Babassu Mesocarp-Based Coating with Amazonian Plant Extracts Obtained Using Natural Deep Eutectic Solvents (NADES) for Cherry Tomato Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Characterization of Uxi Barks and Jambolan Leaves
2.3. NADES Preparation and Characterization
2.4. Production and Characterization of NADES Extracts from Uxi Bark and Jambolan Leaves
2.5. Biopolymer Coating: Development, Characterization and Application on Cherry Tomatoes
2.6. Statistical Analysis
3. Results and Discussion
3.1. NADES Preparation and Characterization
3.2. Characterization of NADES–Uxi Bark Extract and NADES–Jambolan Leaves Extract
Bioactivity Screening Assays
3.3. Biopolymer Coating: Development, Characterization and Application on Cherry Tomatoes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dash, D.R.; Singh, S.K.; Singha, P. Bio-Based Composite Active Film/Coating from Deccan Hemp Seed Protein, Taro Starch and Leaf Extract: Characterizations and Application in Grapes. Sustain. Chem. Pharm. 2024, 39, 101609. [Google Scholar] [CrossRef]
- Anushikha; Deshmukh, R.K.; Kunam, P.K.; Gaikwad, K.K. Guar Gum Based Flexible Packaging Material with an Active Surface Reinforced by Litchi Shell Derived Micro Fibrillated Cellulose and Halloysite Nanotubes. Sustain. Chem. Pharm. 2023, 36, 101302. [Google Scholar] [CrossRef]
- Zhang, D.; Ahlivia, E.B.; Bruce, B.B.; Zou, X.; Battino, M.; Savić, D.; Katona, J.; Shen, L. The Road to Re-Use of Spice By-Products: Exploring Their Bioactive Compounds and Significance in Active Packaging. Foods 2025, 14, 2445. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant Protein-Based Food Packaging Films; Recent Advances in Fabrication, Characterization, and Applications. Trends Food Sci. Technol. 2022, 120, 154–173. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of Biodegradable Polymer-Based Films and Coatings and Their Food Packaging Applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Pavani, M.; Singh, S.K.; Singha, P. Chitosan from Agro-Waste for Food Packaging Applications. In Agro-Waste Derived Biopolymers and Biocomposites; Wiley: Hoboken, NJ, USA, 2024; pp. 267–294. [Google Scholar]
- Gonçalves, L.d.O.; Farias, P.M.D.; Freitas, T.F.d.; Zago, L.; Moreira, R.F.A.; Maniglia, B.C.; Fai, A.E.C. Babassu-Derived Sachet for Pereskia aculeata–Based Oily Sauce: A Bio-Compostable Single-Use Packaging Option. J. Food Sci. 2025, 90, e70690. [Google Scholar] [CrossRef]
- Ferreira, D.C.M.; Molina, G.; Pelissari, F.M. Effect of Edible Coating from Cassava Starch and Babassu Flour (Orbignya phalerata) on Brazilian Cerrado Fruits Quality. Food Bioproc. Technol. 2020, 13, 172–179. [Google Scholar] [CrossRef]
- Galante, M.; Brassesco, M.E.; Maragoni Santos, C.; Beres, C.; Fai, A.E.C.; Cabezudo, I. Grape Pomace as a Natural Source of Antimicrobial Agents for Food Preservation. Front. Nutr. 2025, 12, 1650450. [Google Scholar] [CrossRef]
- Politi, F.A.S.; Mello, J.C.P.d.; Migliato, K.F.; Nepomuceno, A.L.A.; Moreira, R.R.D.; Pietro, R.C.L.R. Antimicrobial, Cytotoxic and Antioxidant Activities and Determination of the Total Tannin Content of Bark Extracts Endopleura Uchi. Int. J. Mol. Sci. 2011, 12, 2757–2768. [Google Scholar] [CrossRef]
- Silva, L.R.; Teixeira, R. Phenolic Profile and Biological Potential of Endopleura Uchi Extracts. Asian Pac. J. Trop. Med. 2015, 8, 889–897. [Google Scholar] [CrossRef]
