Extraction of Bound Polyphenols from Elaeagnus angustifolia L. by Ultrasonic-Assisted Enzymatic Hydrolysis and Evaluation of Its Antioxidant Activity In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Investigation
2.1.1. Effect of Enzyme Ratio on BP Extraction
2.1.2. The Influence of Ultrasound Time on BP Extraction
2.1.3. The Effect of Liquid-to-Solid Ratio on BP Extraction
2.1.4. Effect of pH on BP Extraction
2.2. Response Surface Methodology for Optimizing the BP Extraction Process of E. angustifolia L.
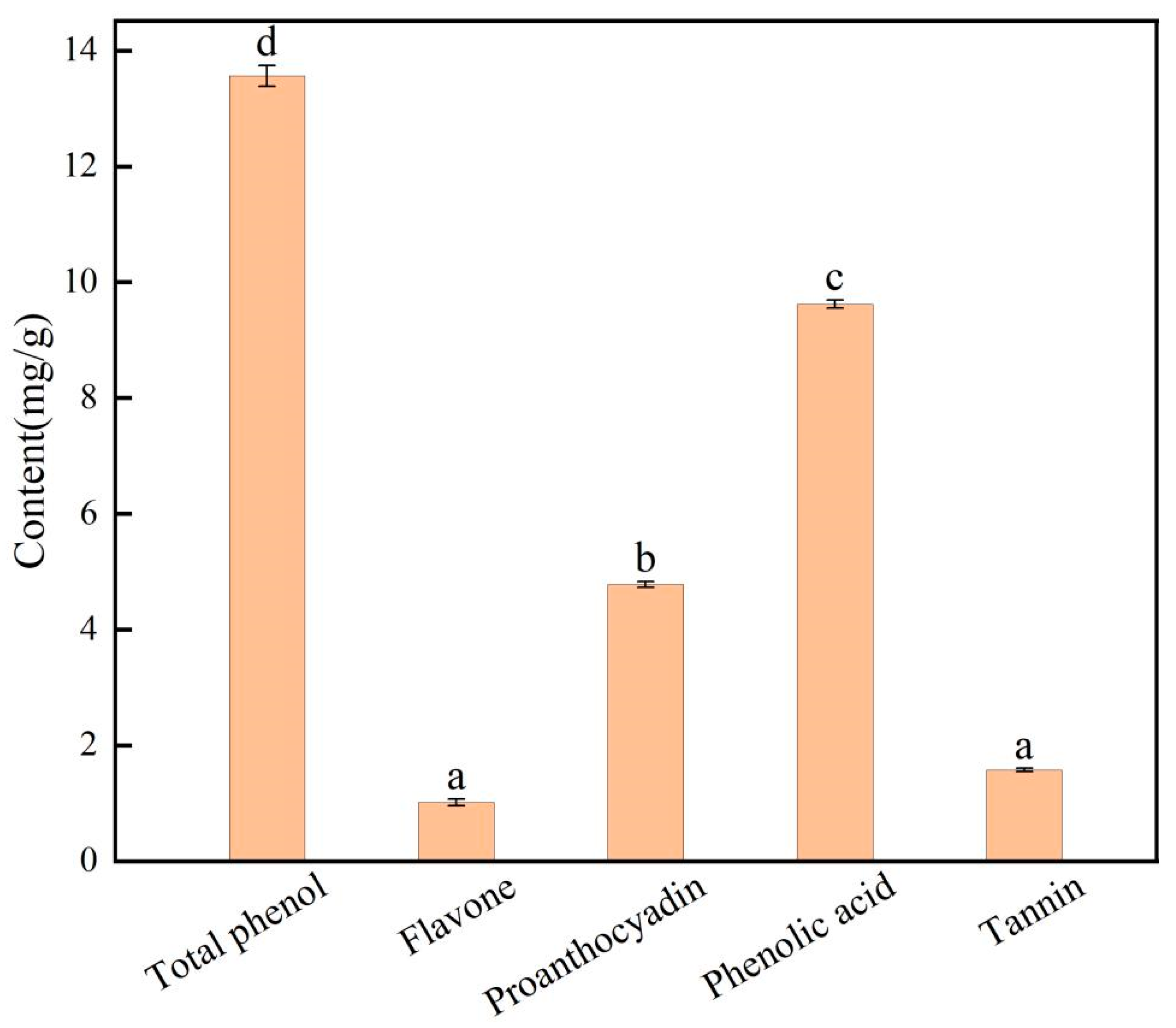
2.3. Determination of Phenols
Phenol Content of E. angustifolia L.
