3.1. Development of Protective Coatings Based on Latex
The latex liquid expelled by the plant during spring pruning cuts was collected. For this purpose, latex was manually collected from the cuts made during pruning. The biopolymer films prepared with the different blends (
Table 1) were observed and analyzed once formed on 120 × 120 mm plates.
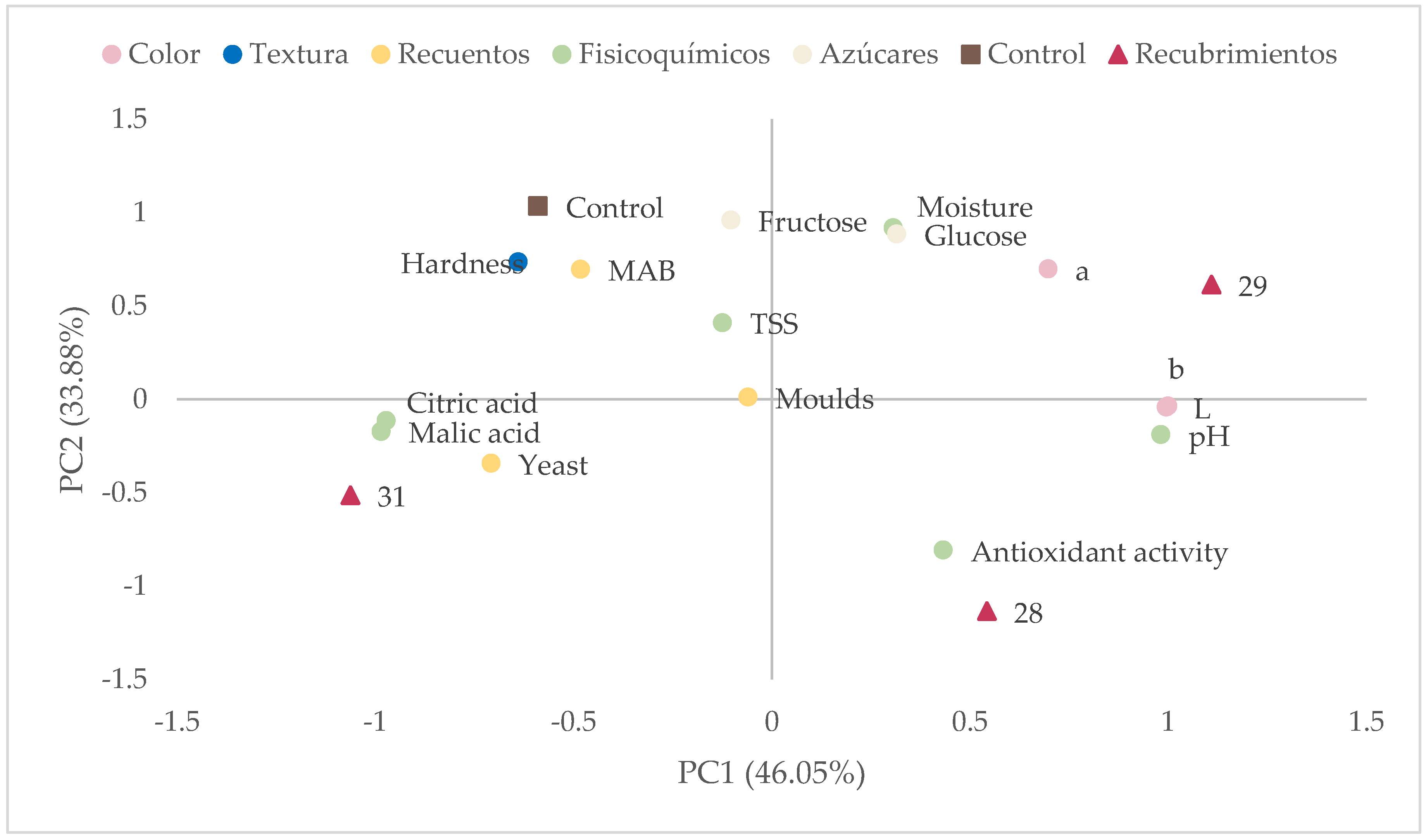
The CIELAB color space coordinates of the different formulations were obtained, including lightness, the red–green coordinate, and the yellow–blue coordinate. The analysis was performed directly on the biopolymer films placed on 120 × 120 mm plates. Lightness values obtained ranged between 86 and 87, without significant differences in the different formulations. Regarding a coordinate (red–green coordinate), all values were below 1, with the minimum value being 0.20 (biopolymer film 20) and the maximum value being 0.74 (biopolymer films 41 and 43). Finally, for the b coordinate (yellow–blue coordinate), values ranged between 1.38 and 2.37, with the lowest value in biopolymer film 43 and the highest values in formulations 35 and 36, with no significant differences in any of these biopolymer films. Thus, these parameters did not seem very decisive for selecting a mixture.