- Oliveira, R.T.; dos Santos Rolim, C.S.; do Nascimento Rolim, L.; de Sousa Gomes, M.L.; Martins, G.A.S.; de Castro, L.M.; do Nascimento, W.M.; Saraiva-Bonatto, E.C.; de Cássia Saraiva Nunomura, R.; Lamarão, C.V.; et al. Endopleura Uchi—A Review about Its Nutritional Compounds, Biological Activities and Production Market. Food Res. Int. 2021, 139, 109884. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Hoscheid, J.; Garcia, V.; de Oliveira Santos Junior, O.; da Silva, C. Phytochemical Extract from Syzygium Cumini Leaf: Maximization of Compound Extraction, Chemical Characterization, Antidiabetic and Antibacterial Activity, and Cell Viability. Processes 2024, 12, 2270. [Google Scholar] [CrossRef]
- Ruan, Z.P.; Zhang, L.L.; Lin, Y.M. Evaluation of the Antioxidant Activity of Syzygium cumini Leaves. Molecules 2008, 13, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Balabram, S.K.; Tessaro, L.; Astolfo, M.E.d.A.; Sponchiado, P.A.I.; Bogusz Junior, S.; Maniglia, B.C. Development of NADES–Annatto Seed Extract for Enhancing 3D Printed Food Designed for Dysphagia Patients. Foods 2025, 14, 1604. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Bogusz Junior, S.; Mitchell, A.E. Green Strategies for Recovery of Bioactive Phenolic Compounds from Agro-Industrial Wastes (Pomegranate Peels, Almond Hulls, and Elderberry Pomace) Using Natural Deep Eutectic Solvents. ACS Food Sci. Technol. 2023, 3, 2144–2156. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Oliveira, L.F.R.; Titato, G.M.; Lanças, F.M.; Correa, D.S. Sustainable Extraction of Value-Added Compounds from Orange Waste Using Natural Deep Eutectic Solvents. J. Mol. Liq. 2025, 431, 127703. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Airouyuwa, J.O.; Mostafa, H.; Ranasinghe, M.; Maqsood, S. Influence of Physicochemical Properties of Carboxylic Acid-Based Natural Deep Eutectic Solvents (CA-NADES) on Extraction and Stability of Bioactive Compounds from Date (Phoenix dactylifera L.) Seeds: An Innovative and Sustainable Extraction Technique. J. Mol. Liq. 2023, 388, 122767. [Google Scholar] [CrossRef]
- Boiteux, J.; Espino, M.; Azcarate, S.; Silva, M.F.; Gomez, F.J.V.; Pizzuolo, P.; de los Angeles Fernandez, M. NADES Blend for Bioactive Coating Design as a Sustainable Strategy for Postharvest Control. Food Chem. 2023, 406, 135054. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Yan, X.; Cai, Z.; Fu, L.; Gu, Q.; Liu, L.; Jin, H.; Fu, Y. Functional Chitosan/Zein Films with Rosa Roxburghii Tratt Leaves Extracts Prepared by Natural Deep Eutectic Solvents. Food Packag. Shelf Life 2022, 34, 101001. [Google Scholar] [CrossRef]
- Elia Dazat, R.; Fernandez, M.d.l.Á.; Espino, M.; Boiteux, J.; Silva, M.F.; Gomez, F.J.V. Biopolymeric Sensor Based on Natural Deep Eutectic Solvents for Monitoring Meat Spoilage. Food Control 2024, 166, 110712. [Google Scholar] [CrossRef]
- Tadele, M.A.; Roy, V.C.; Ho, T.C.; Chun, B.-S. Extraction of Anthocyanin from Purple Sweet Potato Using Ultrasound-Assisted Natural Deep Eutectic Solvents and Its Application for Smart Packaging Film. Food Bioproc. Technol. 2025, 18, 2394–2409. [Google Scholar] [CrossRef]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and Characterisation of a Pectin-Based Edible Film That Contains Mulberry Leaf Extract and Its Bio-Active Components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, P.; Zhu, L. Preparation of Modified Atmosphere Packaging Based on the Respiratory Characteristics of Cherry Tomato and Its Freshness Preservation Application. Sci. Hortic. 2024, 333, 113286. [Google Scholar] [CrossRef]
- Álvarez, A.; Manjarres, J.J.; Ramírez, C.; Bolívar, G. Use of an Exopolysaccharide-Based Edible Coating and Lactic Acid Bacteria with Antifungal Activity to Preserve the Postharvest Quality of Cherry Tomato. LWT 2021, 151, 112225. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Guazi, J.S.; Conti-Silva, A.C.; Nicoletti, V.R. Active Packaging for Postharvest Storage of Cherry Tomatoes: Different Strategies for Application of Microencapsulated Essential Oil. Food Packag. Shelf Life 2021, 29, 100723. [Google Scholar] [CrossRef]
