Chemical, Sensory Variations in Black Teas from Six Tea Cultivars in Jingshan, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Black Tea Samples
2.2. Chemicals and Reagents
2.3. Color Quantitative Analysis
2.4. Quantitative Analysis of Taste
2.5. Determination of Non-Volatile Components
2.5.1. Determination of Tea Pigment
2.5.2. Catechins, Caffeine, Theanine
2.6. Non-Targeted Metabolomics
2.7. Analysis of Aromatic Components
2.7.1. GC-MS Analytical Conditions
2.7.2. Qualitative Analysis
2.7.3. Determination of OAV (Odor Activity Value)
2.7.4. Quantitative Descriptive Analysis (QDA) of Aroma
2.7.5. Molecular Docking
2.8. Statistical Analysis
3. Results and Discussions
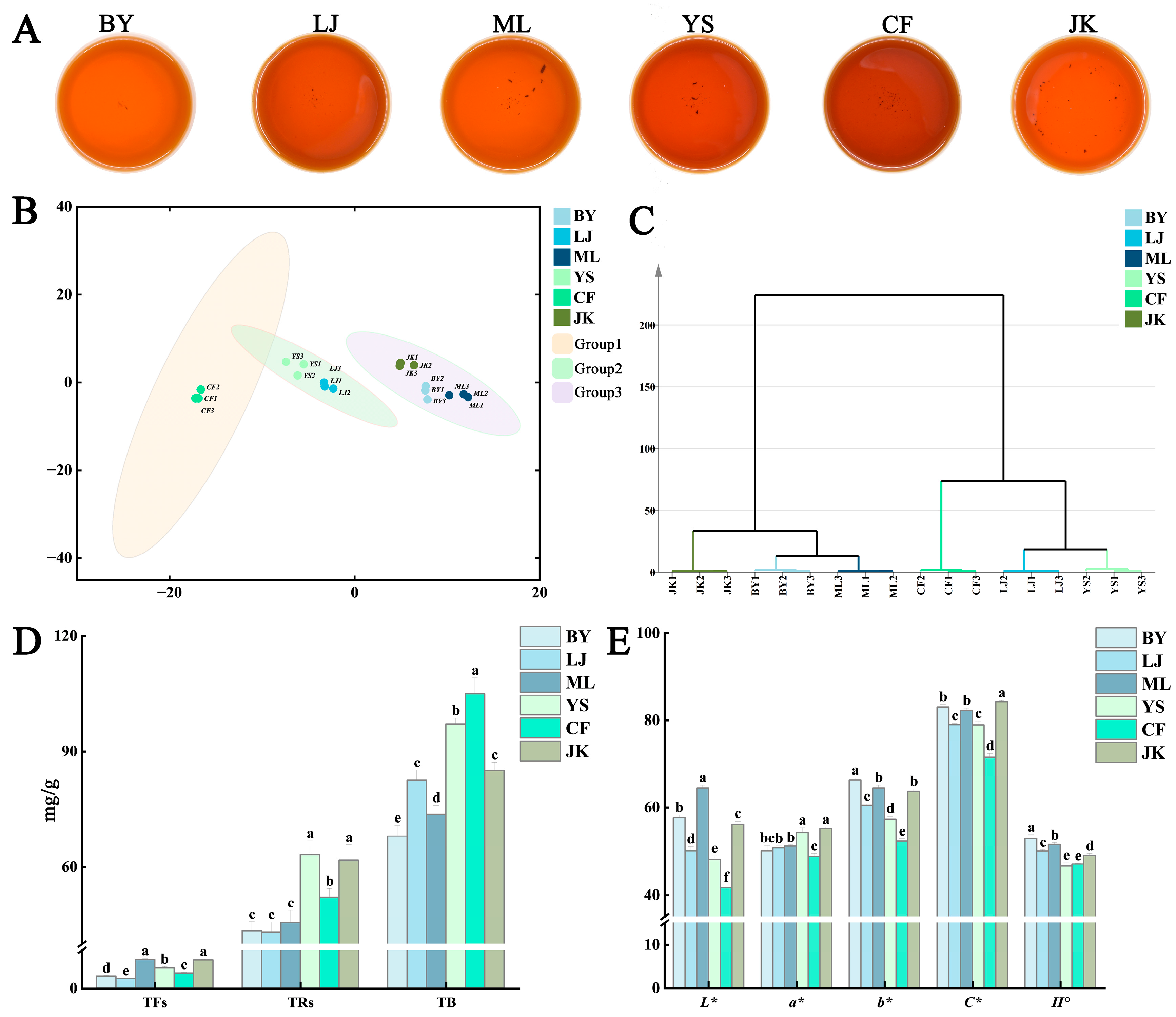
3.1. The Effects of Tea Cultivars on Color and Taste Characteristics of Black Teas
3.1.1. Colorimetric Values and Taste Score
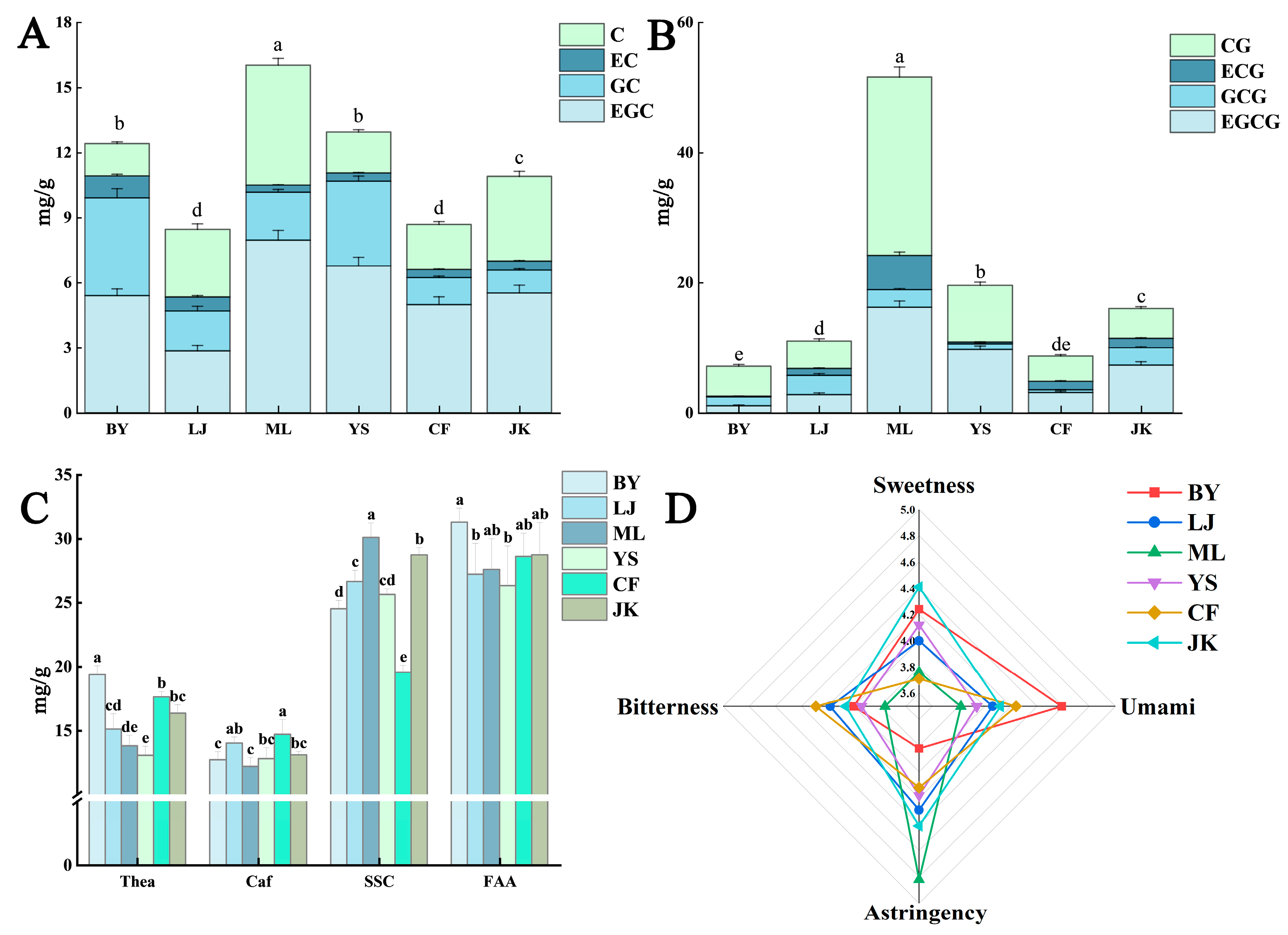