The results for physical property analysis, such as thickness, moisture, and solubility of
Ficus carica L. latex-based coatings, are shown in
Table 2.
The thickness of the biopolymer films varies between 0.04 and 0.09 mm. The lowest value is observed in formulation 10/3/0 (0.04 ± 0.01), while several formulations exhibit the highest (
p < 0.05) thickness of 0.09 mm, including 5/2/0.5, 5/2/1, 5/2.5/1, 10/2/0.5, and 1/2/0.5, among others. The results indicate that the composition of the materials used influences the final structure of the biopolymer film [
28]. Moisture values range between 21.44% (10/2/0.5) and 32.19% (1/3/0). Generally, biopolymer films with lower moisture content tend to be those with formulations containing 10 and 5% latex and 3% citric acid, while formulations with values above 30% include 5/2/1 (30.81 ± 0.76) and 1/3/0 (32.19 ± 6.59). The variability in moisture could be related to the water retention capacity of the components used and their influence on the film structure. Solubility values show greater dispersion, ranging between 77.07% (10/2/0) and 98.73% (5/3/1). It is observed that formulations with higher content of certain compounds tend to have greater solubility, which could be related to the interaction between the polymers and the biopolymer film matrix. Formulations with higher solubility may be more susceptible to degradation in humid environments, which could be a determining factor in their application [
28].
Biopolymer films exhibit considerable variability in their physical properties, depending on the formulation used. Thickness is relatively stable in most samples, while moisture and solubility show significant differences. It is important to consider these characteristics in relation to the final use of the biopolymer films, as properties such as solubility can influence their stability and functionality in specific applications. However, blends with 1% latex and different percentages of citric acid and glycerol showed biopolymer films that were not consistent enough to release, or those that did release broke due to the weakness of the biopolymer film, and some of the 5% films were released but subsequently broke because they were too sticky and not sufficiently elastic. For this reason, the 1% and 5% latex blends were discarded.