- Moeini, A.; Salazar, S.A.; Gargiulo, L.; Dougué Kentsop, R.A.; Mattana, M.; Genga, A.; Josi, C.; Pedram, P.; Cabrera-Barjas, G.; Guerra, S.; et al. Development of Alginate-Based Active Edible Coating with Brassica Juncea and Raphanus Sativus Sprout Extracts to Extend Tomato Shelf-Life. Food Hydrocoll. 2026, 170, 111693. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Arlington, VA, USA, 2016. [Google Scholar]
- Matheus, J.R.V.; de Freitas, T.F.; Zago, L.; Gouvea, R.; Lima, E.R.d.A.; Ferreira, F.N.; de Gois, J.S.; Luchese, C.L.; de Andrade, C.J.; Fai, A.E.C. Olive Leaves Addition on Starch-Pectin Films: Optimization, Characterization, and Evaluation as Edible Hydrosoluble Sachets. Food Bioproc. Technol. 2025, 18, 6582–6601. [Google Scholar] [CrossRef]
- Delgado-González, M.J.; Carmona-Jiménez, Y.; Rodríguez-Dodero, M.C.; García-Moreno, M.V. Color Space Mathematical Modeling Using Microsoft Excel. J. Chem. Educ. 2018, 95, 1885–1889. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S. Utilization of Pomegranate Peel Waste: Natural Deep Eutectic Solvents as a Green Strategy to Recover Valuable Phenolic Compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Haghbakhsh, R.; Marques, R.; Paiva, A.; Carlyle, L.; Duarte, A.R.C. Evaluation of Deep Eutectic Systems as an Alternative to Solvents in Painting Conservation. ACS Sustain. Chem. Eng. 2021, 9, 15451–15460. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic Parameters for Solvents of Interest in Green Chemistry. Green Chem. 2012, 14, 1245. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; Maragoni-Santos, C.; de Freitas, T.F.; Hackbart, E.F.C.; Ribeiro-Santos, R.; Perrone, D.; de Sousa, A.M.F.; Luchese, C.L.; de Andrade, C.J.; Fai, A.E.C. Starch-Pectin Smart Tag Containing Purple Carrot Peel Anthocyanins as a Potential Indicator of Analogous Meat Freshness. Int. J. Biol. Macromol. 2024, 283, 137161. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.F.M.; Mariano, X.M.; Isnard, J.L.; de Souza, G.S.; de Souza Gomes, A.L.; de Carvalho, R.J.T.; Rocha, C.B.; Junior, C.L.S.; Moreira, R.F.A. Evaluation of the Volatile Composition, Toxicological and Antioxidant Potentials of the Essential Oils and Teas of Commercial Chilean Boldo Samples. Food Res. Int. 2019, 124, 27–33. [Google Scholar] [CrossRef]
- Velásquez, P.; Bustos, D.; Montenegro, G.; Giordano, A. Ultrasound-Assisted Extraction of Anthocyanins Using Natural Deep Eutectic Solvents and Their Incorporation in Edible Films. Molecules 2021, 26, 984. [Google Scholar] [CrossRef]
- Filho, L.G.A.d.S.; Castro, K.N.d.C.; Pereira, A.M.L.; Diniz, F.M. Detecção Da Atividade in Vitro de Compostos Naturais à Base de Plantas: Metodologia Científica; Embrapa Meio-Norte: Teresina, Brazil, 2019; Volume 254, pp. 1–14. [Google Scholar]
- Hamidi, M.; Toosi, A.M.; Javadi, B.; Asili, J.; Soheili, V.; Shakeri, A. In Vitro Antimicrobial and Antibiofilm Screening of Eighteen Iranian Medicinal Plants. BMC Complement. Med. Ther. 2024, 24, 135. [Google Scholar] [CrossRef]
- Barone, A.S.; Matheus, J.R.V.; Luchese, C.L.; Marques, M.R.d.C.; de Souza, A.M.F.; Ferreira, W.H.; Moreira, R.F.A.; Fai, A.E.C. Active Antioxidant and Aromatic Films Blended with Persimmon (Diospyros kaki L.) and Orange Peel Flour (Citrus sinensis) as Sustainable Packaging. J. Vinyl Addit. Technol. 2024, 30, 635–650. [Google Scholar] [CrossRef]
- Batu, A. Determination of Acceptable Firmness and Colour Values of Tomatoes. J. Food Eng. 2004, 61, 471–475. [Google Scholar] [CrossRef]
- USDA. United States Standards for Grade of Fresh Tomatoes; USDA: Washington, DC, USA, 1976.
