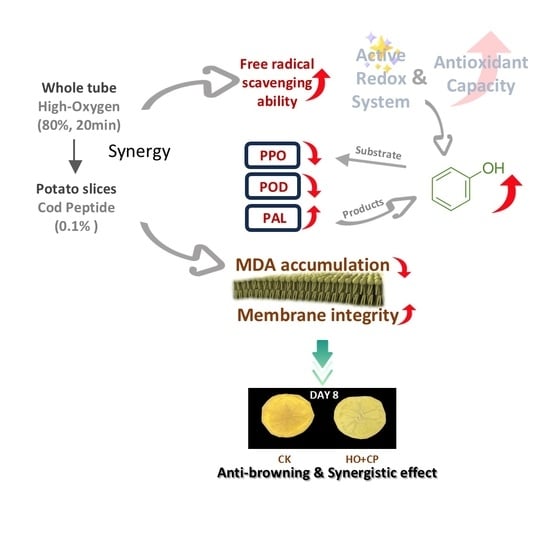
The Super Anti-Browning Effect of High-Oxygen Pretreatment Combined with Cod Peptides on Fresh-Cut Potatoes During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Sample Preparation
2.2. The Color of Fresh-Cut Potatoes
2.3. The Activity of PPO
2.4. The Activity of POD
2.5. The Activity of CAT
2.6. The Activity of SOD
2.7. The Activity of PAL
2.8. Total Phenolic Content
2.9. Membrane Permeability
