | 11.08 ± 0.48 | 10.69 ± 0.54 |
| 2% EO | 10.73 ± 0.31 | 10.89 ± 0.45 | 10.79 ± 0.64 | 11.09 ± 0.52 | 10.76 ± 0.64 |
| h (Hue) | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 54.02 ± 2.47 | 55.00 ± 1.58 | 55.09 ± 3.50 | 48.65 ± 0.67 | 49.53 ± 5.14 |
| 0.5% EO | 52.82 ± 2.77 | 57.04 ± 2.88 | 59.22 ± 3.69 | 48.67 ± 2.88 | 45.04 ± 2.31 |
| 1% EO | 51.46 ± 1.09 | 54.72 ± 2.37 | 47.78 ± 3.33 | 51.57 ± 0.99 | 44.67 ± 1.50 |
| 2% EO | 53.04 ± 1.37 | 53.03 ± 3.24 | 53.08 ± 2.08 | 47.91 ± 2.07 | 45.69 ± 0.90 |
| Delta E * | Days of Storage | ||||
| 7 | 14 | 21 | 28 | ||
| 0% EO | 4.13 ± 0.92 | 6.53 ± 1.27 | 5.45 ± 1.14 | 4.65 ± 1.41 | |
| 0.5% EO | 3.28 ± 0.80 | 3.19 ± 1.14 | 3.88 ± 1.01 | 7.02 ± 1.47 | |
| 1% EO | 1.85 ± 0.20 | 3.82 ± 1.11 | 2.55 ± 0.56 | 6.30 ± 0.61 | |
| 2% EO | 6.79 ± 0.70 | 4.48 ± 0.79 | 5.37 ± 1.71 | 4.94 ± 0.68 | |
| Ham Odor | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 5.0 | 5.5 | 5.0 | 3.0 | 3.0 |
| 0.5% EO | 4.0 | 4.0 | 3.0 | 2.0 | 5.0 |
| 1% EO | 2.0 | 3.5 | 3.0 | 3.0 | 4.0 |
| 2% EO | 1.0 | 1.0 | 2.0 | 1.5 | 2.0 |
| Off-Flavors | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 |
| 0.5% EO | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 1% EO | 1.0 | 1.0 | 2.0 | 1.0 | 1.0 |
| 2% EO | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Thyme Odor | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 1.0 | 1.0 | 1.0 | 2.0 | 1.0 |
| 0.5% EO | 3.0 | 2.0 | 2.0 | 3.0 | 1.0 |
| 1% EO | 5.0 | 2.5 | 3.0 | 4.0 | 3.0 |
| 2% EO | 6.0 | 6.0 | 5.5 | 5.5 | 4.0 |
| Color | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0% EO | 4.0 | 3.5 | 3.0 | 3.0 | 3.0 |
| 0.5% EO | 4.0 | 4.0 | 3.5 | 3.0 | 2.0 |
| 1% EO | 4.0 | 3.0 | 4.0 | 3.0 | 2.0 |
| 2%EO | 4.0 | 4.0 | 3.0 | 3.5 | 2.0 |
| Exudates | Days of Storage | ||||
| 0 | 7 | 14 | 21 | 28 | |
| 0%EO | 1.0 | 2.5 | 6.5 | 4.0 | 4.0 |
| 0.5% EO | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 |
| 1% EO | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 |
| 2%EO | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 |
| Variables | Thyme EO Concentration | Storage Time |
|---|---|---|
| Sig 1 | Sig 1 | |
| Ham odor | ** | ** |
| Off-flavors | NS | * |
| Thyme odor | ** | * |
| Color | NS | ** |
| Exudates | * | ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. https://doi.org/10.3390/foods5030057
Quesada J, Sendra E, Navarro C, Sayas-Barberá E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods. 2016; 5(3):57. https://doi.org/10.3390/foods5030057
Chicago/Turabian StyleQuesada, Jesús, Esther Sendra, Casilda Navarro, and Estrella Sayas-Barberá. 2016. "Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat" Foods 5, no. 3: 57. https://doi.org/10.3390/foods5030057
APA StyleQuesada, J., Sendra, E., Navarro, C., & Sayas-Barberá, E. (2016). Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods, 5(3), 57. https://doi.org/10.3390/foods5030057