- Kumar, A.; Saini, C.S. Edible Composite Bi-Layer Coating Based on Whey Protein Isolate, Xanthan Gum and Clove Oil for Prolonging Shelf Life of Tomatoes. Meas. Food 2021, 2, 100005. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis: 15th Edition. TrAC Trends Anal. Chem. 2005, 9, VI. [Google Scholar] [CrossRef]
- Rather, J.A.; Makroo, H.A.; Showkat, Q.A.; Majid, D.; Dar, B.N. Recovery of Gelatin from Poultry Waste: Characteristics of the Gelatin and Lotus Starch-Based Coating Material and Its Application in Shelf-Life Enhancement of Fresh Cherry Tomato. Food Packag. Shelf Life 2022, 31, 100775. [Google Scholar] [CrossRef]
- da Silva, N.; Junqueira, V.C.A.; Silveira, N.F.A.; Taniwaki, M.H.; Santos, R.F.S.; Gomes, R.A.R. Manual de Métodos de Análise Microbiológica de Alimentos e Água [Food and Water Microbiological Analysis Methods Manual]; Varela: São Paulo, Brazil, 2010. [Google Scholar]
- Paixão, J.A.; Tavares Filho, E.; Bolini, H.M.A. Investigation of Alcohol Factor Influence in Quantitative Descriptive Analysis and in the Time-Intensity Profile of Alcoholic and Non-Alcoholic Commercial Pilsen Beers Samples. Beverages 2020, 6, 73. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.d.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as Potential Solvents for Anthocyanin and Pectin Extraction from Myrciaria Cauliflora Fruit By-Product: In Silico and Experimental Approaches for Solvent Selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Jurić, T.; Uka, D.; Holló, B.B.; Jović, B.; Kordić, B.; Popović, B.M. Comprehensive Physicochemical Evaluation of Choline Chloride-Based Natural Deep Eutectic Solvents. J. Mol. Liq. 2021, 343, 116968. [Google Scholar] [CrossRef]
- Chevé-Kools, E.; Choi, Y.H.; Roullier, C.; Ruprich-Robert, G.; Grougnet, R.; Chapeland-Leclerc, F.; Hollmann, F. Natural Deep Eutectic Solvents (NaDES): Green Solvents for Pharmaceutical Applications and Beyond. Green Chem. 2025, 27, 8360–8385. [Google Scholar] [CrossRef]
- Mohd Fuad, F.; Mohd Nadzir, M.; Harun@Kamaruddin, A. Hydrophilic Natural Deep Eutectic Solvent: A Review on Physicochemical Properties and Extractability of Bioactive Compounds. J. Mol. Liq. 2021, 339, 116923. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; Nogueira, T.B.d.B.; Pereira, A.P.A.; Correia, T.R.; de Sousa, A.M.F.; Pastore, G.M.; Pelissari, F.M.; Miyahira, R.F.; Fai, A.E.C. Antibacterial Films Made with Persimmon (Diospyros kaki L.), Pectin, and Glycerol: An Experimental Design Approach. J. Food Sci. 2021, 86, 4539–4553. [Google Scholar] [CrossRef]