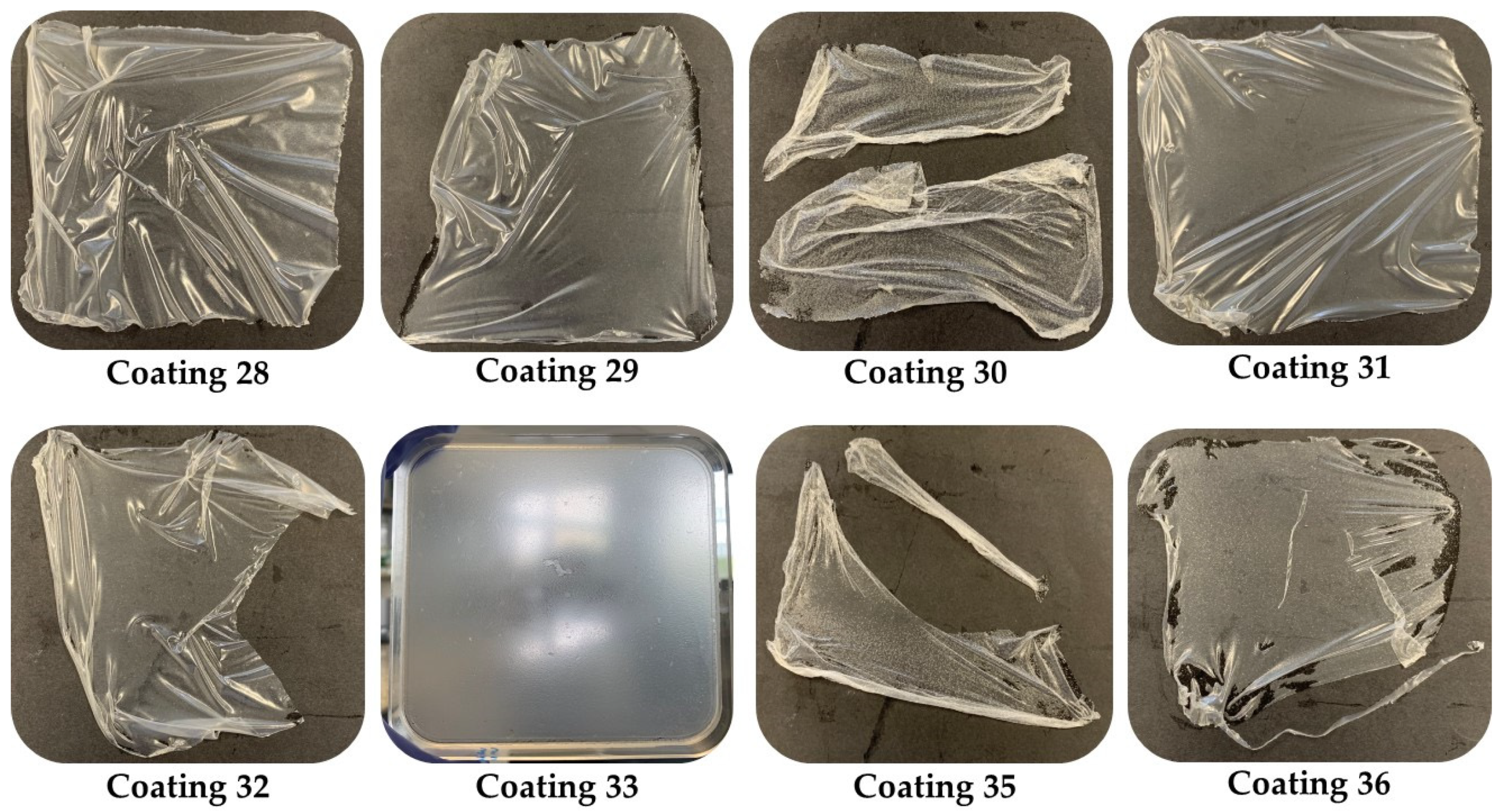
Figure 1 shows the plates of the 10% latex films; all of them were released except for film 33, observing that this percentage gave more elasticity and resistance to the films. Some of these coatings were completely released, as shown below.
Based on the physicochemical properties observed, it was determined that only the formulations containing 10% latex (specifically mixtures 28 to 36) would be subjected to antimicrobial activity testing.