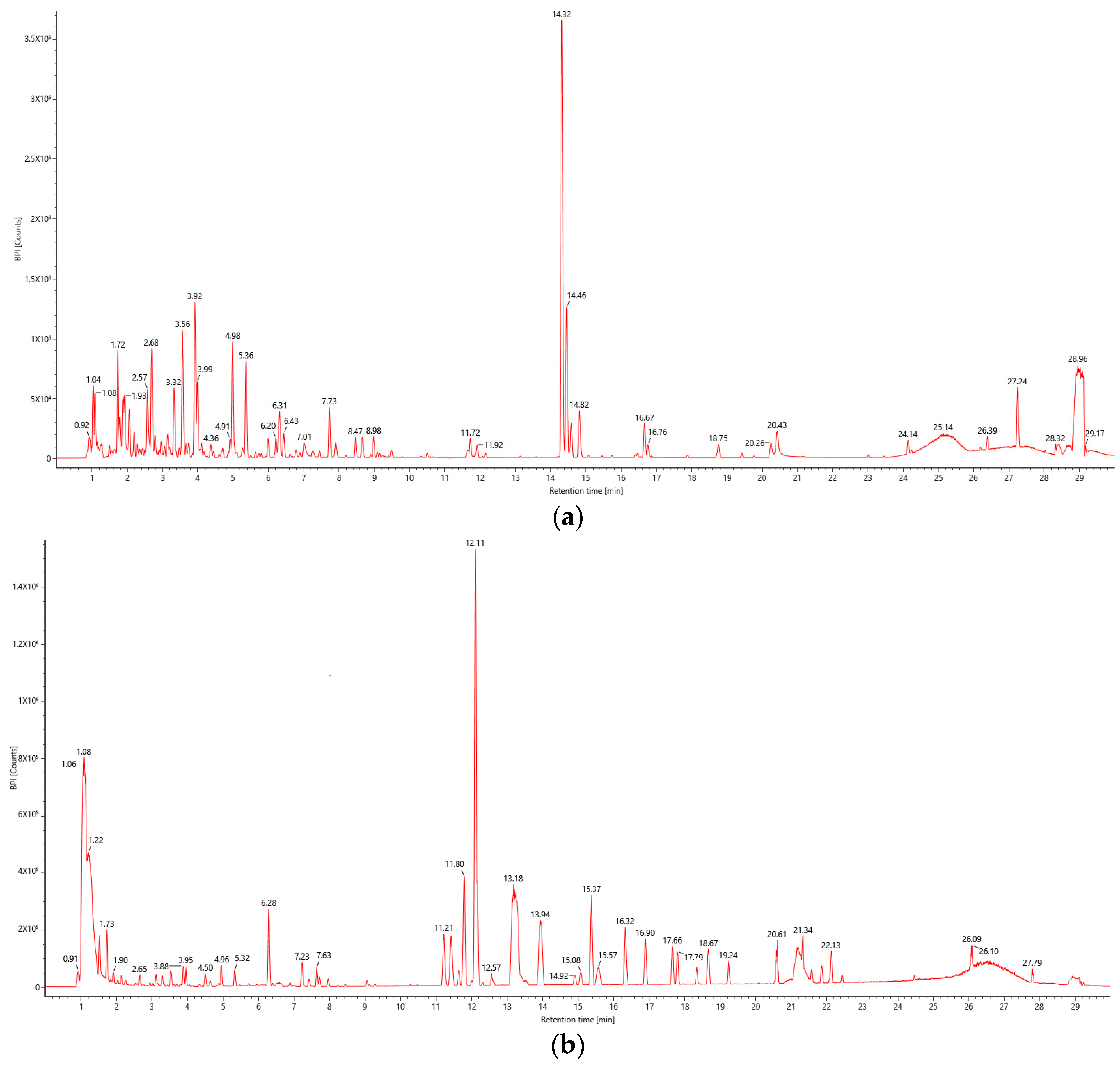
2.4. Qualitative Analysis of BPs of E. angustifolia L.
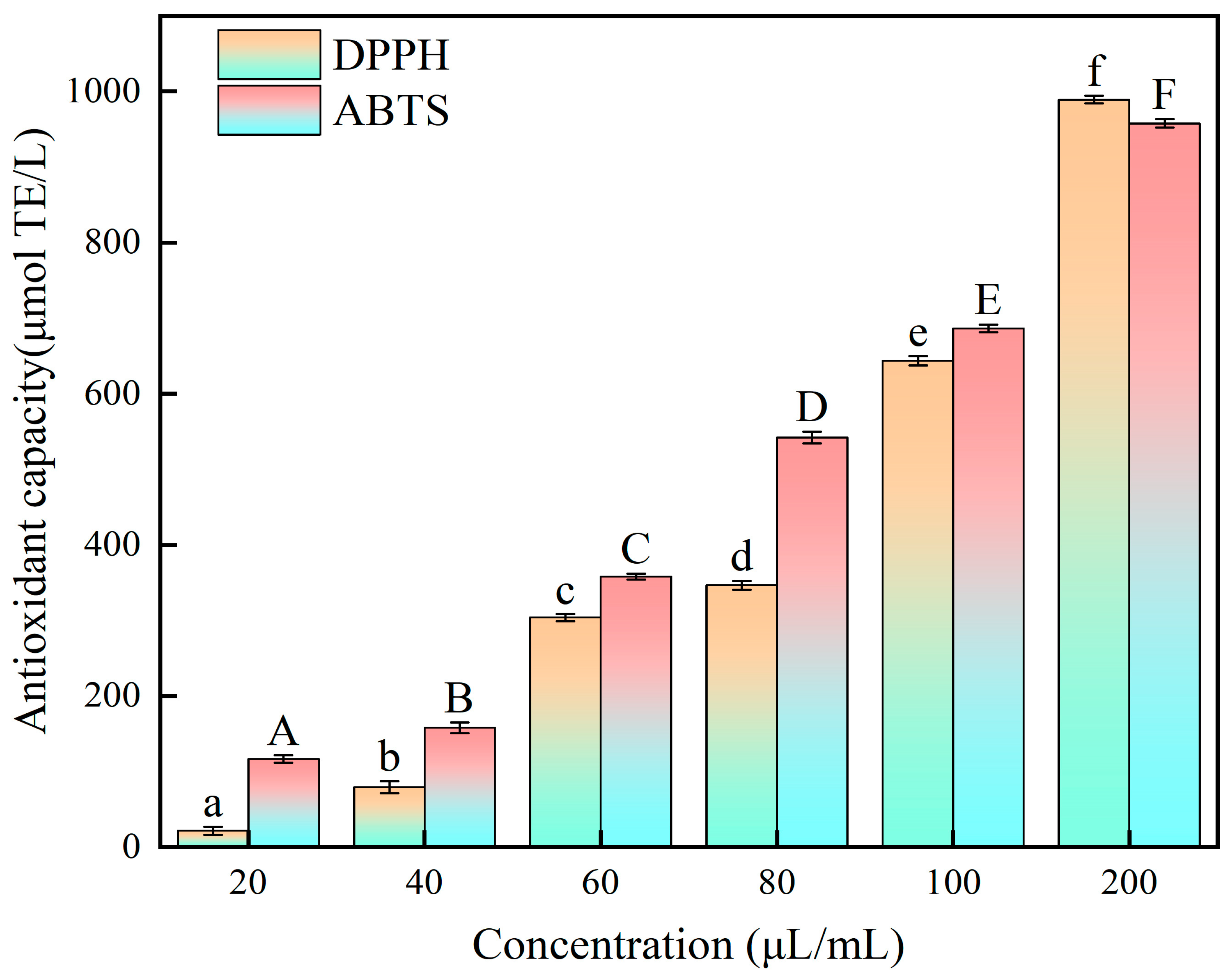
2.5. Antioxidant Analysis of E. angustifolia L. BP Extracts In Vitro
3. Materials and Methods
3.1. Materials and Reagents
3.2. Test Instruments
3.3. Removal of FP from E. angustifolia L.
3.4. Extraction of BP from E. angustifolia L.
3.5. Design of Single-Factor Experiment for BP Extraction of E. angustifolia L.
3.6. Experimental Design of Response Surface
3.7. Determination of Phenolic Compound Content
3.7.1. Determination of Total Phenol
3.7.2. Flavone Determination
3.7.3. Determination of Proanthocyanidins
3.7.4. Determination of Phenolic Acid
3.7.5. Determination of Total Tannins
3.8. Qualitative Analysis of BP in E. angustifolia L.
3.9. Study on Antioxidant Activity In Vitro of BP Extract of E. angustifolia L.
3.9.1. DPPH Radical Scavenging Assay
3.9.2. Determination of ABTS Free Radical Scavenging Ability
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.X.; Chen, X.Y.; Liu, W.; Cui, H.; Yuan, T. Triterpenoid saponin and lignan glycosides from the traditional medicine Elaeagnus angustifolia flowers and their cytotoxic activities. Molecules 2020, 25, 462. [Google Scholar] [CrossRef] [PubMed]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shahlari, M.; Sohraby, M.; Shahlari, N.; Hamidpour, R. Russian olive (Elaeagnus angustifolia L.): From a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J. Tradit. Complement. Med. 2017, 7, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, Y.; Zhu, Y.; Zeng, X.; Liang, X.; Xie, J.; Wu, F. Ethnobotany of wild edible plants in multiethnic areas of the gansu–ningxia–inner mongolia junction zone. J. Ethnobiol. Ethnomed. 2022, 18, 53. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fang, S.J.; Zhang, Z.; Qi, H.M.; Bo, X.M.; Wei, J.; Wu, J. Optimization of extraction process of polysaccharide from Elaeagnus angustifolia flower and its proliferation effect on probiotics. Food Ind. Sci. Technol. 2023, 44, 134–142. [Google Scholar] [CrossRef]
- Jabeen, A.; Sharma, A.; Gupta, I.; Kheraldine, H.; Vranic, S.; Al Moustafa, A.-E.; Al Farsi, H.F. Elaeagnus angustifolia plant extract inhibits epithelialmesenchymal transition and induces apoptosis via HER2 inactivation and JNK pathway in HER2-positive breast cancer cells. Molecules 2020, 25, 4240. [Google Scholar] [CrossRef]
- Yang, S.; Liu, T.; Zhang, H.X.; Li, H.Y.; Zhang, L. Growth performance and physiological characteristics of Elaeagnus angustifolia seedlings under salt stress. J. Fujian For. Univ. 2014, 34, 64–70. [Google Scholar] [CrossRef]
- Kang, J.; Cui, J.Y. Comparison of nutritional components of Elaeagnus angustifolia in different regions of Xinjiang. Food Res. Dev. 2008, 29, 133–134. [Google Scholar]
- Du, H.; Chen, J.; Tian, S.; Gu, H.; Li, N.; Sun, Y.; Ru, J.; Wang, J. Extraction optimization, preliminary characterization and immunological activities in vitro of polysaccharides from Elaeagnus angustifolia L. pulp. Carbohydr. Polym. 2016, 151, 348–357. [Google Scholar] [CrossRef]
- Abela, R.; Ji, B.; Zhou, F. Research progress on nutrition, active ingredients and pharmacological effects of Elaeagnus angustifolia fruit. Food Ind. Sci. Technol. 2018, 39, 343–352. [Google Scholar] [CrossRef]