- Guo, C.; Lv, L.; Liu, Y.; Ji, M.; Zang, E.; Liu, Q.; Zhang, M.; Li, M. Applied Analytical Methods for Detecting Heavy Metals in Medicinal Plants. Crit. Rev. Anal. Chem. 2023, 53, 339–359. [Google Scholar] [CrossRef]
- Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200. [Google Scholar] [CrossRef]
- Rajendran, P.; Somasundaram, P.; Dufossé, L. Microbial Pigments: Eco-Friendly Extraction Techniques and Some Industrial Applications. J. Mol. Struct. 2023, 1290, 135958. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.; Zhang, Y.; Guo, P.; Wu, H.; Gao, R.; Liu, T. Natural Deep Eutectic Solvent (NADES)-Aided Extraction of Bioactive Compounds from Cotton Byproducts for Agricultural Applications: Extraction Optimization, Structural Identification, and Bioactivity Evaluation. Ind. Crops Prod. 2025, 233, 121389. [Google Scholar] [CrossRef]
- Burel, C.; Kala, A.; Purevdorj-Gage, L. Impact of PH on Citric Acid Antimicrobial Activity against Gram-negative Bacteria. Lett. Appl. Microbiol. 2021, 72, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Liu, J. Active Packaging Films and Edible Coatings Based on Polyphenol-rich Propolis Extract: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, T.; Li, S.; Jia, S.; Yang, X.; Cui, Y.; Ma, H.; Yan, S.; Zhang, S. Nature Nano-Barrier: HPMC/MD-Based Lactobacillus Plantarum Pickering Emulsion to Extend Cherry Tomato Shelf Life. Foods 2025, 14, 2729. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jiang, J.; Tian, Y.; Guo, Y.; He, T.; Xie, Y.; Wu, K.; Zhu, F.; Jiang, F. Improved Konjac Glucomannan/Curdlan-Based Emulsion Coating by Mung Bean Protein Addition for Cherry Tomato Preservation. Int. J. Biol. Macromol. 2025, 291, 139080. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Pereira Ribeiro, C.P.; Uliana, N.R.; Cassetari Rodrigues, M.B.; da Rosa, C.G.; Ferrareze, J.P.; Veeck, A.P.d.L.; Nunes, M.R. Bioactive and PH-Sensitive Films Based on Carboxymethyl Cellulose and Blackberry (Morus nigra L.) Anthocyanin-Rich Extract: A Perspective Coating Material to Improve the Shelf Life of Cherry Tomato (Solanum lycopersicum L. var. cerasiforme). Biocatal. Agric. Biotechnol. 2021, 33, 101989. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, Z.; Cai, R.; Yue, T.; Cui, L. Preparation and Characterization of Chitosan–Nano-ZnO Composite Films for Preservation of Cherry Tomatoes. Foods 2021, 10, 3135. [Google Scholar] [CrossRef]