The antibacterial activity was tested against pathogenic bacteria from the Spanish Type Culture Collection, specifically,
Staphylococcus aureus CECT 976,
Bacillus cereus CECT 131,
Listeria monocytogenes CECT 911, and
Listeria innocua CECT 910 as Gram-positive bacteria, and
Escherichia coli CECT 4267 and
Salmonella choleraesuis CECT 4395 as Gram-negative bacteria. Regarding the results obtained, no antibacterial activity was observed in any of the samples tested against
Bacillus cereus,
Listeria monocytogenes, or
Listeria innocua. However, activity was observed against
Salmonella choleraesuis in all tested combinations and against
Escherichia coli in combinations 31 and 33, which showed inhibition zones of 6 ± 0.10 mm and 4 ± 0.05 mm, respectively. For
Staphylococcus aureus, only formulation 34, containing 3% citric acid, showed activity, with an inhibition zone of 3 ± 0.01 mm. This indicates that most formulations do not have a significant effect on these bacteria, which could be related to the composition of the biofilms and their mechanism of action. No activity was observed in the controls performed with citric acid alone and glycerol alone. The analyzed biofilms showed variability in their antibacterial activity depending on the target bacteria. There is limited information in the literature regarding the antibacterial activity of fig latex; however, there are studies involving latex from other
Ficus species or rubber. Salem et al. [
29] evaluated the activity of nanoparticles from aqueous extracts of latex and leaves of
Ficus sycomorus. Their results align with ours, as they also observed higher activity against
Salmonella sp. and
Escherichia coli among the Gram-negative bacteria and
Staphylococcus aureus among the Gram-positive bacteria. On the other hand, Ref. [
30] conducted a comparative study of the antibacterial effects of latex from
Calotropis procera and also corroborated that the antibacterial activity of latex was moderate.
Regarding antifungal activity, analyses were carried out to evaluate the activity against molds responsible for fruit spoilage and mycotoxigenic molds (
Table 3 and
Table 4).
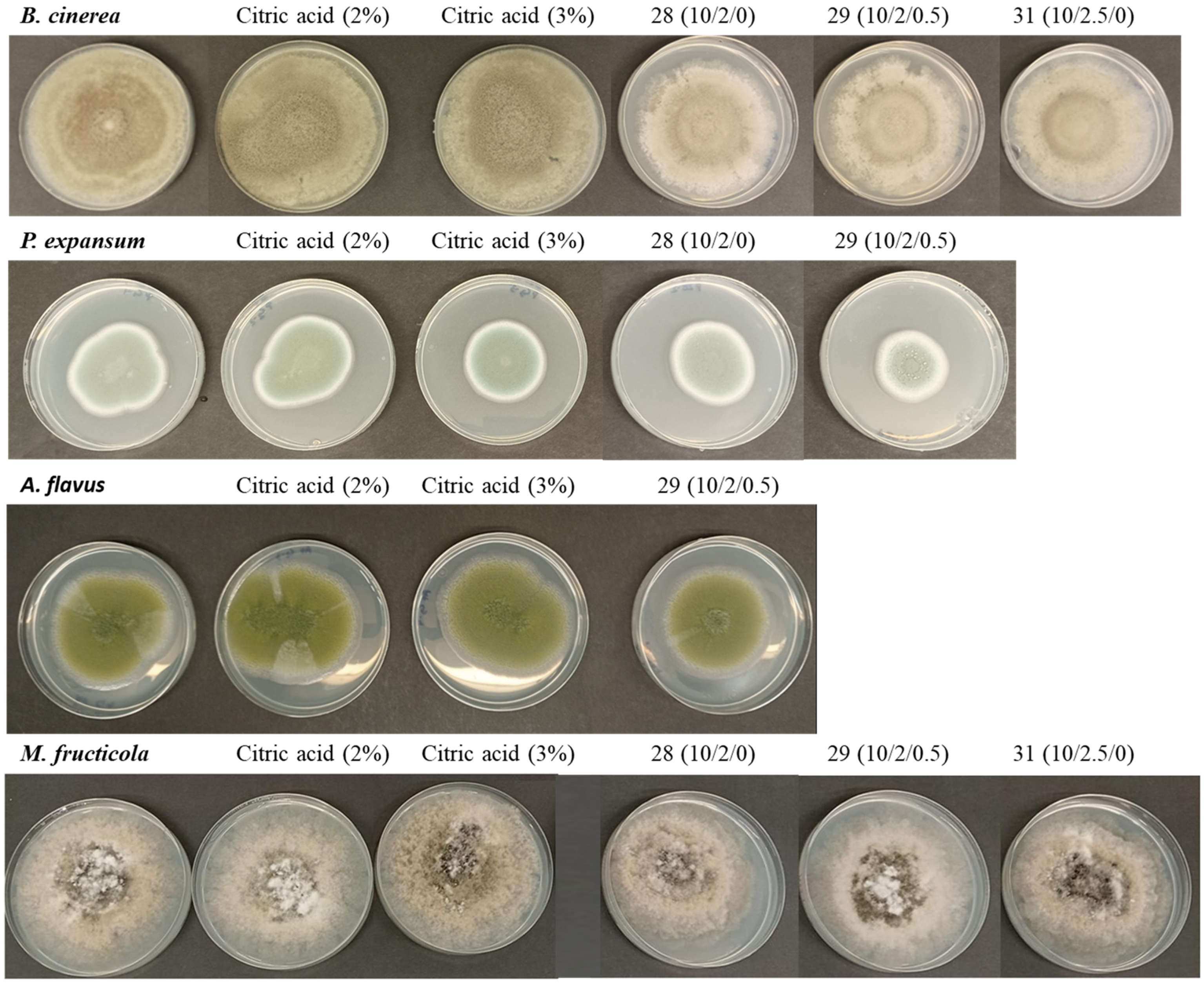
Table 3 presents the growth (in mm) of
Monilinia fructicola and
Botrytis cinerea after 7 days of incubation at 25 °C under various conditions, including a control, treatments with citric acid, and biofilms with different formulations. Overall, it is observed that some biofilms were able to reduce fungal growth compared to the control, although the effects vary depending on the species and the specific formulation.
For