- Wang, T.; Lin, X.N.; Liu, N.; Liu, Y.G. The main active substances of Elaeagnus angustifolia and their application in food. Chin. Fruit Veg. 2023, 43, 42–48. [Google Scholar] [CrossRef]
- Olagaray, K.E.; Bradford, B.J. Plant flavonoids to improve productivity of ruminants—A review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Weng, W.; Zhang, Q.M.; Li, S.K.; Zhu, J.F. Research progress on antiviral effect and mechanism of plant polyphenols. Chin. J. Tradit. Chin. Med. 2020, 35, 6236–6240. [Google Scholar]
- Liu, X.; Ma, Q.; Sun, Y.; Ju, W.; Kumrungsee, T.; Zhou, Z.; Wang, L.; Cao, R. Effects of processings and complexation on solubility, antioxidant and antibacterial properties of buckwheat polyphenols. Food Bioprocess Technol. 2024, 17, 1583–1595. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Yan, C.Z.; Ye, F.Y.; Zhao, G.H. Research progress of polyphenols in food. Food Sci. 2015, 36, 249–254. [Google Scholar] [CrossRef]
- Zhang, M.W. Research progress on phenolic compounds and antioxidant activity of whole grains. Food Process. 2016, 41, 28–36. [Google Scholar]
- Zhou, Z. The distribution of phenolic acids in rice. Food Chem. 2004, 87, 401–406. [Google Scholar] [CrossRef]
- Mora-Rochin, S.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O.; Sánchez-Peña, P.; Reyes-Moreno, C.; Milán-Carrillo, J. Phenolic content and antioxidant activity of tortillas produced from pigmented maize processed by conventional nixtamalization or extrusion cooking. J. Cereal Sci. 2010, 52, 502–508. [Google Scholar] [CrossRef]
- Boateng, I.D. Mechanisms, capabilities, limitations, and economic stability outlook for extracting phenolics from agro-byproducts using emerging thermal extraction technologies and their combinative effects. Food Bioprocess Technol. 2024, 17, 1109–1140. [Google Scholar] [CrossRef]
- Lv, J.J.; Li, L.; Zhang, N. Research progress on the release and functional activity of bound polyphenols in plant foods. Food Ind. Sci. Technol. 2025, 46, 404–413. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Fabiano-Tixier, A.; Nutrizio, M.; Režek Jambrak, A.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Raes, K.; Van Camp, J. Combined alkaline hydrolysis and ultrasound-assisted extraction for the release of nonextractable phenolics from cauliflower (Brassica oleracea var. botrytis) waste. J. Agric. Food Chem. 2014, 62, 3371–3376. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Ma, H. Effects of pretreatment and type of hydrolysis on the composition, antioxidant potential and hepg2 cytotoxicity of bound polyphenols from tartary buckwheat (Fagopyrum tataricum L. Gaerth) hulls. Food Res. Int. 2021, 142, 110187. [Google Scholar] [CrossRef]
- Zhao, K.H.; Zhang, R.F.; Su, D.X.; Dong, L.H.; Liu, L.; Wei, Z.C.; Zhang, M.W. Research progress on phenolic compounds and antioxidant activity of whole grains. Chin. J. Food Sci. 2017, 17, 183–196. [Google Scholar] [CrossRef]
- Li, W.T. Study on the Composition, Gastrointestinal Bioaccessibility and Antioxidant Activity of Different Bound Polyphenols in Six Kinds of Legumes. Master’s Thesis, Nanchang University, Nanchang, China, 2018. [Google Scholar]
- Anica, D.; Shilpa, S.; Tulasi, S. Progress in Ameliorating Beneficial Characteristics of Microbial Cellulases by Genetic Engineering Approaches for Cellulose Saccharification. Front. Microbiol. 2020, 11, 1387. [Google Scholar]