- Constantino, L.V.; de Araujo, S.R.; Suzuki Fukuji, A.S.; Nogueira, A.F.; de Lima Filho, R.B.; Zeffa, D.M.; Nicio, T.T.; Oliveira, C.; Azeredo Gonçalves, L.S. Post-Harvest Characterization and Sensory Analysis of Roma Tomato Cultivars under Organic Cultivation: A Strategy Using Consumers and Chefs. Int. J. Gastron. Food Sci. 2022, 29, 100564. [Google Scholar] [CrossRef]
- Senevirathna, S.M.A.A.; Jayathunge, K.G.L.R.; Prasanga, G.L.R.; Wijesekara, W.L.I. Plant-Derived Composite Edible Coatings for Prolonging the Postharvest Life of Lime (Citrus aurantiifolia) and Tomato (Solanum lycopersicum L.) under Ambient Storage. ACS Food Sci. Technol. 2024, 4, 1700–1713. [Google Scholar] [CrossRef]
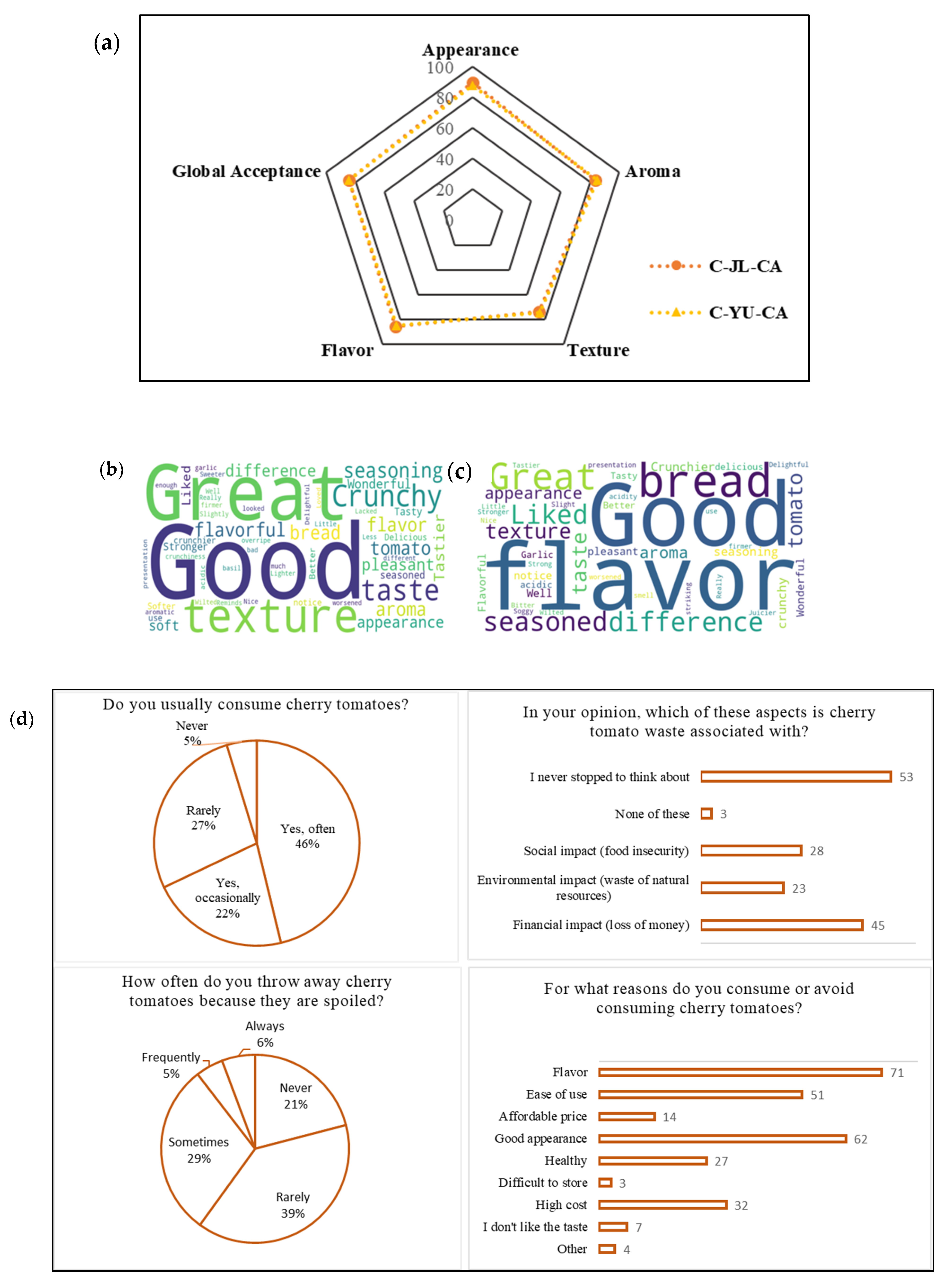
- Marques, C.; Correia, E.; Dinis, L.-T.; Vilela, A. An Overview of Sensory Characterization Techniques: From Classical Descriptive Analysis to the Emergence of Novel Profiling Methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef]
- Marwood, S.; Byrne, N.; McCarthy, O.; Heavin, C.; Barlow, P. Examining the Relationship between Consumers’ Food-Related Actions, Wider Pro-Environmental Behaviours, and Food Waste Frequency: A Case Study of the More Conscious Consumer. Sustainability 2023, 15, 2650. [Google Scholar] [CrossRef]


| Classical Solvent | ||||
|---|---|---|---|---|
| Extract ID | Sample | Solvent | ||
| YU-E | Uxi bark | EtOH/H2O 60% (v/v). | ||
| JL-E | Jambolan Leaves | EtOH/H2O 60% (v/v). | ||
| Natural Deep Eutectic Solvents (NADES) | ||||
| Extract ID | Sample | HBA | HBD | Molar ratio |
| YU-CA | Uxi bark | Choline Chloride | Citric acid | 1:1 |
| YU-G | Choline Chloride | Glucose | 1:1 | |
| YU-U | Choline Chloride | Urea | 1:2 | |
| JL-CA | Jambolan leaves | Choline Chloride | Citric acid | 1:1 |
| JL-G | Choline Chloride | Glucose | 1:1 | |
| JL-U | Choline Chloride | Urea | 1:2 | |
| Properties | CC-CA | CC-G | CC-U |
|---|---|---|---|
| pH at 25 °C | 0 ± 0 c | 5 ± 0 b | 7 ± 0 a |
| Density at 25 °C (g cm−3) | 1.234 ± 0.038 a | 1.239 ± 0.009 a | 1.152 ± 0.037 c |
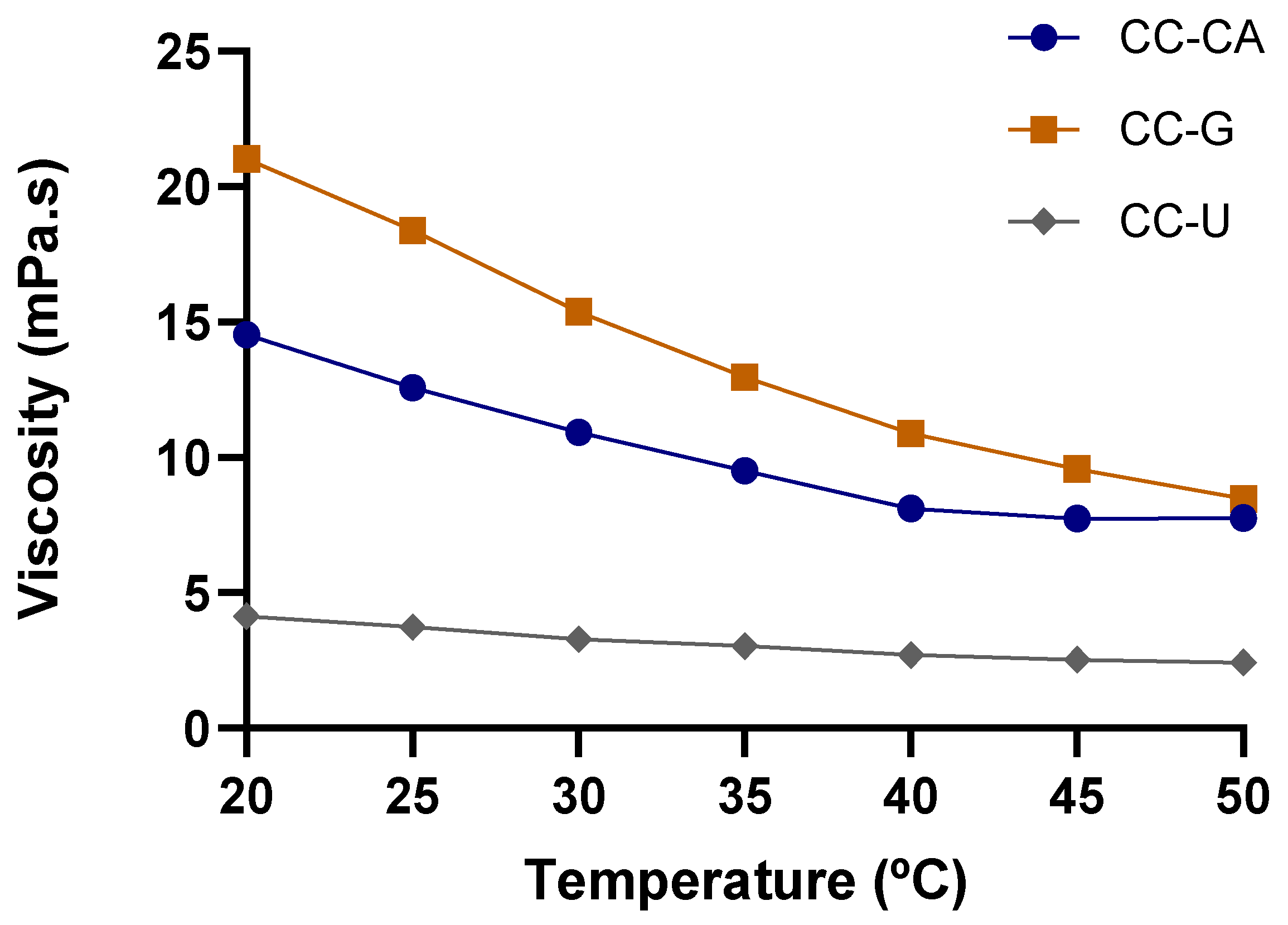
| Viscosity at 25 °C (mPa s) | 12.566 ± 0.111 b | 18.375 ± 0.430 a | 3.742 ± 0.115 c |
| ENR (kcal mol−1) | 44.6 ± 0.1 c | 49.9 ± 0.1 a | 49.4 ± 0.1 b |