3.1.2. The Principal Non-Volatile Components in Tea Leaves
Catechins, Caffeine, Soluble Sugars, and Amino Acids
Tea Pigments
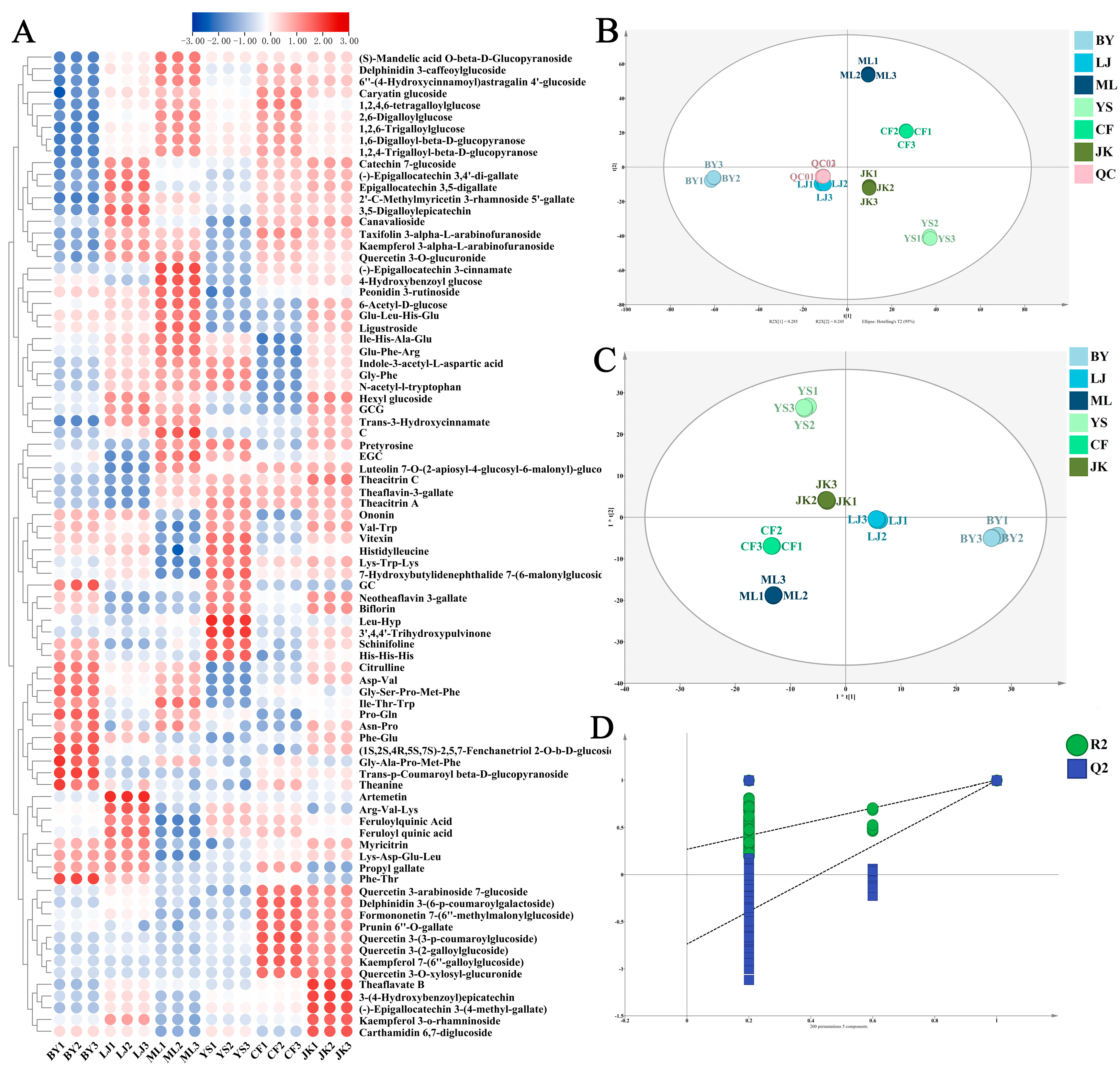
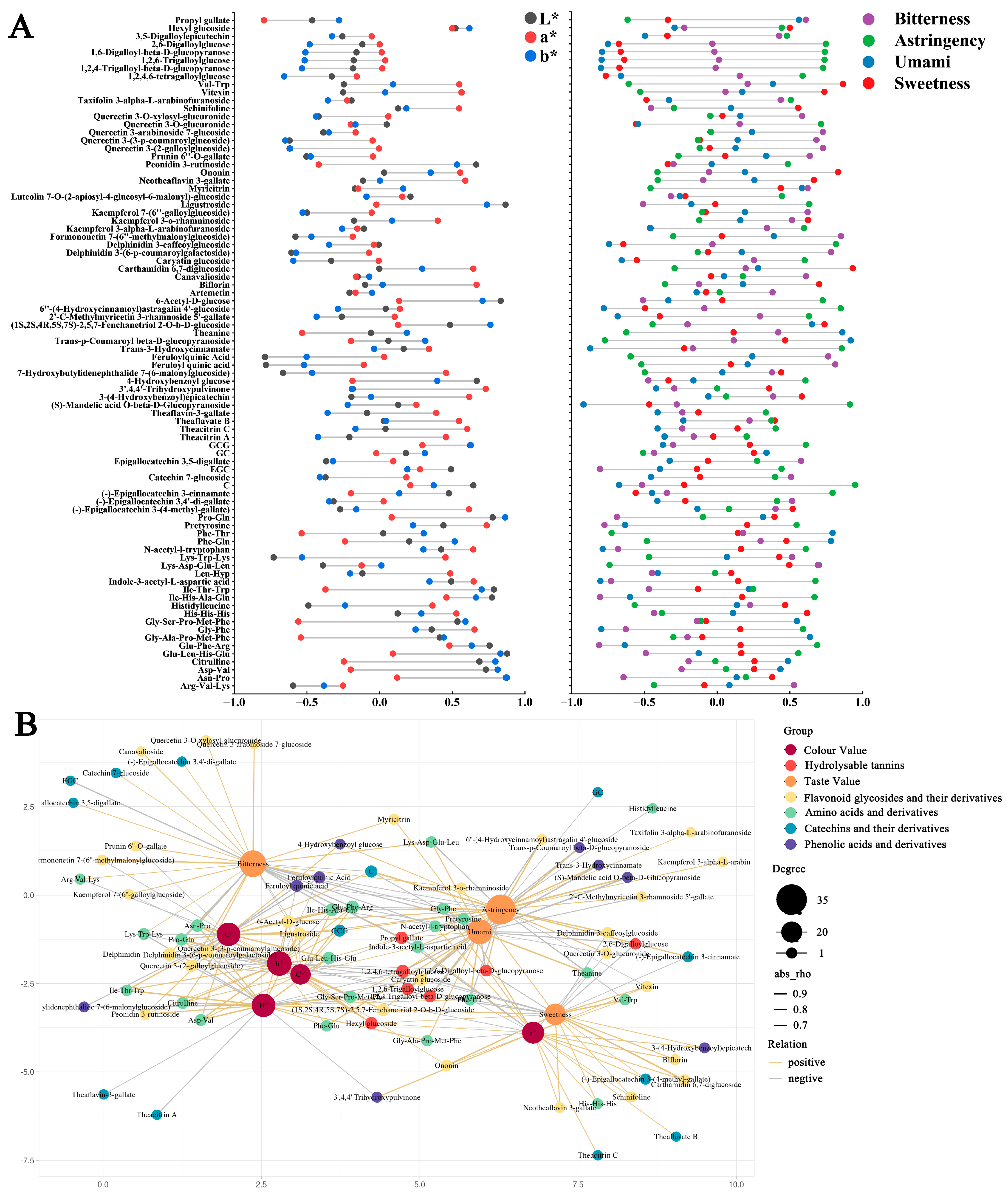
3.1.3. The Multivariate Analysis Results of LC-MS-Based Metabolomics
Non-Volatile Differential Metabolites
Differential Metabolites Related to the Color and Taste of Tea Soups
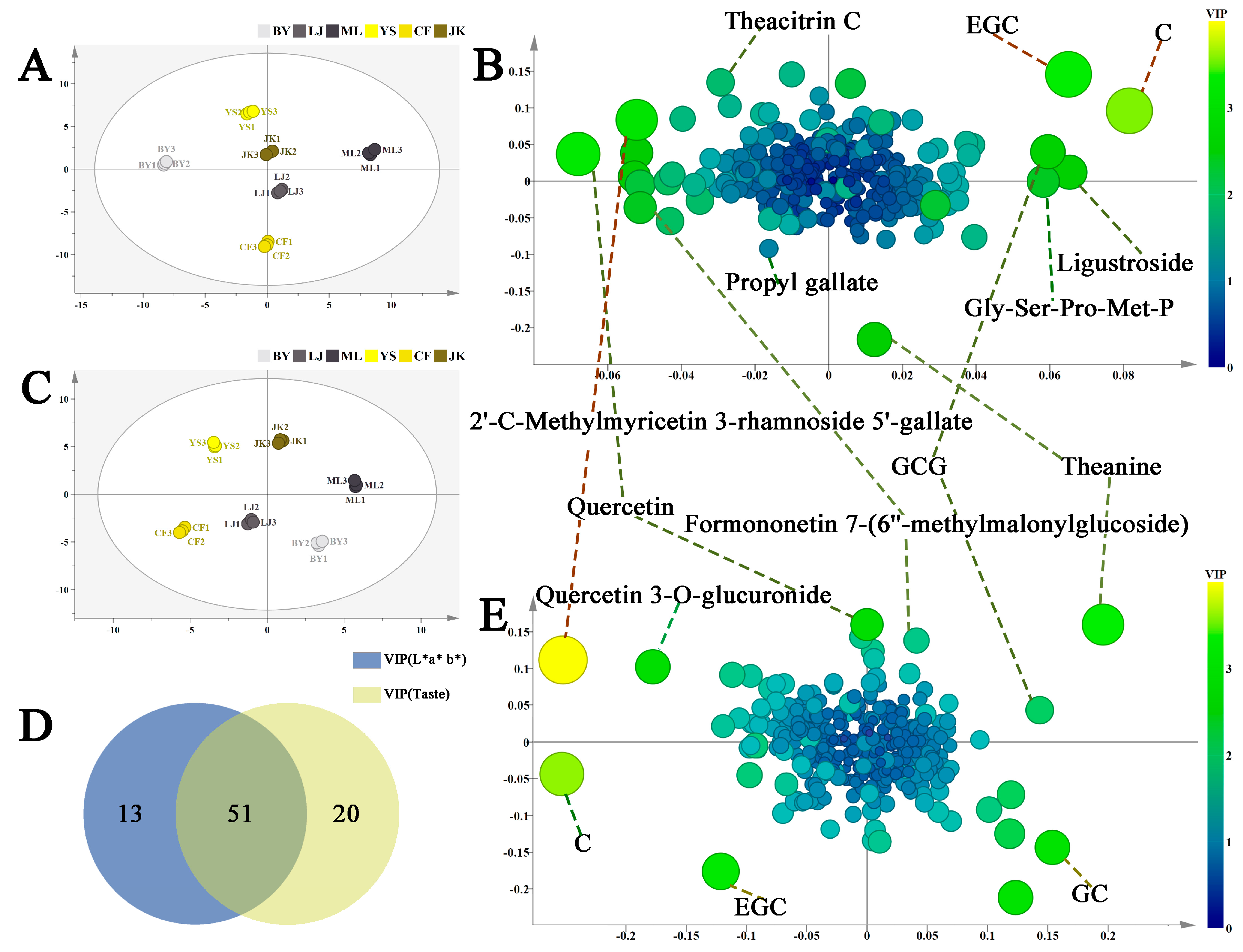
3.1.4. Correlation and Regression Analysis of Metabolites Associated with the Color and Taste of Tea Soups
Amino Acids and Their Derivatives
Catechins and Their Derivatives
Phenolic Acids
Flavonoid Glycosides and Their Derivatives
Hydrolyzable Tannins
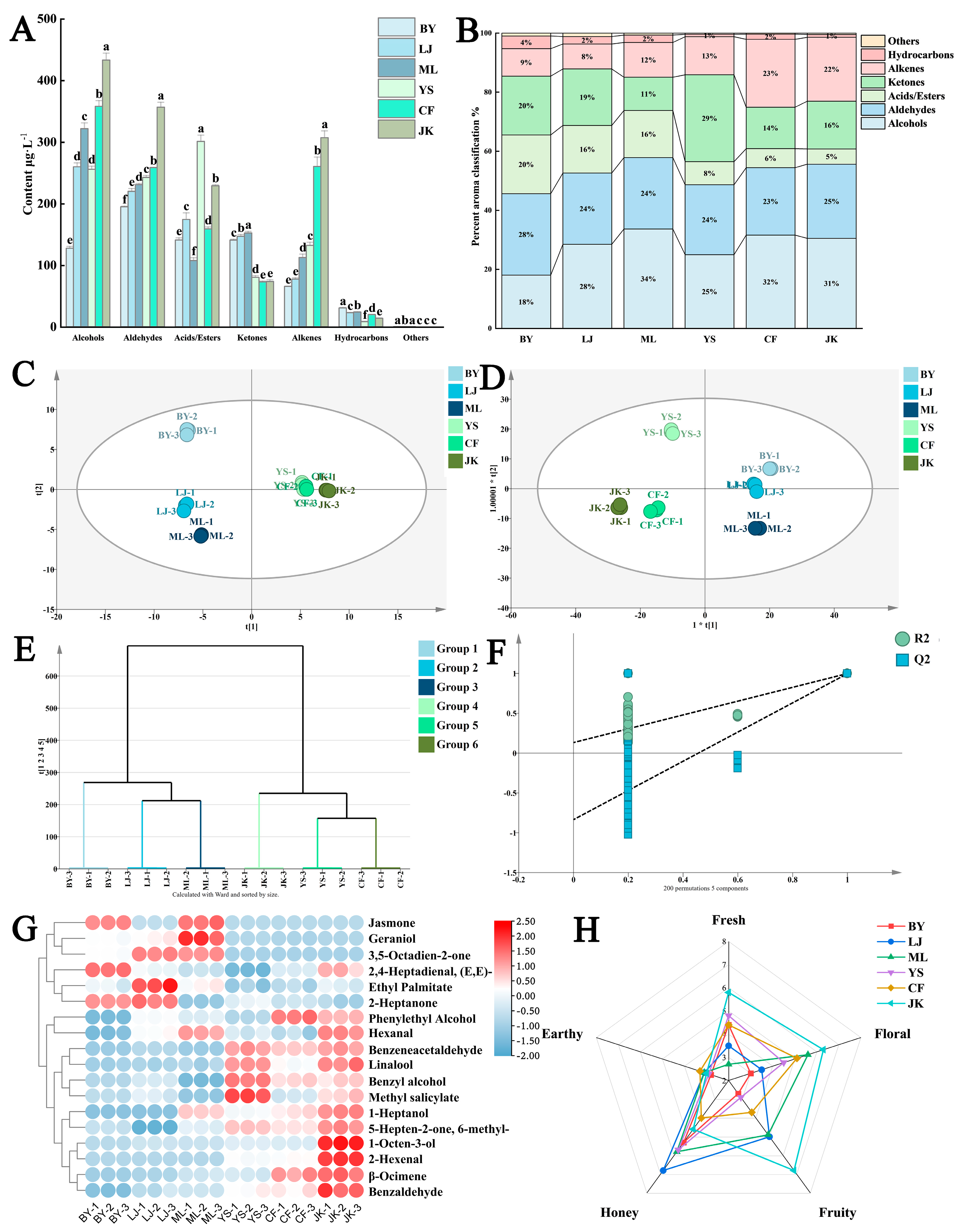
3.2. The Impact of Tea Cultivars on the Aroma of Black Tea
3.2.1. Comparison of the Types and Compositions of Volatile Substances
3.2.2. Analysis of the Discrepancies in Aroma Substances