- Ding, Y.; Tang, S.Y.; Hou, H.X.; Dong, H.Z.; Zhang, J.L. Effects of hydrolysis conditions on the release of phenolic acids in wheat bran. Chin. Food Nutr. 2012, 18, 52–58. [Google Scholar]
- Mu, T.H.; Ye, J.; Yang, M.J.; Miao, J.L.; Wang, X.J.; Zhou, X.T.; Zhang, W.W. Study on extraction process optimization and storage stability of pomegranate peel polyphenols. Food Ind. Sci. Technol. 2021, 42, 142–146. [Google Scholar] [CrossRef]
- Boateng, I.D.; Mustapha, A.; Daubert, C.R.; Kuehnel, L.; Kumar, R.; Flint-Garcia, S.; Agliata, J.; Wan, C.; Somavat, P. Novel two-pot microwave extraction of purple corn pericarp’s phenolics and evaluation of the polyphenol-rich extract’s product quality, bioactivities, and structural properties. Food Bioprocess Technol. 2023, 16, 2668–2691. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.Q.; Li, X.F. Optimization of ultrasonic-assisted extraction process and antioxidant activity of polyphenols from bayberry core-shell. Packag. Food Mach. 2021, 39, 8–12, 83. [Google Scholar] [CrossRef]
- Liu, R.Q.; Zong, N.Y.; Yang, S.M.; Peng, R.; Li, R.Y. Optimization of ultrasonic-assisted enzymatic extraction of polyphenols from Zizyphus mauritiana and its antioxidant activity. Chem. Eng. 2024, 38, 1–6+11. [Google Scholar] [CrossRef]
- Jiao, P.; He, X.; Ma, S.; Wang, S.; Niu, Q. Ultrasonic-Assisted Extraction of Antioxidants from Perilla frutescens Leaves Based on Tailor-Made Deep Eutectic Solvents: Optimization and Antioxidant Activity. Molecules 2023, 28, 7554. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Tao, H.L.; Cai, L.J.; Wei, S.S.; Yuan, Y.M.; Yang, W.J.; Hu, Q.H. Response surface test optimized the ultrasonic-assisted extraction process of polyphenols from Moringa oleifera leaves and its antioxidant activity. Food Sci. 2016, 37, 24–30. [Google Scholar] [CrossRef]
- Gao, Y.; Feng, Y.; Lv, Z.; Su, J.Y.; Feng, Y. Enzymatic hydrolysis to release sweet potato bound phenols. Food Res. Dev. 2022, 43, 118–125. [Google Scholar] [CrossRef]
- Hemwimol, S.; Pavasant, P.; Shotipruk, A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrason. Sonochem. 2006, 13, 543–548. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, S.; Fu, L. Study on enzymatic extraction of pomegranate peel polyphenols. Chin. J. Food Sci. 2012, 12, 56–65. [Google Scholar] [CrossRef]
- Hatiguli, A.; Shi, L.L.; Jia, X.Y.; Shi, M.H. Optimization of extraction process of total flavonoids and polyphenols from Elaeagnus moorcroftii based on response surface methodology. Chin. Folk. Med. 2025, 34, 34–43. [Google Scholar]
- Honda, S.; Ishida, R.; Hidaka, K.; Masuda, T. Stability of Polyphenols under Alkaline Conditions and the Formation of a Xanthine Oxidase Inhibitor from Gallic Acid in a Solution at pH 7.4. Food Sci. Technol. Res. 2019, 25, 123–129. [Google Scholar] [CrossRef]
- Arigò, A.; Česla, P.; Šilarová, P.; Calabrò, M.L.; Česlová, L. Development of extraction method for characterization of free and bonded polyphenols in barley (Hordeum vulgare L.) Grown in czech republic using liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 245, 829–837. [Google Scholar] [CrossRef]
- Wu, N.; Zhu, B.S.; Zhang, X.; Dong, F.Y.; Liu, J.; Cheng, F.; Deng, Z.Y.; Ban, Y. Changes of active components and antioxidant capacity of Phyllanthus emblica fruit and ethanol extract during simulated digestion in vitro. Food Ind. Technol. 2023, 44, 349–358. [Google Scholar] [CrossRef]