| Samples | Extracts | L* | a* | b* | Cab* | ΔE | pH Range | Visual Appearance |
|---|---|---|---|---|---|---|---|---|
| Uxi bark | YU-CA | 24.29 ± 0.04 a | 0.46 ± 0.05 a | −0.13 ± 0.0 a | 0.45 ± 0.02 d | 0.61 ± 0.01 a | 0–1 |  |
| YU-G | 27.68 ± 1.52 b | 0.17 ± 0.09 b | −0.13 ± 0.13 a | 0.41 ± 0.03 c | 4.04 ± 0.59 b | 3 |  | |
| YU-U | 24.31 ± 0.03 a | −0.24 ± 0.05 c | −0.04 ± 0.04 a | 0.24 ± 0.04 b | 1.32 ± 0.07 a | 7–8 |  | |
| YU-E | 24.84 ± 0.04 a | 0.65 ± 0.02 a | −0.05 ± 0.03 a | 0.65 ± 0.02 a | - | 4–5 |  | |
| Jambolan leaves | JL-CA | 25.07 ± 0.07 a | 0.66 ± 0.1 a | 0.87 ± 0.04 a | 1.1 ± 0.06 a | 2.02 ± 0.13 a | 0–1 |  |
| JL-G | 28.42 ± 0.58 b | −0.03 ± 0.11 b | −0.04 ± 0.18 b | 0.13 ± 0.04 b | 6.11 ± 0.11 b | 4–5 |  | |
| JL-U | 28.28 ± 0.58 b | 0.08 ± 0.05 b | 2.05 ± 0.55 c | 2.05 ± 0.55 a | 2.16 ± 0.66 a | 6–7 |  | |
| JL-E | 26.28 ± 0.05 a | 0.66 ± 0.11 a | 1.77 ± 0.05 c | 1.89 ± 0.04 a | - | 4–5 |  |
| S. aureus | E. coli | P. aeruginosa | Salmonella | |||
|---|---|---|---|---|---|---|
| Disk diffusion assay | ||||||
| Extracts | Uxi bark | YU-CA | +++ | + | +++ | ++ |
| YU-G | + | − | − | − | ||
| YU-U | + | − | − | − | ||
| YU-E | + | − | − | − | ||
| Jambolan leaves | JL-CA | +++ | + | +++ | +++ | |
| JL-G | ++ | − | − | − | ||
| JL-U | +++ | − | − | − | ||
| JL-E | + | − | − | − | ||
| Solvent | NADES | CC-CA | +++ | + | +++ | ++ |
| CC-G | − | − | − | − | ||
| CC-U | − | − | − | − | ||
| Positive controls | Antibiotics | Azithromycin | +++ | +++ | +++ | +++ |
| Imipenem | +++ | +++ | +++ | +++ | ||
| Ciprofloxacin | +++ | +++ | +++ | +++ | ||
| Minimum inhibitory concentration | ||||||
| Extracts | YU-CA (mg mL−1) | 8.36 | 4.18 | 1.045 | 8.36 | |
| JL-CA (mg mL−1) | 8.36 | 8.36 | 0.52 | 8.36 | ||
| Minimum bactericidal concentration | ||||||
| Extracts | YU-CA (mg mL−1) | 16.72 | 33.44 | 2.09 | 33.44 | |
| JL-CA (mg mL−1) | 33.44 | 66.87 | 2.09 | 16.72 | ||
| Coatings | C-YU-CA (mg mL−1) | 750 | 750 | 93.75 | 750 | |
| C-JL-CA (mg mL−1) | 375 | 750 | 187.5 | 750 | ||
| Storage Time (d) | |||||
|---|---|---|---|---|---|
| Parameter | Sample | 0 | 3 | 7 | 9 |
| pH | Control | 3.89 ± 0.03 aA | 4.42 ± 0.19 aB | 4.21 ± 0.19 aB | 4. 04 ± 0.11 aB |
| C-YU-CA | 4.13 ± 0.18 aA | 4.21 ± 0.14 aA | 4.33 ± 0.18 aA | 4.16 ± 0.1 aA | |
| C-JL-CA | 4.01 ± 0.1 aA | 3.96 ± 0.02 aA | 4.31 ± 0.21 aA | 4.05 ± 0.16 aA | |