3.2.3. Screening and Analysis of Key Aroma-Contributing Substances
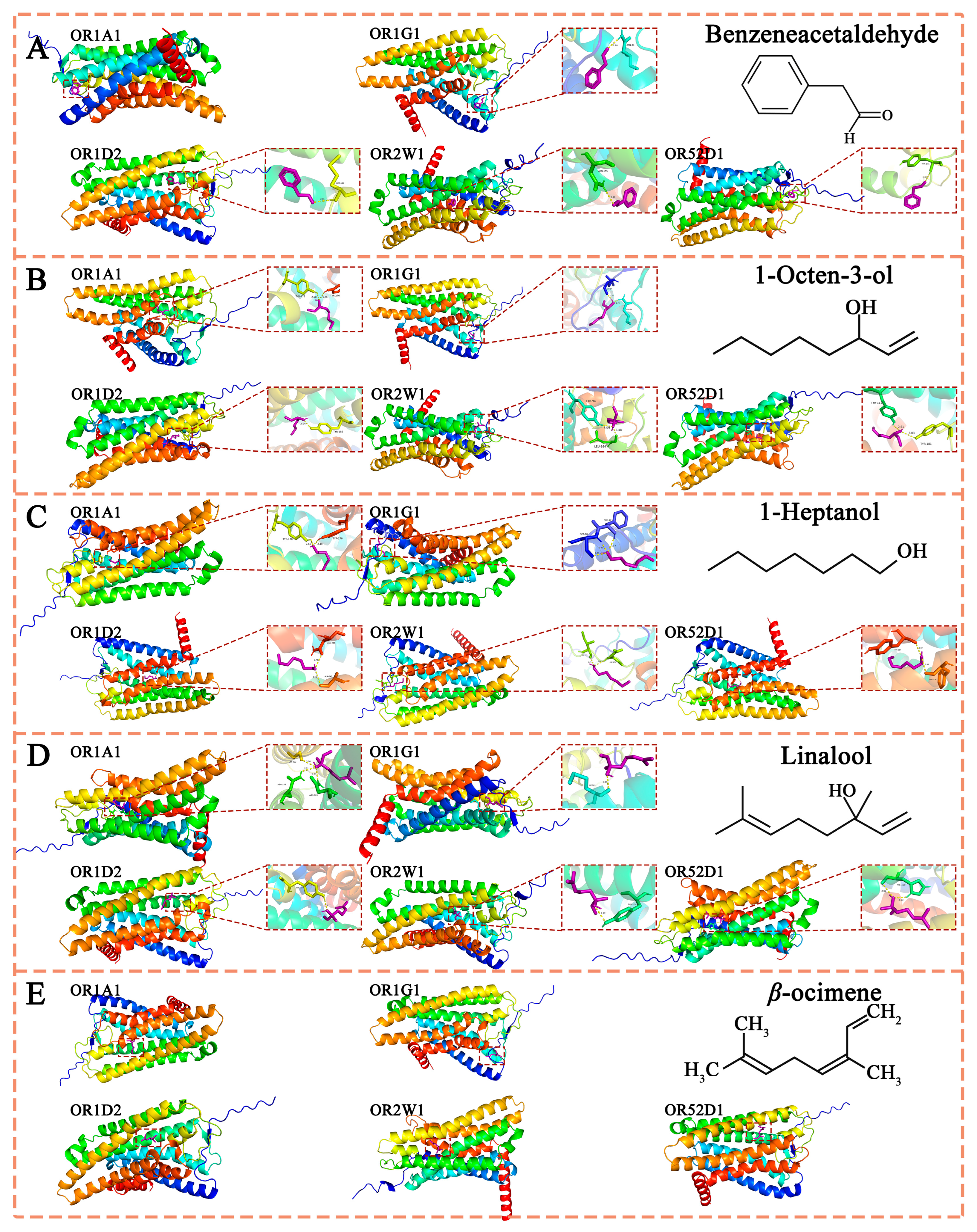
3.2.4. Molecular Docking Analysis of the Combination and Interaction Between Aroma Substances and Aroma Receptors
The Binding Interaction Region Between ORs and Aroma Compounds
The Comparison of Binding Energies Between Different ORs and Aroma Substances
The Types of Interaction Forces Between Olfactory Receptors (ORs) and Aroma Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Zhu, C.; Zhao, S.; Zhang, S.; Wang, W.; Fu, H.; Li, X.; Zhou, C.; Chen, L.; Lin, Y.; et al. De Novo Transcriptome and Phytochemical Analyses Reveal Differentially Expressed Genes and Characteristic Secondary Metabolites in the Original Oolong Tea (Camellia sinensis) Cultivar ‘Tieguanyin’ Compared with Cultivar ‘Benshan’. BMC Genom. 2019, 20, 265. [Google Scholar] [CrossRef] [PubMed]