- Li, D.; Wan Ibadullah, W.Z.; Shukri, R.; Duan, Q.; Gu, Y.; Mustapha, N.A. The effects of different extraction methods on the yield, microstructure, and antioxidant activity of polysaccharides from Rhodomyrtus tomentosa berry. Food Bioprocess Technol. 2024, 18, 3719–3731. [Google Scholar] [CrossRef]
- Xu, X.; Wang, D.; Li, Y.; Deyrup, S.T.; Zhang, H. Plant-derived lignans as potential antiviral agents: A systematic review. Phytochem. Rev. 2021, 21, 239–289. [Google Scholar] [CrossRef] [PubMed]
- Rong-Jyh, L.; Chuan-Min, Y.; Tzung-Han, C.; Feng-Yu, C.; Guey-Horng, W.; Ya-Ping, T.; Lin, W.; Ting-Wei, H.; Hui-Chuan, W.; Leong-Perng, C.; et al. Antioxidant, anti-adipocyte differentiation, antitumor activity and anthelmintic activities against anisakis simplex and Hymenolepis nana of yakuchinone a from Alpinia oxyphylla. BMC Complement. Altern. Med. 2013, 13, 237. [Google Scholar]
- Baek, J.Y.; Song, J.H.; Choi, S.Y. Protective effect of furosin isolated from Euphorbia helioscopia against glutamate-induced ht22 cell death. J. Korean Med. 2018, 39, 35–43. [Google Scholar] [CrossRef]
- Yao, J.; Ge, C.; Duan, D.; Zhang, B.; Cui, X.; Peng, S.; Liu, Y.; Fang, J. Activation of the phase ii enzymes for neuroprotection by ginger active constituent 6-dehydrogingerdione in pc12 cells. J. Agric. Food. Chem. 2014, 62, 5507–5518. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Ghany, T.M.A. In vitro repress of breast cancer by bio-product of edible Pleurotus ostreatus loaded with chitosan nanoparticles. Appl. Biol. Chem. 2023, 66, 33. [Google Scholar] [CrossRef]
- Liang, H.M.; Ren, J.B.; Li, Y.K.; Gao, D.Y.; Hu, W.X. Improved DPPH and ABTS free radical methods were used to evaluate the antioxidant capacity of different grape seed oils. Chin. J. Grain Oil 2018, 33, 85–91. [Google Scholar] [CrossRef]
- López-Téllez, J.M.; Del Pilar Cañizares-Macías, M. Evaluation of tomato (Lycopersicon esculentum p. Mill.) By-product extracts obtained by different extraction methods as exploitation strategy of high-value polyphenols. Food Bioprocess Technol. 2024, 17, 3217–3235. [Google Scholar] [CrossRef]
- Qi, W.S.; Rong, L.; Tao, J.Z.; Ying, W.; Jin, T.; Hua, T.S. Evaluation of antioxidant active ingredients of spikenard essential oil by ultra-fast gas chromatography electronic nose and radical scavenging mechanism. Ind. Crops Prod. 2020, 151, 112489. [Google Scholar]
- Yu, L.; Beta, T. Identification and Antioxidant Properties of Phenolic Compounds during Production of Bread from Purple Wheat Grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef] [PubMed]
- Melanie, P.; Sandra, K.; Thomas, H.; Ute, S.; Oliver, M.; Peter, E. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.L.; Wang, B.; Liu, C.; Zubaidan, A.; Gao, C.Y. Antioxidant activity and digestive enzyme inhibition of polyphenol extracts from Allium mongolicum Regel. Chin. J. Food 2024, 24, 164–177. [Google Scholar]
- Qin, Y.Z.; He, Y.; Feng, Y.T.; Fan, F.Y. Optimization of ultrasonic-assisted alkaline extraction of bound polyphenols from Rosa sterilis and analysis of its antioxidant activity. Food Ind. Sci. Technol. 2024, 45, 1–9. [Google Scholar]
- Xiao, Y.T.; Yan, C.; Wang, F.; Wang, R.H.; Zhu, W. Comparative Study on Polyphenol Components and Antioxidant Activity of Indica and Japonica Rice Bran. Chin. J. Grain Oil 2023, 38, 76–81. [Google Scholar]