M. fructicola, the growth in the control was 80.50 ± 2.09 mm, while citric acid at 2% and 3% showed a slight reduction (79.10 ± 1.47 mm and 78.00 ± 0.89 mm, respectively), with no statistically significant differences compared to the control. However, some biofilms were more effective in reducing growth, particularly formulation 28 with 75.33 ± 0.29 mm and 29 with 76.05 ± 0.18 mm, which showed significant differences (
p < 0.05) compared to the control (
Figure 2). In the case of
B. cinerea, the control also showed a growth of 81.80 ± 1.86 mm, and citric acid at 2% and 3% did not have a significant inhibitory effect. However, some biofilms significantly reduced growth, especially formulations 28 and 29, which showed significant differences compared to the control (
Figure 2). These results indicate that some biofilms were able to reduce the growth of
M. fructicola and
B. cinerea, but their effectiveness depends on the specific formulation. Formulations 28 and 29 were the most effective in inhibiting the growth of both fungi. This suggests the need to optimize the formulations to enhance their antifungal activity.
There are no studies on the inhibition of these fungi with latex, but [
31] studied the antifungal activity of a proteolytic fraction P1G10 from the latex of
Vasconcellea cundinamarcensis against the cell growth and cell wall integrity of
Botrytis cinerea, and they propose it as a promising antifungal agent for controlling disease-causing agents in the food agroindustry.
Table 4 presents the growth (in mm) of
Aspergillus niger,
Aspergillus flavus, and
Penicillium expansum after 7 days of incubation at 25 °C under various treatments, including a control, citric acid, and biofilms with different formulations. It is observed that the effects of the treatments vary depending on the fungal species, and some formulations significantly reduce growth compared to the control.
For
A. niger, the growth in the controls and samples did not show a clear inhibitory effect, suggesting that these formulations were not effective on this species. However, for
A. flavus, some biofilms were able to decrease its growth, particularly formulation 29, with 57.83 ± 0.58 mm, which showed significant differences (
p < 0.05) compared to the control (
Figure 2). Finally, for
P. expansum, biofilm formulations 29 and 31 showed significant differences (
p < 0.05) compared to the control (
Figure 2). The results indicate that some biofilm formulations were able to reduce the growth of
A. flavus and
P. expansum, particularly formulation 29, which showed the greatest inhibition in both species. These findings highlight the importance of optimizing formulations and evaluating their impact on each fungal species to improve their effectiveness as antifungal agents. There are no studies on the effect of latex on these mycotoxigenic species; however, it is known that the bioactive compounds in plant latex are a potential source of antifungals against postharvest pathogens [
32].