2.10. Malondialdehyde Content
2.11. Antioxidant Capacity
2.12. Statistical Analysis
3. Results and Discussion
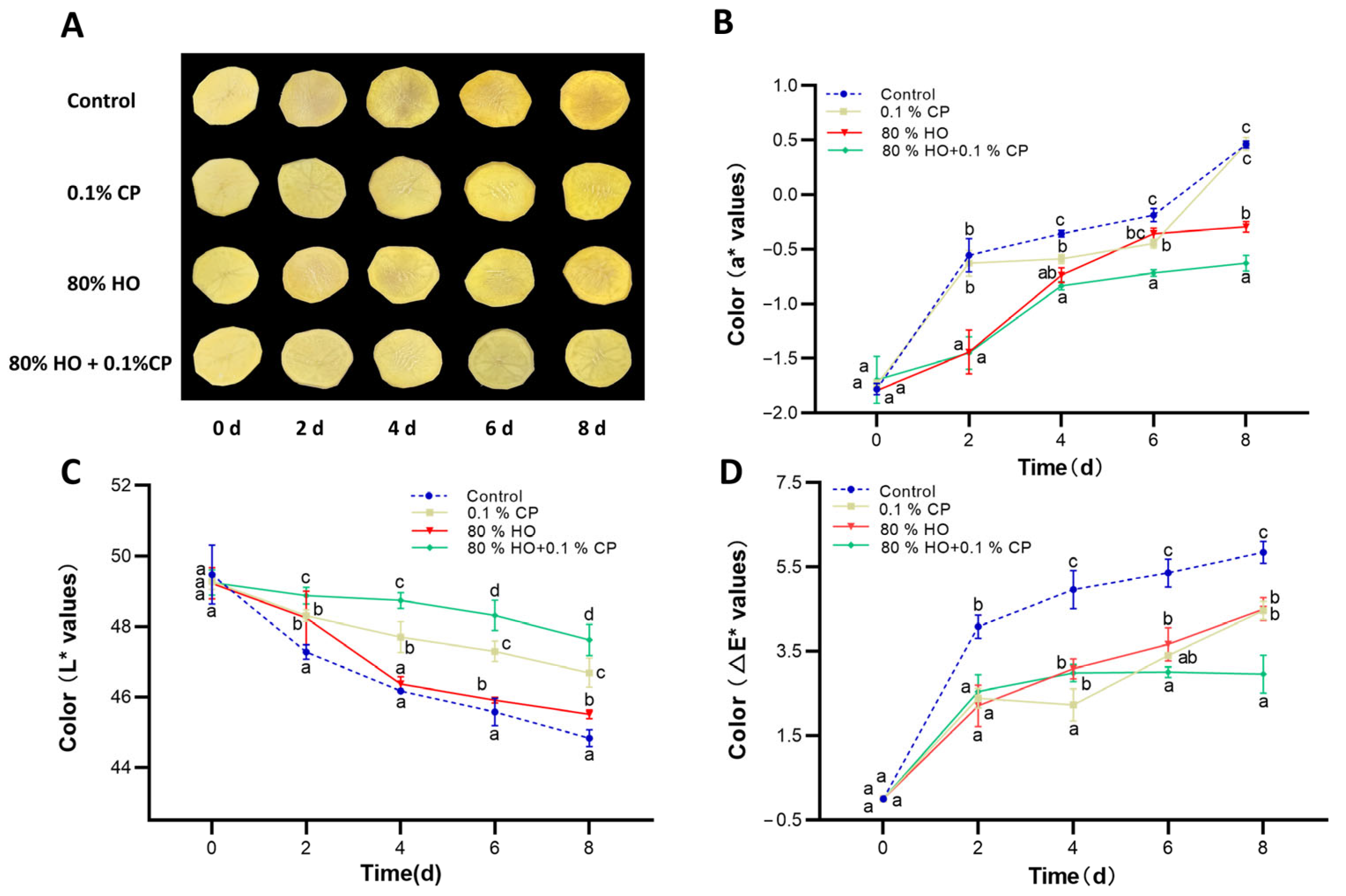
3.1. Changes in Color and Overall Visual Characteristics
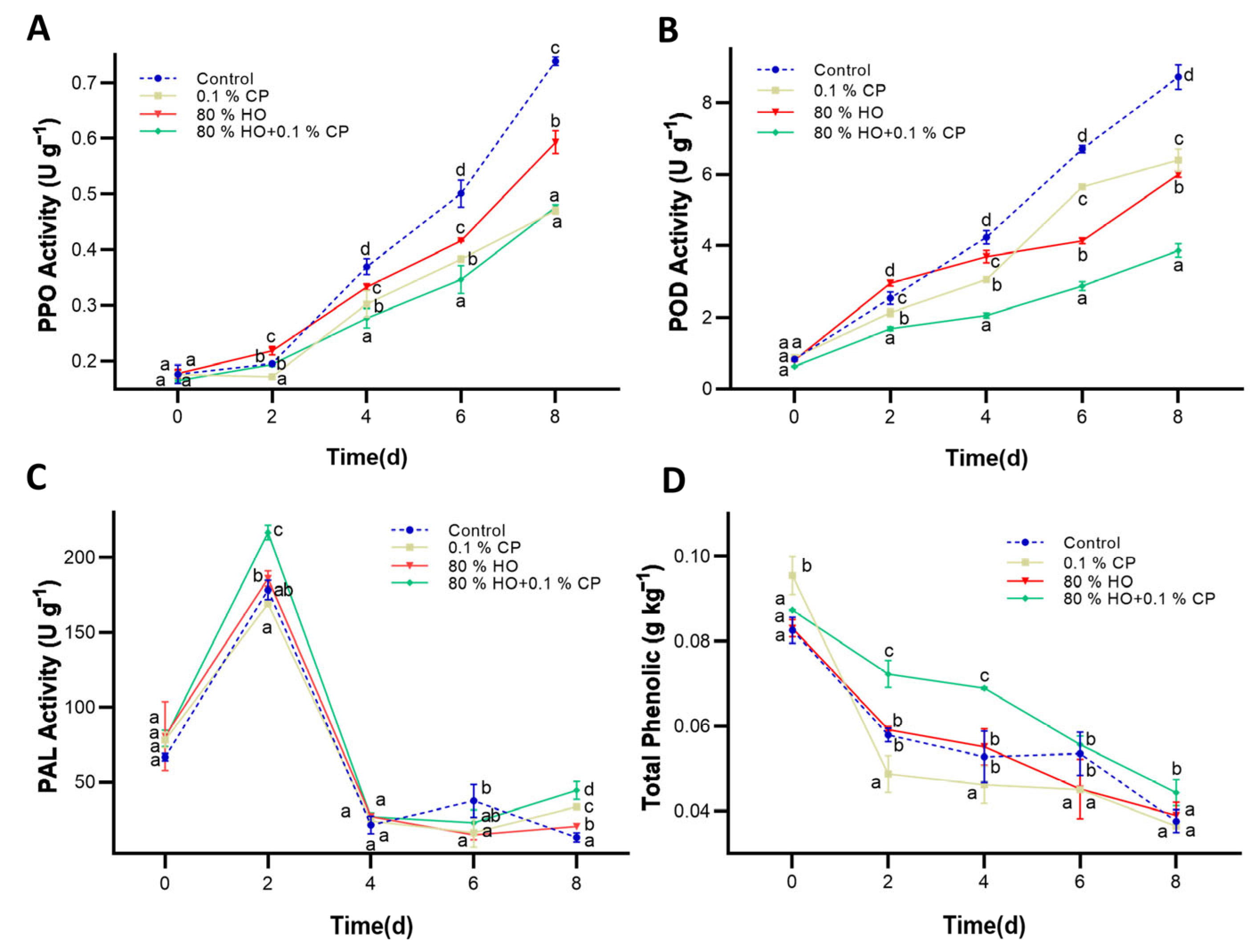
3.2. PPO, POD, and PAL Activities and Total Phenolic Content
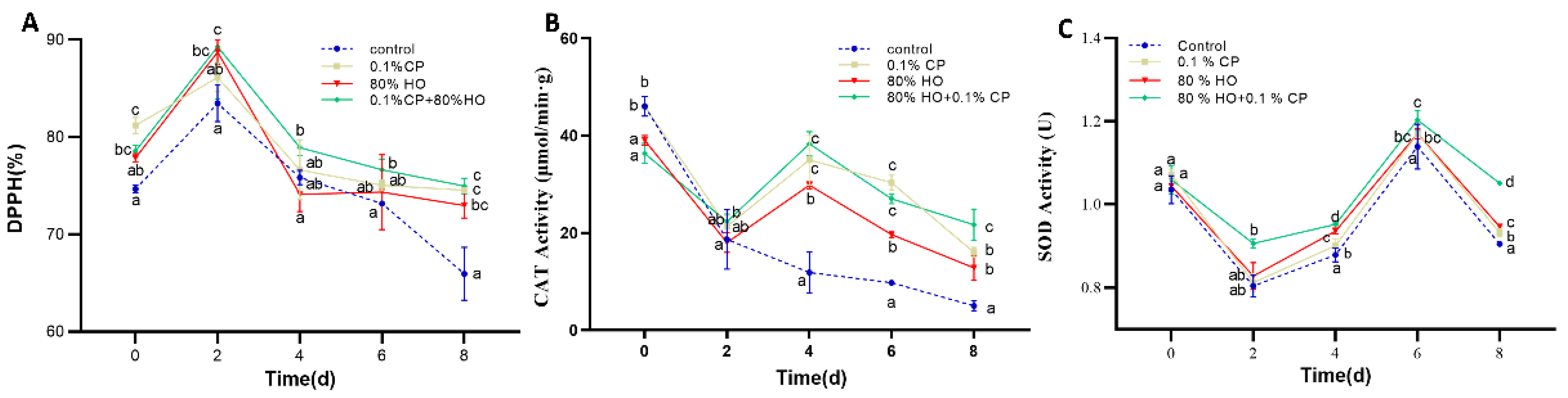
3.3. Antioxidant Capacity (DPPH) and ROS Scavenging Capacity (CAT and SOD Activities)