- Nyarukowa, C.; Van Reenen, M.; Koech, R.; Mason, S.; Lindeque, Z.; Kamunya, S.; Mose, R.; Apostolides, Z. Untargeted Metabolomics Reveals Differences between Commercial and Non-Commercial Camellia sinensis Cultivars Used in Black Tea Production. J. Plant Biochem. Biotechnol. 2022, 31, 803–814. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Rothenberg, D.O.N.; Zhang, K.; Lin, Y.; Jin, K.; Li, J.; Zhou, H.; Su, H.; Ji, R.; et al. Multiomics Correlation Analysis on the Mechanism of Flavor Substance Formation during the Processing of “Huanong 53” Black Tea. LWT 2024, 214, 117086. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Luo, W.; Tang, J.; Tuo, Y.; Liao, N.; Zhuang, D.; Yang, K.; Lin, J.; Zhang, Y.; et al. Study on Metabolic Variation Reveals Metabolites Important for Flavor Development and Antioxidant Property of Hainan Dayezhong Black Tea. Food Res. Int. 2024, 196, 115112. [Google Scholar] [CrossRef]
- Owuor, P.O.; Obanda, M.; Nyirenda, H.E.; Mandala, W.L. Influence of Region of Production on Clonal Black Tea Chemical Characteristics. Food Chem. 2008, 108, 263–271. [Google Scholar] [CrossRef]
- Moreira, J.; Aryal, J.; Guidry, L.; Adhikari, A.; Chen, Y.; Sriwattana, S.; Prinyawiwatkul, W. Tea Quality: An Overview of the Analytical Methods and Sensory Analyses Used in the Most Recent Studies. Foods 2024, 13, 3580. [Google Scholar] [CrossRef]
- Long, P.; Su, S.; Han, Z.; Granato, D.; Hu, W.; Ke, J.; Zhang, L. The effects of tea plant age on the color, taste, and chemical char-acteristics of Yunnan Congou black tea by multi-spectral omics insight. Food Chem. X 2024, 21, 101190. [Google Scholar] [CrossRef]
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. State Administration for Market Regulation, Standardization Administration of PRC: Beijing, China, 2018.
- Huang, L.; Deng, G.; Fan, Y.; Wang, W.; Yu, T.; Wei, Y.; Gao, J.; Ning, J.; Wang, Y. The Processing of Shaking and Standing Improves the Taste Quality of Summer Black Tea. Food Res. Int. 2005, 201, 115545. [Google Scholar] [CrossRef]
- Hua, J.; Xu, Q.; Yuan, H.; Wang, J.; Wu, Z.; Li, X.; Jiang, Y. Effects of Novel Fermentation Method on the Biochemical Com-ponents Change and Quality Formation of Congou Black Tea. J. Food Compos. Anal. 2021, 96, 103751. [Google Scholar] [CrossRef]
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea. State Administration for Market Regulation, Standardization Administration of PRC: Beijing, China, 2018.
- GB/T 23193-2017; Determination of Theanine in Tea-Using High Performance Liquid Chromatography. State Administration for Market Regulation, Standardization Administration of PRC: Beijing, China, 2017.