3.2. Shelf-Life Study of Dried Figs Coated with Selected Combinations
After analyzing the obtained biofilms, the optimal formulations with 10% latex were chosen for the shelf-life study. The selected formulations were 28, 29, and 31. Regarding citric acid content, formulations 28 and 29 were constituted of 2% citric acid, while formulation 31 contained 2.5%. Additionally, glycerol was present only in formulation 29 at a minimal amount of 0.5%.
Figure 3 shows the different batches of figs after applying the selected coatings by spraying. As observed, the application of the coatings did not alter the visual appearance of the dried figs. Biofilms are typically transparent or slightly opaque, allowing the natural appearance of the fruit to remain visible. This is important for consumer acceptance, as coated fruits retain their original color and shine [
33].
Microbiological counts of mesophilic aerobic bacteria (Log CFU/g) during the shelf-life study of different batches of dried figs, evaluated at days 0, 30, and 60, are presented in
Table 5. Uncoated control was compared with different coatings formulated with biofilms.
Regarding mesophilic aerobic bacteria, all batches had levels above 3 Log CFU/g on day 0 of coating application. After 30 days, these values significantly decreased in the batch with coating 28, which showed significantly lower values (p < 0.05) compared to the other batches. On day 60, a significant reduction in mesophilic aerobic bacteria was observed, with the batch coated with formulation 28 being the only one where bacterial levels were below the detection limit.
Regarding mold counts, on day 0, all batches had values close to 3 Log CFU/g, with the control and batch 28 showing the highest values (p < 0.05). These levels significantly decreased over the storage period, reaching values greater than 1 Log CFU/g in the control batch and batch 31 on day 60. Coating 28 showed the greatest effect against mold growth, as it was the only batch where no mold growth was detected at the end of the storage period. Yeasts were below the detection limit in all analyzed batches.
Formulate coatings with natural antimicrobial agents that help prevent the growth of pathogens and molds on the surface of the fruit [
34]. These antimicrobial agents can include various compounds such as essential oils, plant extracts, and other biopolymers with antimicrobial properties.
These results suggest that the evaluated coatings are effective and represent an additional benefit in terms of the microbiological safety and stability of dry figs during storage.
The analysis of color parameters during the shelf-life study shows differences in the L (lightness), a* (red–green), and b* (yellow–blue) coordinates, indicating changes in the appearance of the figs throughout the storage period (
Table 6).
The lightness (L) decreased over time in all batches, suggesting a progressive darkening of the fruit. In the control batch, lightness decreased from 57.67 on day 0 to 46.02 on day 60, reflecting evident deterioration. However, the coated batches showed a smaller decrease in lightness, especially coating 29, which maintained more stable values (51.15 on day 0 and 51.42 on day 60). This suggests that the coatings may help delay the darkening of the fruit, which is a positive indicator in terms of visual quality. Regarding the a* coordinate, which indicates the intensity of the red color, a progressive increase was observed in all batches throughout storage. In the control batch, values increased from 13.56 on day 0 to 16.22 on day 60, indicating a shift towards more reddish tones. The coated batches also showed an increase in this coordinate, but without significant differences between them. The b* coordinate, representing the intensity of the yellow color, showed a slight decrease, not significant, over time in most batches, which may be related to the degradation of natural fruit pigments.
The results suggest that the applied coatings helped reduce the loss of lightness and maintain more stable color characteristics of the figs during storage compared to the control batch. Coatings play a crucial role in protecting the color of fruits during storage and distribution [
33]. Coatings act as a physical barrier that limits the fruit’s exposure to oxygen, which is essential for preventing the oxidation of natural pigments. This not only enhances visual appearance but can also increase consumer acceptance and reduce food waste.
Table 7 presents the evaluation of moisture, hardness, and antioxidant activity of dehydrated figs during 60 days of storage, revealing significant differences among the analyzed batches. The applied coatings appear to influence moisture retention, texture, and preservation of antioxidant compounds over time.