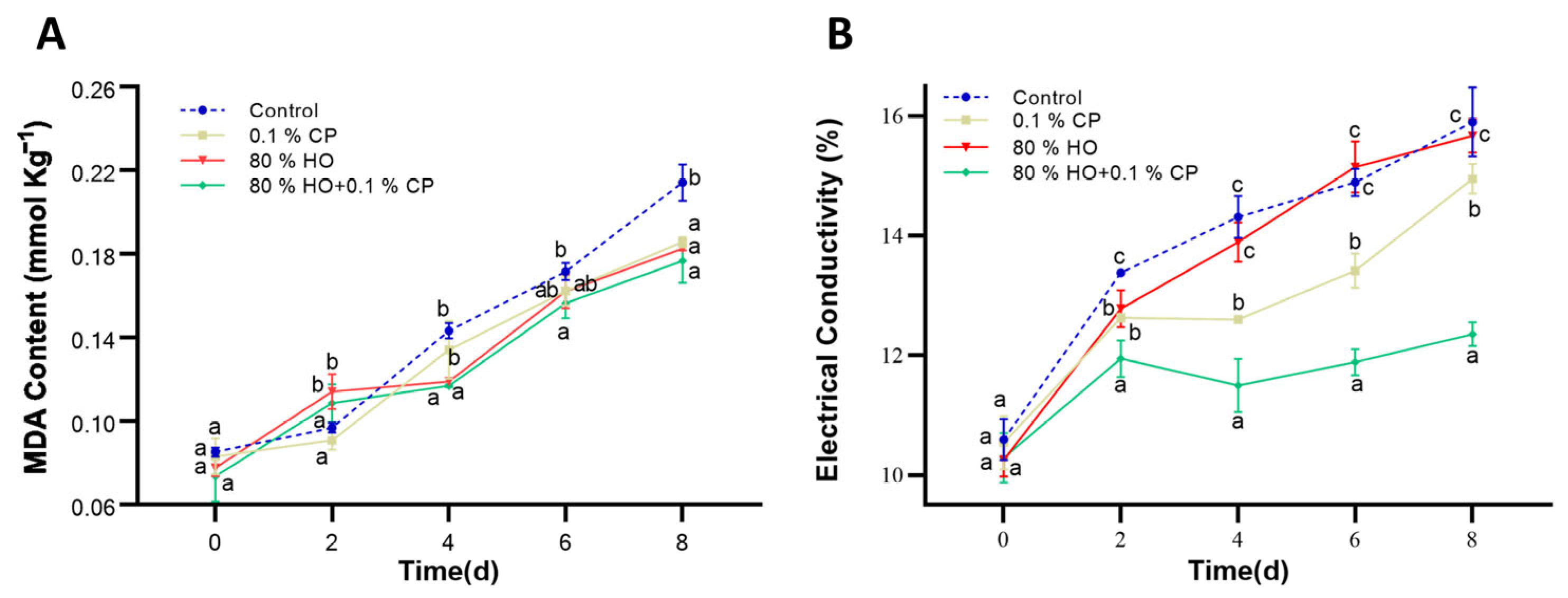
3.4. Membrane Permeability and Lipid Peroxidation Indicator (MDA Content)
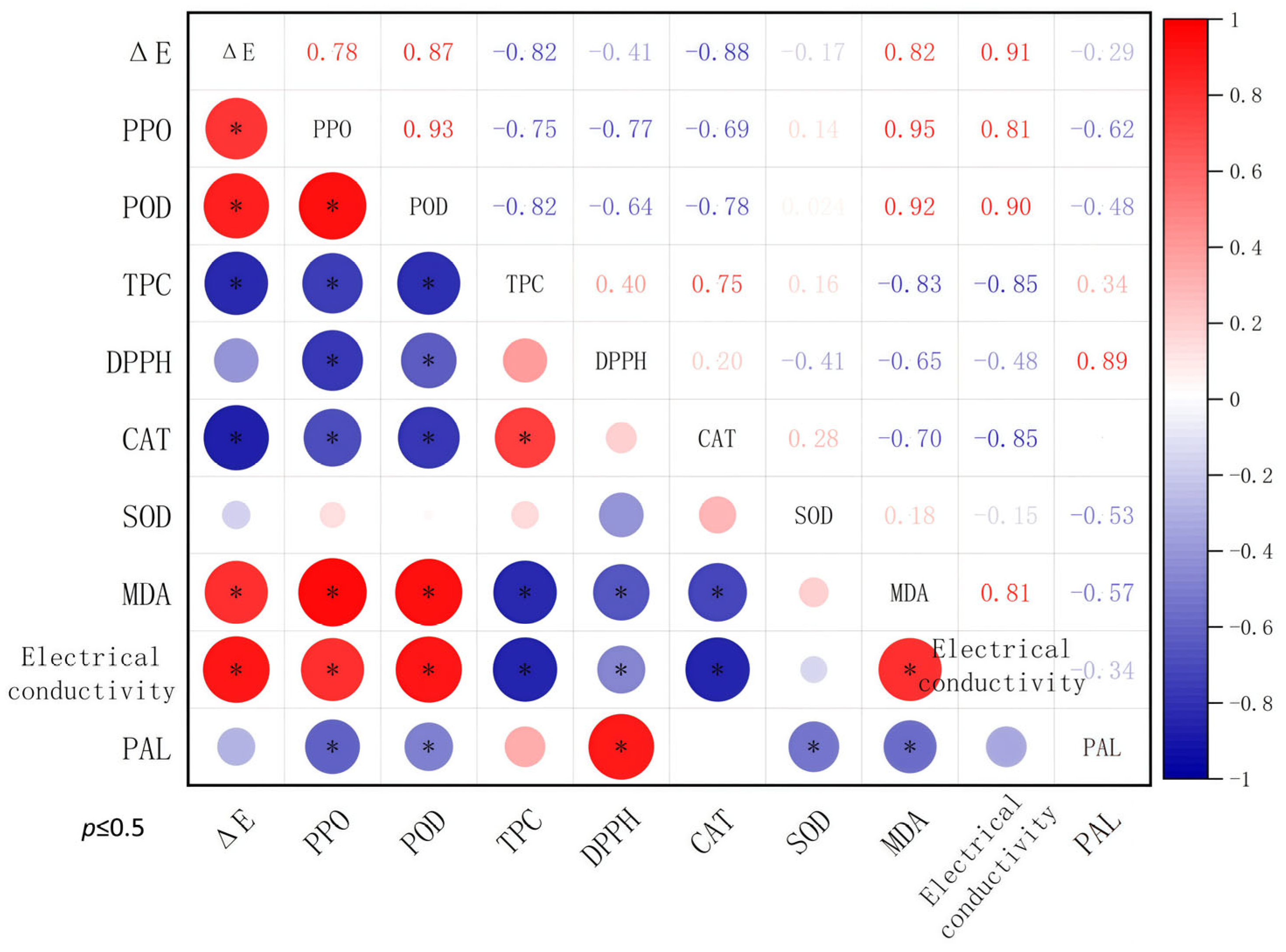
3.5. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorente-Mento, J.M.; Valverde, J.M.; Serrano, M.; Pretel, M.T. Fresh-cut salads: Consumer acceptance and quality parameter evolution during storage in domestic refrigerators. Sustainability 2022, 14, 3473. [Google Scholar] [CrossRef]
- Lalley, H. Pre-Cut Produce Sales Are Booming. Here’s What’s New. Available online: https://www.winsightgrocerybusiness.com/fresh-food/pre-cut-produce-sales-are-booming-heres-whats-new (accessed on 7 October 2023).
- Statista. Total Department Sales of Fresh Produce in the U.S. from 2019 to 2022. Available online: https://www.statista.com/statistics/1277676/fresh-produce-sales-in-the-us/ (accessed on 7 October 2023).
- Chang, X.; Feng, Y.; Dong, T.; Wang, Q. Inhibitory effect and the involved mechanism of furaneol on enzymatic browning of potatoes. Postharvest Biol. Technol. 2024, 216, 113076. [Google Scholar] [CrossRef]
- Romagnoli. Potatoes, One of the Most Widely Consumed Foods in the World. Available online: https://www.romagnolipatate.it/en/blog/news/the-potato-one-of-the-most-widely-consumed-foods-in-the-world (accessed on 7 October 2023).