- Yue, C.; Peng, H.; Li, W.; Tong, Z.; Wang, Z.; Yang, P. Untargeted Metabolomics and Transcriptomics Reveal the Mechanism of Metabolite Differences in Spring Tender Shoots of Tea Plants of Different Ages. Foods 2022, 11, 2303. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, S.; Li, T.; Wei, Y.; Gu, Z.; Su, Z.; Ning, J.; Wang, Y.; Hou, Z. Characterization of the Key Volatile Compounds in Longjing Tea (Camellia sinensis) with Different Aroma Types at Different Steeping Temperatures by GC‒MS and GC‒IMS. LWT 2024, 200, 116183. [Google Scholar] [CrossRef]
- The National Institute of Standards and Technology. NIST Inorganic Crystal Structure Database, NIST Standard Reference Database; The National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. [Google Scholar]
- Goodacre, R.; Broadhurst, D.; Smilde, A.K.; Kristal, B.S.; Baker, J.D.; Beger, R.; Bessant, C.; Connor, S.; Capuani, G.; Craig, A.; et al. Proposed Minimum Reporting Standards for Data Analysis in Metabolomics. Metabolomics 2007, 3, 231–241. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Guo, J.; Xia, Q.; Zhao, G.; Zhou, H.; Xie, F. Metabolic Profiling of Chinese Tobacco Leaf of Different Geographical Origins by GC-MS. J. Agric. Food Chem. 2013, 61, 2597–2605. [Google Scholar] [CrossRef]
- Xiong, Z.; Feng, W.; Xia, D.; Zhang, J.; Wei, Y.; Li, T.; Huang, J.; Wang, Y.; Ning, J. Distinguishing Raw Pu-Erh Tea Production Regions through a Combination of HS-SPME-GC-MS and Machine Learning Algorithms. LWT 2023, 185, 115140. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Jiang, Z.; Zhao, X. GC × GC-ToF-MS Combined with Multivariate Statistical Methods to Explore the Effects of L. Paracasei Fermentation on Bread Flavor Characteristics. Food Chem. 2024, 435, 137643. [Google Scholar] [CrossRef]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste Characterisation of Green Tea Catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Xu, M.; Wei, K.; Dai, Q.; Wan, X.; Leong, O.; Lin, R.; Cui, C.; Hou, R. The Chemical Basis of Aroma/Taste and Color Formation in Green Tea Infusion during Cold Brewing Revealed by Metabolomics Analysis. Food Chem. 2025, 479, 143788. [Google Scholar] [CrossRef]
- McMullen, M.K.; Whitehouse, J.M.; Shine, G.; Whitton, P.A.; Towell, A. Caffeine in Hot Drinks Elicits Cephalic Phase Responses Involving Cardiac Activity. Food Funct. 2012, 3, 931. [Google Scholar] [CrossRef]
- Piyasena, K.N.P.; Hettiarachchi, L.S.K.; Jayawardhane, S.A.D.P.S.; Edirisinghe, E.N.U.; Jayasinghe, W.S. Evaluation of Inherent Fructose, Glucose and Sucrose Concentrations in Tea Leaves (Camellia sinensis L.) and in Black Tea. Appl. Food Res. 2022, 2, 100100. [Google Scholar] [CrossRef]
- Deb, S.; Borah, A. Exploring the Molecular Mechanisms and Therapeutic Benefits of L-Theanine in Counteracting Inflammation. PharmaNutrition 2024, 30, 100419. [Google Scholar] [CrossRef]
- Zhang, Y.-z.; Zhang, L.-y. Effect of Raw Tea’s Fermentation Degree and Biochemical Substrates on Tea Cream Stability of Tea Drink. J. Tea Sci. 2010, 30, 533–543. [Google Scholar]
- Yang, Z.; Zhu, Y.; Xie, Y.; Yu, L. Impact of Different Aspergillus Fermentation Combinations on the Quality Components and Color of Summer and Autumn Tea Infusion. Sci. Technol. Food Ind. 2025, 46, 127–135, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Shen, X.; Wu, X.; Qi, H.; Wei, D.; Deng, M.; Wang, K.; Liu, A.; Chen, L. Oxidation Characteristics of Catechins in Suspended Fermentation of Different Varieties’ Tea Leaves. J. Food Compos. Anal. 2024, 135, 106596. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, X.; Zhao, J.; Yang, C.S.; Zhang, S.; Huang, J.; Wang, K.; Liu, Z.; Zhu, M. Theaflavins: An Underexploited Functional Compound in Black Tea. Trends Food Sci. Technol. 2024, 154, 104755. [Google Scholar] [CrossRef]
- Narukawa, M.; Toda, Y.; Nakagita, T.; Hayashi, Y.; Misaka, T. L-Theanine Elicits Umami Taste via the T1R1 + T1R3 Umami Taste Receptor. Amino Acids 2014, 46, 1583–1587. [Google Scholar] [CrossRef]
- Rhyu, M.-R.; Kim, Y.; Misaka, T. Suppression of hTAS2R16 Signaling by Umami Substances. Int. J. Mol. Sci. 2020, 21, 7045. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.-Q.; Granato, D.; Xu, Y.-Q.; Ho, C.-T. Association between Chemistry and Taste of Tea: A Review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, Y.N.; Chen, J.X.; Wang, F.; Du, Q.Z.; Yin, J.F. Quantitative analyses of the bitterness and as-tringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef]