- Ordóñez, R.M.; Cardozo, M.L.; Zampini, I.C.; Isla, M.I. Evaluation of antioxidant activity and genotoxicity of alcoholic and aqueous beverages and pomace derived from ripe fruits of Cyphomandra betacea sendt. J. Agric. Food Chem. 2010, 58, 331–337. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, W.J.; Zhang, Y.T.; Buerhan, J.; Li, Y.P.; Ding, J.B.; Li, R.R.; Yang, J. Comparison of alkali hydrolysis and acid hydrolysis extraction process of bound polyphenols from Lycii Fructus and product analysis. Res. Dev. Nat. Prod. 2024, 36, 632–643. [Google Scholar] [CrossRef]
- Hoyos, M.N.; Huertas, L.F.V.; Vargas, J.D.C.; Aguilar, V.L.; Corella, D.A.; Baudrit, J.R.V.; Esquivel, L.G.R.; Kopper, A.S.; Navarro, A.M.; Sibaja, A.M.A. HRMS Characterization and Antioxidant Evaluation of Costa Rican Spent Coffee Grounds as a Source of Bioactive Polyphenolic Extracts. Foods 2025, 14, 448. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, L.; Li, L.; Lv, J.J.; Dong, Y.Y. Optimization of ultrasonic-assisted extraction process of polyphenols from Taraxacum mongolicum root and its antioxidant and hypoglycemic activities in vitro. Food Ind. Technol. 2024, 45, 200–208. [Google Scholar] [CrossRef]
- Zhao, D.; Dai, Q.; Zhang, Y.; Shen, H.; Mao, Y.; Zhou, X.; Chen, X.; Pang, H.; Wang, H.; Liu, L. Identification of Miscanthus floridulus as a promising anti-alzheimer’s disease and antidiabetic agent through bioactivity evaluations and chemical composition analyses. Arab. J. Chem. 2024, 17, 106037. [Google Scholar] [CrossRef]
- Lv, Q.; Luo, F.; Zhao, X.; Liu, Y.; Hu, G.; Sun, C.; Li, X.; Chen, K. Identification of proanthocyanidins from litchi (Litchi chinensis sonn.) Pulp by lc-esi-q-tof-ms and their antioxidant activity. PLoS ONE 2015, 10, 120480. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A.; Joel Karunakaran, R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from india. Lwt—Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.P.; Li, J.L.; Jin, Y.P. Study on the dynamic changes of total flavonoids, total flavonols and total phenolic acids in the leaves of Schisandra chinensis at different harvest times. Shizhen Tradit. Chin. Med. 2021, 32, 2500–2502. [Google Scholar]
- Xin, Q.Y.; Min, X.H.; Tian, Z.T.; Xue, L.; Ying, H.; Da, W.H.; Mei, G.X.; Zhi, Y.W. Ultra-high performance liquid chromatography/ion mobility-quadrupole time-of-flight mass spectrometry and database-driven automatic peak annotation for the rapid profiling and characterization of the multicomponents from Stephaniae tetrandrae radix (Fang-Ji). World J. Tradit. Chin. Med. 2021, 7, 120–134. [Google Scholar]
- Li, J.F.; Diao, J.J.; Cheng, Z.H.; Gao, X.; Chang, J.M. Analysis of chemical constituents of grape seed extract and determination of multi-component content based on ultra-high performance liquid chromatography-tandem mass spectrometry. Food Ferment. Ind. 2024, 50, 303–312. [Google Scholar] [CrossRef]
- Sathasivam, R.; Kim, N.S.; Lim, J.; Yang, S.H.; Kim, B.; Park, H.W.; Kim, J.K.; Park, S.U. Comprehensive analysis of primary and secondary metabolites and antioxidant activities provides insights into metabolic profiling of different organs of Pimpinella brachycarpa nakai. Food Chem. 2025, 468, 142394. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chem. 2021, 339, 127879. [Google Scholar] [CrossRef]

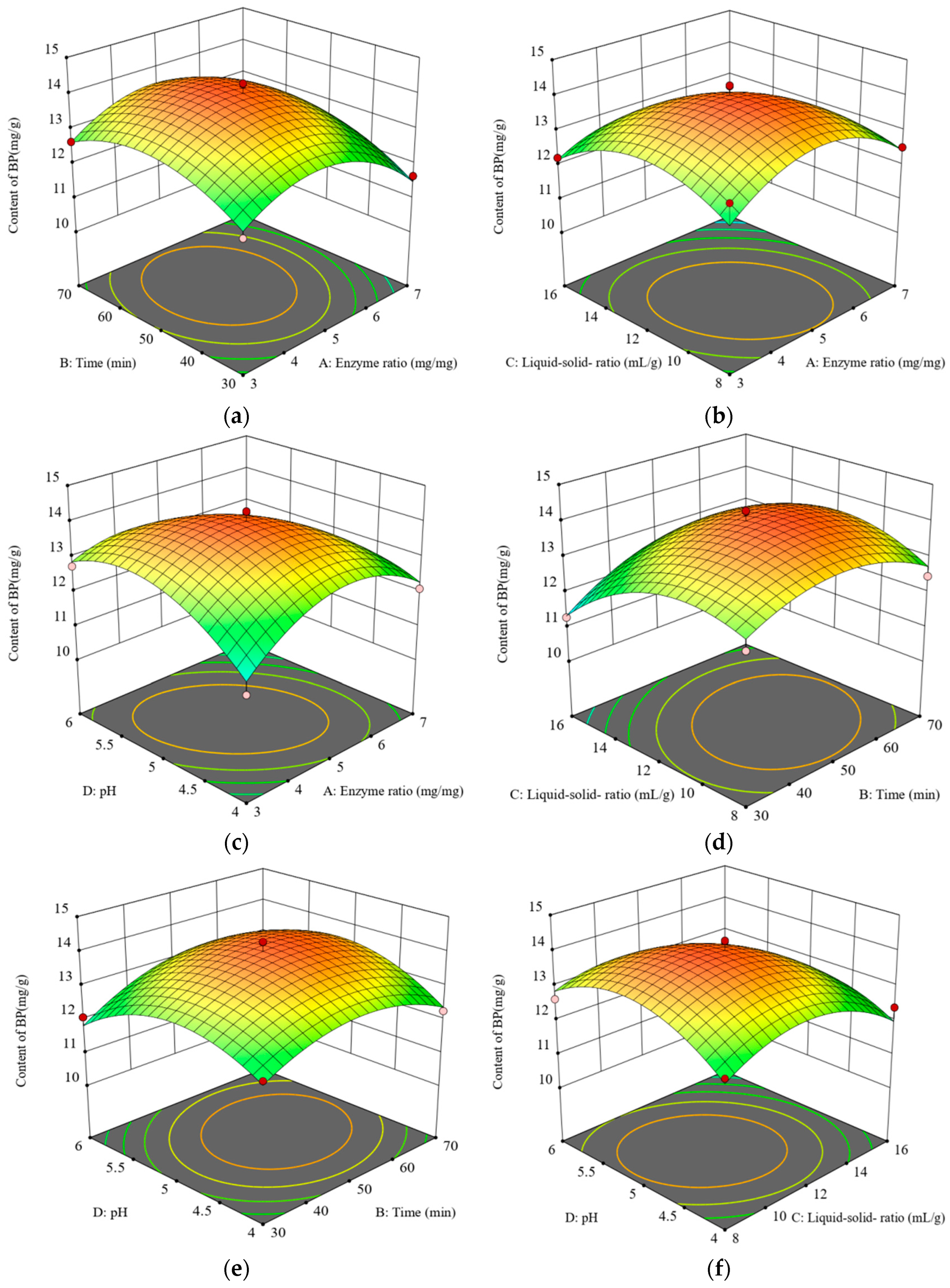