- Nokthai, P.; Lee, V.S.; Shank, L. Molecular Modeling of Peroxidase and Polyphenol Oxidase: Substrate Specificity and Active Site Comparison. Int. J. Mol. Sci. 2010, 11, 3266–3276. [Google Scholar] [CrossRef]
- Kou, R.; Peng, M.; Zheng, J.; Hou, S.; Ma, L.; Liu, X. Short-Time High-Oxygen Pre-Treatment Delays Lignification of Loquat (Eriobotrya japonica Lindl.) During Low-Temperature Storage. Foods 2025, 14, 201. [Google Scholar] [CrossRef]
- Xin, Z.; Qianqian, L.; Cong, H.; Qingmin, C.; Wenxiao, J.; Maorun, F. Combined treatment of ɛ-polylysine and UV-C inhibited aerobic bacteria growth, and maintained the color, texture, flavor of vacuum-packed fresh-cut lettuce slices. Postharvest Biol. Technol. 2024, 218, 113156. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, S.; Jia, S.; Gui, B.; Wei, Y.; Chen, Y.; Ye, J.; Xu, F.; Ding, P.; Shao, X. Broccoli stem extract enhances browning inhibition in fresh-cut peach fruit by inhibiting polyphenol oxidase activity and improving antioxidant capacity. Postharvest Biol. Technol. 2025, 222, 113399. [Google Scholar] [CrossRef]
- Chen, C.; Meng, F.-B.; Lv, H.-J.; Gou, Z.-Z.; Qiu, J.; Li, Y.-C. Study on the bacteriostasis of lemon essential oil and the application of lemon essential oil nanoemulsion on fresh-cut kiwifruit. Front. Sustain. Food Syst. 2024, 8, 1394831. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Yang, Q.; Yang, H.; Li, Y.; Zhou, B.; Li, T.; Gao, Y.; Qiao, L. Cod peptides inhibit browning in fresh-cut potato slices: A potential anti-browning agent of random peptides for regulating food properties. Postharvest Biol. Technol. 2018, 146, 36–42. [Google Scholar] [CrossRef]
- Niaz, N.; Hu, W.; Pan, S.; Ali, K.; Niaz, S. Heat shock combined with chemical treatment partially recovered chilling injury and improved antioxidant capacity in banana. Postharvest Biol. Technol. 2024, 216, 113077. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Lu, Y.; Yang, Q.; Li, Y.; Deng, X.; Liu, Y.; Du, X.; Qiao, L.; Zheng, J. Effect of high oxygen pretreatment of whole tuber on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 301, 125287. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Murtaza, A.; Iqbal, A.; Zhang, J.; Zhu, L.; Xu, X.; Pan, S.; Hu, W. High-pressure carbon dioxide treatment alleviates browning development by regulating membrane lipid metabolism in fresh-cut lettuce. Food Control 2022, 134, 108749. [Google Scholar] [CrossRef]
- Thakur, P.; Nayyar, H. Facing the cold stress by plants in the changing environment: Sensing, signaling, and defending mechanisms. In Plant Acclimation to Environmental Stress; Springer: New York, NY, USA, 2012; pp. 29–69. [Google Scholar]
- Gibbs, D.J.; Holdsworth, M.J. Every breath you take: New insights into plant and animal oxygen sensing. Cell 2020, 180, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Ran, Y.; Li, L.; Chen, L.; Lin, Q.; Liang, F.; Li, J.; Li, X.; Tang, Y. Short-term high oxygen pre-stimulation inhibits browning of fresh-cut watercored Fuji apples. Postharvest Biol. Technol. 2022, 191, 111959. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Antioxidant content of fresh-cut pears stored in high-O2 active packages compared with conventional low-O2 active and passive modified atmosphere packaging. J. Agric. Food Chem. 2008, 56, 932–940. [Google Scholar] [CrossRef]
- Ban, Q.; Liu, T.; Ning, K.; Fan, J.; Cui, Q.; Guo, Y.; Zai, X. Effect of calcium treatment on the browning of harvested eggplant fruits and its relation to the metabolisms of reactive oxygen species (ROS) and phenolics. Food Sci. Nutr. 2021, 9, 5567–5574. [Google Scholar] [CrossRef]