Initial moisture varied among the batches, with the control presenting 24.31%, while the coatings showed higher values, especially batch 29 with 30.85%. However, as storage progressed, all batches showed a reduction in moisture content, although the coated batches tended to retain more water than the control. At the end of the 60 days, the control had 18.53%, while batch 29 maintained a higher level (19.08%). This suggests that the coatings may help reduce water loss of the product over time.
In terms of hardness, initial values were relatively low in all batches but increased significantly after 60 days, especially in the control batch (1.12 N), indicating a progressive hardening of the fruit. The coatings showed lower hardness values at the end of storage, with batch 28 reaching 0.57 N and batch 29 reaching 0.70 N. This suggests that the coatings may have reduced the rate of hardening, possibly due to greater moisture retention and less dehydration.
The behavior of antioxidant activity varied among the batches. In the control, antioxidant activity decreased drastically from 27.35 mg Trolox/100 g at the beginning to only 4.23 mg Trolox/100 g at the end of storage, indicating significant degradation of antioxidant compounds over time. In contrast, the coated batches showed better preservation of antioxidant activity, with batch 28 reaching a value even higher than the initial after 60 days (34.03 mg Trolox/100 g), while batches 29 and 31 also maintained high levels (27.31 and 27.58 mg Trolox/100 g, respectively). These results indicate that the coatings may have protected the antioxidant compounds, possibly by reducing exposure to oxidation or degradation caused by moisture loss.
The results suggest that the coatings applied to dried figs have a positive impact on moisture conservation, reduction in hardening, and preservation of antioxidant activity. In particular, batch 29 showed the best balance between water retention, lower hardness, and stable antioxidant activity. These findings highlight the importance of using coatings to improve the quality and shelf life of dried figs.
By reducing moisture loss, coatings help maintain the texture and color of the fruit. Dehydration can cause the fruit to become dull and lose its natural shine, but coatings preserve internal moisture, thus maintaining its fresh appearance. In addition, coatings can contain natural antioxidants, which help protect the fruit’s pigments against oxidative damage [
33].
The study of the evolution of pH and citric and malic acids in dehydrated figs during storage reveals significant variations among different batches and over time (
Table 8). These parameters are crucial as they influence microbiological stability, sensory characteristics, and the quality of the final product.
The pH remained stable until day 30, but a significant decrease was observed in all batches by day 60. The citric acid content varied throughout storage. Initially, the coated batches had slightly higher concentrations compared to the control (1.03–1.14 g/L vs. 0.76 g/L). By day 30, all batches experienced an increase in citric acid concentration, but on day 60, citric acid levels decreased in all batches. The behavior of malic acid was similar to that of citric acid. The results indicate that the applied coatings can slightly modify the pH evolution since a tendency was observed in coatings 28 and 29 to delay this process.
The evolution of soluble solids (°Brix), glucose, and fructose in dehydrated figs over 60 days of storage shows significant variations that may be related to changes in sugar concentration and water migration within the fruit (
Table 9).
Soluble solids values increased in all batches, reaching between 85.37 and 89.03 °Brix by the end of the storage period. This suggests a progressive concentration of sugars due to moisture loss. The higher initial values in the control could be due to the absence of a protective barrier that retained water content. Glucose showed a drastic decrease in all batches after day 0. However, by day 30, values dropped significantly and this trend continued until day 60. This decrease may be related to sugar degradation processes during storage or the conversion of glucose into other compounds. The coatings do not seem to prevent this reduction, although they may modulate the rate of degradation.
The behavior of fructose was similar to that of glucose. This behavior supports the hypothesis that reducing sugars are undergoing some form of transformation or degradation during storage, possibly due to non-enzymatic browning reactions, such as the Maillard reaction, or interactions with other compounds present in the dehydrated fig matrix.
The coatings seem to affect the initial concentrations of soluble solids and sugars but do not prevent their evolution during storage. This suggests that while the coatings may influence product preservation, they are not completely effective in halting sugar transformation over the storage period.