| No. | A | B | C | D | Y (mg/g) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 0 | 12.598 |
| 2 | 0 | 1 | 0 | 1 | 12.850 |
| 3 | 0 | −1 | −1 | 0 | 12.499 |
| 4 | 0 | 1 | 0 | −1 | 12.268 |
| 5 | 0 | 0 | 0 | 0 | 14.270 |
| 6 | −1 | 0 | −1 | 0 | 13.000 |
| 7 | −1 | 0 | 1 | 0 | 12.224 |
| 8 | 1 | 0 | 1 | 0 | 10.544 |
| 9 | 1 | 0 | 0 | −1 | 12.100 |
| 10 | −1 | 0 | 0 | 1 | 12.744 |
| 11 | 1 | 0 | 0 | 1 | 11.137 |
| 12 | −1 | 1 | 0 | 0 | 12.645 |
| 13 | 0 | −1 | 0 | −1 | 12.356 |
| 14 | 0 | −1 | 1 | 0 | 11.287 |
| 15 | 0 | 0 | 0 | 0 | 13.677 |
| 16 | 0 | 0 | −1 | −1 | 12.477 |
| 17 | 0 | 0 | 0 | 0 | 13.970 |
| 18 | 1 | 0 | −1 | 0 | 12.532 |
| 19 | 0 | 0 | 1 | 1 | 11.551 |
| 20 | 0 | 1 | −1 | 0 | 12.470 |
| 21 | −1 | −1 | 0 | 0 | 12.063 |
| 22 | −1 | 0 | 0 | −1 | 11.313 |
| 23 | 0 | 0 | 0 | 0 | 13.853 |
| 24 | 0 | −1 | 0 | 1 | 12.082 |
| 25 | 0 | 0 | 0 | 0 | 14.138 |
| 26 | 1 | −1 | 0 | 0 | 11.657 |
| 27 | 0 | 1 | 1 | 0 | 12.492 |
| 28 | 0 | 0 | 1 | −1 | 12.389 |
| 29 | 0 | 0 | −1 | 1 | 12.627 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 21.36 | 14 | 1.53 | 12.68 | <0.0001 | ** |
| A | 0.9759 | 1 | 0.9759 | 8.11 | 0.0129 | * |
| B | 0.951 | 1 | 0.951 | 7.9 | 0.0139 | * |
| C | 2.18 | 1 | 2.18 | 18.13 | 0.0008 | ** |
| D | 0.0006 | 1 | 0.0006 | 0.0053 | 0.9428 | |
| AB | 0.0322 | 1 | 0.0322 | 0.2673 | 0.6132 | |
| AC | 0.3669 | 1 | 0.3669 | 3.05 | 0.1026 | |
| AD | 1.43 | 1 | 1.43 | 11.91 | 0.0039 | ** |
| BC | 0.3803 | 1 | 0.3803 | 3.16 | 0.0971 | |
| BC | 0.1834 | 1 | 0.1834 | 1.52 | 0.2373 | |
| CD | 0.2441 | 1 | 0.2441 | 2.03 | 0.1762 | |
| A2 | 7.83 | 1 | 7.83 | 65.11 | <0.0001 | ** |
| B2 | 3.75 | 1 | 3.75 | 31.17 | <0.0001 | ** |
| C2 | 5.34 | 1 | 5.34 | 44.37 | <0.0001 | ** |
| D2 | 5.63 | 1 | 5.63 | 46.82 | <0.0001 | ** |
| Residual | 1.68 | 14 | 0.1203 | |||
| Misfit term | 1.4 | 10 | 0.1402 | 1.99 | 0.2654 | |
| Pure error | 0.2822 | 4 | 0.0706 | |||
| Summation | 23.04 | 28 |
| Regression Equation | Correlation Coefficient | |
|---|---|---|
| Total phenols | y = 1.8223x + 0.0579 | 0.9955 |
| Flavone | y = 1.1897x + 0.0007 | 0.9990 |
| Proanthocyanidins | y = 0.572x − 0.0006 | 0.9961 |
| Phenolic acid | y = 1.0768x + 0.0901 | 0.9976 |
| Tannin | y = 1.458x + 0.024 | 0.9984 |
| ID | RT (min) | Chemical Formula | First-Order Mass Spectrometry | Secondary Fragments (m/z) | Component Name | Type | |
|---|---|---|---|---|---|---|---|
| Neutral Mass (m/z) | Observed (m/z) | ||||||
| 1 | 2.66 | C9H8O2 | 148.0523 | 147.045 | 103.0546 | 3,4-Dihydrocoumarin | Coumarin |
| 2 | 2.76 | C16H14O3 | 226.0952 | 225.0879 | 147.0448 | 4′-Methylpinosylvin | Lignin |
| 3 | 3.46 | 482.1894 | 527.1876 | 271.1294 331.1504 395.1465 | 2-Butenoic acid, 2-methyl-, (6R,7R,8R,14aS)-5,6,7,8-tetrahydro-1,2-dimethoxy-6,7-dimethyl-3-oxo-3H,14H-benzo [1,8] cycloocta [1,2,3-cd] [1,3] dioxolo [4,5-g] benzofuran-8-yl ester, (2Z)-rel-(-) | Lignin | |
| 4 | 3.62 | C17H16O2 | 252.1106 | 251.1033 | 221.0924 | Effusol | Polyphenol |
| 5 | 4.06 | C19H18O3 | 294.1216 | 293.1143 | 275.1032 | 1,7-Bis (4-hydroxyphenyl) hepta-4,6-dien-3-one | Flavone |
| 6 | 4.28 | C25H30O8 | 358.1732 | 403.1714 | 241.1191 | Kadsurenin C | Lignin |
| 7 | 4.98 | C29H38O9 | 530.2535 | 531.2607 | 349.0756 | Angeloylgomisin Q | Lignin |
| 8 | 4.67 | C20H24O3 | 312.1771 | 335.1663 | 119.0494 | Yakuchinone A | Polyphenol |