- Kahn, V. Effect of proteins, protein hydrolyzates and amino acids on o-dihydroxyphenolase activity of polyphenol oxidase of mushroom, avocado, and banana. J. Food Sci. 1985, 50, 111–115. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Bagal, U.R.; Leebens-Mack, J.H.; Lorenz, W.W.; Dean, J.F. The phenylalanine ammonia lyase (PAL) gene family shows a gymnosperm-specific lineage. BMC Genom. 2012, 13, S1. [Google Scholar] [CrossRef]
- Timperio, A.M.; Egidi, M.G.; Zolla, L. Proteomics applied on plant abiotic stresses: Role of heat shock proteins (HSP). J. Proteom. 2008, 71, 391–411. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Lu, Y.; Li, Y.; Li, T.; Zhou, B.; Qiao, L. Effect of purslane (Portulaca oleracea L.) extract on anti-browning of fresh-cut potato slices during storage. Food Chem. 2019, 283, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, K.; Wang, N.; Du, J. Effects of aqueous chlorine dioxide treatment on polyphenol oxidases from Golden Delicious apple. LWT-Food Sci. Technol. 2007, 40, 1362–1368. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Fan, X.; Tang, Y.; Yun, J. Response of antioxidant activity and sensory quality in fresh-cut pear as affected by high O2 active packaging in comparison with low O2 packaging. Food Sci. Technol. Int. 2012, 18, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jiang, A.; Feng, K.; Gu, S.; Xu, D.; Hu, W. Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biol. Technol. 2019, 157, 110958. [Google Scholar] [CrossRef]
- Dong, T.; Shi, J.; Jiang, C.-Z.; Feng, Y.; Cao, Y.; Wang, Q. A short-term carbon dioxide treatment inhibits the browning of fresh-cut burdock. Postharvest Biol. Technol. 2015, 110, 96–102. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Muñoz, T.; Escribano, M.A.I.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, X.; Zheng, J.; Wang, T.; You, X.; Liu, H.; Liu, X. The effect of ultrasonic on reducing anti-browning minimum effective concentration of purslane extract on fresh-cut potato slices during storage. Food Chem. 2021, 343, 128401. [Google Scholar] [CrossRef]
- Qiao, L.; Gao, M.; Zheng, J.; Zhang, J.; Lu, L.; Liu, X. Novel browning alleviation technology for fresh-cut products: Preservation effect of the combination of Sonchus oleraceus L. extract and ultrasound in fresh-cut potatoes. Food Chem. 2021, 348, 129132. [Google Scholar] [CrossRef]
- Li, X.; Luo, S.; Shen, J.; Li, C.; Kadeer, W.; Chen, L.; Li, X.; Jiang, Y.; Tang, Y. Synergistic anti-browning effects of short-term high oxygen pre-stimulation and supercooled storage on fresh-cut potatoes by regulating polyphenol biosynthesis and membrane lipid oxidation. Postharvest Biol. Technol. 2025, 219, 113257. [Google Scholar] [CrossRef]
- Sui, X.; Meng, Z.; Dong, T.; Fan, X.; Wang, Q. Enzymatic browning and polyphenol oxidase control strategies. Curr. Opin. Biotechnol. 2023, 81, 102921. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Zhao, W.; Zheng, Y.; Wang, Y.; Wang, P.; Ma, Y.; Zhao, X. Low frequency ultrasound treatment enhances antibrowning effect of ascorbic acid in fresh-cut potato slices. Food Chem. 2022, 380, 132190. [Google Scholar] [CrossRef] [PubMed]
- Sheyhakinia, S.; Bamary, Z.; Einali, A.; Valizadeh, J. The induction of salt stress tolerance by jasmonic acid treatment in roselle (Hibiscus sabdariffa L.) seedlings through enhancing antioxidant enzymes activity and metabolic changes. Biologia 2020, 75, 681–692. [Google Scholar] [CrossRef]
- Vitti, M.C.D.; Sasaki, F.F.; Miguel, P.; Kluge, R.A.; Moretti, C.L. Activity of enzymes associated with the enzymatic browning of minimally processed potatoes. Braz. Arch. Biol. Technol. 2011, 54, 983–990. [Google Scholar] [CrossRef]