| TA (mg of citric acid 100 g−1 cherry tomatoes) | Control | 3.99 ± 0.07 aA | 3.69 ± 0.48 aA | 2.79 ± 0.02 aB | 2.96 ± 0.11 aB |
| C-YU-CA | 3.99 ± 0.59 aA | 4.14 ± 0.49 aA | 3.07 ± 0.53 aA | 2.77 ± 0.37 aA | |
| C-JL-CA | 3.82 ± 0.84 aA | 4.55 ± 0.45 aA | 2.68 ± 0.27 aA | 2.53 ± 0.55 aB | |
| Salmonella sp. in 25 g | Control | Absence | Absence | Absence | Absence |
| C-YU-CA | Absence | Absence | Absence | Absence | |
| C-JL-CA | Absence | Absence | Absence | Absence | |
| E. coli (CFU g−1) | Control | <10 | <10 | <10 | <10 |
| C-YU-CA | <10 | <10 | <10 | <10 | |
| C-JL-CA | <10 | <10 | <10 | <10 | |
| TPC (CFU g−1) | Control | <10 | <10 | <10 | 2 × 10 ± 0.0 a |
| C-YU-CA | <10 | <10 | <10 | 10 ± 0.0 b | |
| C-JL-CA | <10 | <10 | <10 | 10 ± 0.0 b | |
| Yeast and mold (CFU g−1) | Control | <10 | <10 | <10 | 2 × 10 ± 0.0 a |
| C-YU-CA | <102 | <102 | <102 | <102 | |
| C-JL-CA | <102 | <102 | <102 | 2 × 10 ± 0.0 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Maragoni-Santos, C.; Cortat, C.M.G.; Zago, L.; Bogusz Junior, S.; Pinto, T.C.A.; de Gois, J.S.; Maniglia, B.C.; Fai, A.E.C. Babassu Mesocarp-Based Coating with Amazonian Plant Extracts Obtained Using Natural Deep Eutectic Solvents (NADES) for Cherry Tomato Preservation. Foods 2026, 15, 74. https://doi.org/10.3390/foods15010074
Maragoni-Santos C, Cortat CMG, Zago L, Bogusz Junior S, Pinto TCA, de Gois JS, Maniglia BC, Fai AEC. Babassu Mesocarp-Based Coating with Amazonian Plant Extracts Obtained Using Natural Deep Eutectic Solvents (NADES) for Cherry Tomato Preservation. Foods. 2026; 15(1):74. https://doi.org/10.3390/foods15010074
Chicago/Turabian StyleMaragoni-Santos, Carollyne, Camila Marcolongo Gomes Cortat, Lilia Zago, Stanislau Bogusz Junior, Tatiana Castro Abreu Pinto, Jefferson Santos de Gois, Bianca Chieregato Maniglia, and Ana Elizabeth Cavalcante Fai. 2026. "Babassu Mesocarp-Based Coating with Amazonian Plant Extracts Obtained Using Natural Deep Eutectic Solvents (NADES) for Cherry Tomato Preservation" Foods 15, no. 1: 74. https://doi.org/10.3390/foods15010074
APA StyleMaragoni-Santos, C., Cortat, C. M. G., Zago, L., Bogusz Junior, S., Pinto, T. C. A., de Gois, J. S., Maniglia, B. C., & Fai, A. E. C. (2026). Babassu Mesocarp-Based Coating with Amazonian Plant Extracts Obtained Using Natural Deep Eutectic Solvents (NADES) for Cherry Tomato Preservation. Foods, 15(1), 74. https://doi.org/10.3390/foods15010074