| 9 | 6.18 | C9H10O3 | 166.0626 | 165.0553 | 120.0214 121.0289 | Paeonol | Polyphenol |
| 10 | 6.23 | 374.1686 | 373.1613 | 357.0982 | Cubeb oleoresin | Lignin | |
| 11 | 7.23 | C30H34O8 | 522.2272 | 523.2345 | 221.1184 | Benzoylgomisin H | Polyphenol |
| 12 | 9.09 | C18H18O2 | 266.1297 | 284.1635 | 221.0928 | Juncusol | Polyphenol |
| 13 | 11.22 | 446.1216 | 445.1143 | 121.0292 313.0719 401.0882 | 3′-Methoxydaidzein | Flavone | |
| 14 | 12.11 | C19H14O7 | 356.09 | 401.0882 | 121.0293 313.0720 | 3-(1,3-Benzodioxol-5-ylmethyl)-3,4-dihydro-5,7-dihydroxy-8-methyl-4-oxo-2H-1-benzopyran-6-carboxaldehyde | Flavone |
| 15 | 13.15 | C16H18O5 | 290.113 | 289.1057 | 207.1029 | 5-O-Methylvisamminol | Flavone |
| 16 | 13.32 | C10H10O4 | 194.0615 | 239.0597 | 116.9948 | Kakoul | Coumarin |
| 17 | 13.37 | 342.0738 | 341.0665 | 109.0289 177.0556 | 3,8-Dihydroxy-4,10-dimethoxy-7-oxo-[2] benzopyrano [2] [1]benzopyran-7-(5H)-one | Polyphenol | |
| 18 | 13.71 | C14H14O6 | 250.0841 | 249.0767 | 132.9904 165.0165 177.0921 | 3-Acetyl-3,4-dihydro-5,6-dimethoxy-2 (1) H-benzopyranone | Coumarin |
| 19 | 13.95 | C32H28O11 | 600.1668 | 599.1595 | 221.1184 | Neosappanone A | Polyphenol |
| 20 | 15.54 | C15H14O6 | 594.1373 | 593.13 | 549.1037 | Epiafzelechin-(2β-O-7,4β-8)-ent-Epicatechin | Flavone |
| 21 | 15.6 | C30H26O13 | 594.1403 | 595.1476 | 112.9853 | Tiliroside | Flavone |
| 22 | 15.69 | C18H16O7 | 328.0951 | 327.0878 | 121.0291 | 5-Hydroxy-3′,4′,7-trimethoxyflavone | Flavone |
| 23 | 16.38 | C27H22O19 | 650.0752 | 649.0679 | 426.9899 | Furosin | Tannin |
| 24 | 17.47 | C20H20O5 | 340.1305 | 339.1232 | 277.0829 291.0989 | 6,7-dimethoxy-2-(2-(4-methoxyphenyl)ethyl)chromone | Flavone |
| 25 | 17.8 | C17H22O4 | 292.1678 | 291.1605 | 245.1545 | 6-Dehydrogingerdione | Polyphenol |
| 26 | 20.6 | C14H22O | 206.1671 | 205.1599 | 189.1281 | 2-Octylphenol | Polyphenol |
| 27 | 26.08 | C21H26O10 | 438.1507 | 437.1434 | 183.0118 195.0626 | Sec-O-Glucosylhamaudol | Polyphenol |
| Factor | Levels | |||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Enzyme ratio (mg/mg) | A | 3:1 | 5:1 | 7:1 |
| Time(min) | B | 30 | 50 | 70 |
| Liquid-to-solid ratio(mL/g) | C | 8 | 12 | 16 |
| pH | D | 4 | 5 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Li, L.; Liang, Z.; Wu, W.; Zhang, N.; Jia, Q. Extraction of Bound Polyphenols from Elaeagnus angustifolia L. by Ultrasonic-Assisted Enzymatic Hydrolysis and Evaluation of Its Antioxidant Activity In Vitro. Foods 2025, 14, 1567. https://doi.org/10.3390/foods14091567
Lv J, Li L, Liang Z, Wu W, Zhang N, Jia Q. Extraction of Bound Polyphenols from Elaeagnus angustifolia L. by Ultrasonic-Assisted Enzymatic Hydrolysis and Evaluation of Its Antioxidant Activity In Vitro. Foods. 2025; 14(9):1567. https://doi.org/10.3390/foods14091567
Chicago/Turabian StyleLv, Jingjing, Lu Li, Zilong Liang, Wenyue Wu, Na Zhang, and Qinghua Jia. 2025. "Extraction of Bound Polyphenols from Elaeagnus angustifolia L. by Ultrasonic-Assisted Enzymatic Hydrolysis and Evaluation of Its Antioxidant Activity In Vitro" Foods 14, no. 9: 1567. https://doi.org/10.3390/foods14091567
APA StyleLv, J., Li, L., Liang, Z., Wu, W., Zhang, N., & Jia, Q. (2025). Extraction of Bound Polyphenols from Elaeagnus angustifolia L. by Ultrasonic-Assisted Enzymatic Hydrolysis and Evaluation of Its Antioxidant Activity In Vitro. Foods, 14(9), 1567. https://doi.org/10.3390/foods14091567