- Supapvanich, S.; Prathaan, P.; Tepsorn, R. Browning inhibition in fresh-cut rose apple fruit cv. Taaptimjaan using konjac glucomannan coating incorporated with pineapple fruit extract. Postharvest Biol. Technol. 2012, 73, 46–49. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Lin, Y.; Zhang, S.; Chen, Y.; Jiang, X. The roles of metabolism of membrane lipids and phenolics in hydrogen peroxide-induced pericarp browning of harvested longan fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Gao, H.; Chai, H.; Cheng, N.; Cao, W. Effects of 24-epibrassinolide on enzymatic browning and antioxidant activity of fresh-cut lotus root slices. Food Chem. 2017, 217, 45–51. [Google Scholar] [CrossRef]
- Lim, W.Y.; Wong, C.W. Inhibitory effect of chemical and natural anti-browning agents on polyphenol oxidase from ginger (Zingiber officinale Roscoe). J. Food Sci. Technol. 2018, 55, 3001–3007. [Google Scholar] [CrossRef]
- Gacche, R.; Shete, A.; Dhole, N.; Ghole, V. Reversible inhibition of polyphenol oxidase from apple using L-cysteine. Indian J. Chem. Technol. 2006, 13, 459–463. [Google Scholar]
- Girelli, A.M.; Mattei, E.; Messina, A.; Tarola, A.M. Inhibition of polyphenol oxidases activity by various dipeptides. J. Agric. Food Chem. 2004, 52, 2741–2745. [Google Scholar] [CrossRef]
- Khan, M.R.; Chinsirikul, W.; Sane, A.; Chonhenchob, V. Combined effects of natural substances and modified atmosphere packaging on reducing enzymatic browning and postharvest decay of longan fruit. Int. J. Food Sci. Technol. 2020, 55, 500–508. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.D.; Coleman, M.L.; Pugh, C.W. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell. Mol. Life Sci. 2009, 66, 3539–3554. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The hyperoxic-hypoxic paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Ipson, B.R.; Fisher, A.L. Roles of the tyrosine isomers meta-tyrosine and ortho-tyrosine in oxidative stress. Ageing Res. Rev. 2016, 27, 93–107. [Google Scholar] [CrossRef]
- Liang, L.; Wang, C.; Li, S.; Chu, X.; Sun, K. Nutritional compositions of Indian Moringa oleifera seed and antioxidant activity of its polypeptides. Food Sci. Nutr. 2019, 7, 1754–1760. [Google Scholar] [CrossRef]
- Tessema, B.; Sack, U.; Serebrovska, Z.; König, B.; Egorov, E. Effects of hyperoxia on aging biomarkers: A systematic review. Front. Aging 2022, 2, 783144. [Google Scholar] [CrossRef]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Jiang, Y.; Li, A.; Peng, M.; Wang, T.; Kou, R.; Kang, J.; Liu, X. The Super Anti-Browning Effect of High-Oxygen Pretreatment Combined with Cod Peptides on Fresh-Cut Potatoes During Storage. Foods 2025, 14, 1564. https://doi.org/10.3390/foods14091564
Zheng J, Jiang Y, Li A, Peng M, Wang T, Kou R, Kang J, Liu X. The Super Anti-Browning Effect of High-Oxygen Pretreatment Combined with Cod Peptides on Fresh-Cut Potatoes During Storage. Foods. 2025; 14(9):1564. https://doi.org/10.3390/foods14091564
Chicago/Turabian StyleZheng, Jiaxuan, Yishan Jiang, Aiguang Li, Mengfei Peng, Ting Wang, Runlei Kou, Ji Kang, and Xia Liu. 2025. "The Super Anti-Browning Effect of High-Oxygen Pretreatment Combined with Cod Peptides on Fresh-Cut Potatoes During Storage" Foods 14, no. 9: 1564. https://doi.org/10.3390/foods14091564
APA StyleZheng, J., Jiang, Y., Li, A., Peng, M., Wang, T., Kou, R., Kang, J., & Liu, X. (2025). The Super Anti-Browning Effect of High-Oxygen Pretreatment Combined with Cod Peptides on Fresh-Cut Potatoes During Storage. Foods, 14(9), 1564. https://doi.org/10.3390/foods14091564