- Yamazaki, T.; Sagisaka, M.; Ikeda, R.; Nakamura, T.; Matsuda, N.; Ishii, T.; Nakayama, T.; Watanabe, T. The Human Bitter Taste Receptor hTAS2R39 Is the Primary Receptor for the Bitterness of Theaflavins. Biosci. Biotechnol. Biochem. 2014, 78, 1753–1756. [Google Scholar] [CrossRef]
- Liu, Z.; Ran, Q.; Li, Q.; Yang, T.; Dai, Y.; Zhang, T.; Fang, S.; Pan, K.; Long, L. Interaction between Major Catechins and Umami Amino Acids in Green Tea Based on Electronic Tongue Technology. J. Food Sci. 2023, 88, 2339–2352. [Google Scholar] [CrossRef]
- Wei, Y.; Yin, X.; Zhao, M.; Zhang, J.; Li, T.; Zhang, Y.; Wang, Y.; Ning, J. Metabolomics Analysis Reveals the Mechanism Underlying the Improvement in the Color and Taste of Yellow Tea after Optimized Yellowing. Food Chem. 2023, 428, 136785. [Google Scholar] [CrossRef]
- Yu, F.; Chen, C.; Chen, S.; Wang, K.; Huang, H.; Wu, Y.; He, P.; Tu, Y.; Li, B. Dynamic changes and mechanisms of organic acids during black tea manufacturing process. Food Control. 2022, 132, 108535. [Google Scholar] [CrossRef]
- Warren, D.B.; McPhee, E.; Birru, W.A.; Benameur, H.; Chalmers, D.K.; Pouton, C.W. Location of Solvated Probe Molecules Within Nonionic Surfactant Micelles Using Molecular Dynamics. J. Pharm. Sci. 2019, 108, 205–213. [Google Scholar] [CrossRef]
- Kareem, N.S.; Zahid, N.I.; Sabah, K.J. Liquid Crystal and Emulsifying Properties of Carboxymethyl Glucoside as Water-Soluble Surfactant. Liq. Cryst. 2024, 1–14. [Google Scholar] [CrossRef]
- Murshid, N.; Wang, X. Iron-Carbonyl Aqueous Vesicles (MCsomes) by Hydration of [Fe(CO){CO(CH2)5CH3}(Cp)(PPh3)] (FpC6): Highly Integrated Colloids with Aggregation-Induced Self-Enhanced IR Absorption (AI-SEIRA). Chem. Weinh. Bergstr. Ger. 2015, 21, 19223–19230. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Wang, J.; Hua, J.; Wang, J.; Yang, Y.; Dong, C.; Zhou, Q.; Jiang, Y.; Deng, Y.; et al. Rutin, γ-Aminobutyric Acid, Gallic Acid, and Caffeine Negatively Affect the Sweet-Mellow Taste of Congou Black Tea Infusions. Molecules 2019, 24, 4221. [Google Scholar] [CrossRef]
- Yamauchi, N.; Yakushiji, K.; Tago, A.; Saito, R.; Sogame, Y.; Ogata, M.; Kobayashi, Y. Fabrication of a Sugar-Immobilized Fluorescent PMMA Shell on a Ni Core Particle via Soap-Free Emulsion Polymerization. Colloid Polym. Sci. 2022, 300, 213–221. [Google Scholar] [CrossRef]
- Iwashina, T.; Omori, Y.; Kitajima, J.; Akiyama, S.; Suzuki, T.; Ohba, H. Flavonoids in Translucent Bracts of the Himalayan Rheum Nobile (Polygonaceae) as Ultraviolet Shields. J. Plant Res. 2004, 117, 101–107. [Google Scholar] [CrossRef]
- Vallejo-Becerra, V.; Marín-Zamora, M.E.; Vásquez-Bahena, J.M.; Rojas-Melgarejo, F.; Hidalgo-Lara, M.E.; García-Ruiz, P.A. Immobilization of Recombinant Invertase (Re-INVB) from Zymomonas Mobilis on d-Sorbitol Cinnamic Ester for Production of Invert Sugar. J. Agric. Food Chem. 2008, 56, 1392–1397. [Google Scholar] [CrossRef]
- Belloir, C.; Brulé, M.; Tornier, L.; Neiers, F.; Briand, L. Biophysical and Functional Characterization of the Human TAS1R2 Sweet Taste Receptor Overexpressed in a HEK293S Inducible Cell Line. Sci. Rep. 2021, 11, 22238. [Google Scholar] [CrossRef]
- Keville, K.M.; Franses, E.I.; Caruthers, J.M. Preparation and Characterization of Monodisperse Polymer Microspheroids. J. Colloid Interface Sci. 1991, 144, 103–126. [Google Scholar] [CrossRef]
- Wang, C.; He, Z.; Zhang, C.; Du, L.; Xiao, D.; Xu, Y. Sensory and Instrumental Analysis-Guided Exploration of Odor-Active Compounds Recovery with Oil during the Water-Boiling Extraction of Pu-Erh Tea. Food Res. Int. 2020, 134, 109243. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.-P.; Zhang, Y.; Dai, W.-D.; Guo, L.; Tan, J.-F.; Peng, Q.-H.; et al. Identification and Quantification of Key Odorants in the World’s Four Most Famous Black Teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.; Jiang, H.; Sun, H.; Zhang, C.; Zhao, H.; Li, X.; Yan, F.; Chen, C.; Xu, Z. Aroma Characterization of Hanzhong Black Tea (Camellia sinensis) Using Solid Phase Extraction Coupled with Gas Chromatography–Mass Spectrometry and Olfactometry and Sensory Analysis. Food Chem. 2019, 274, 130–136. [Google Scholar] [CrossRef]
- Sharma, P.; Tudu, B.; Bhuyan, L.P.; Tamuly, P.; Bhattacharyya, N.; Bandyopadhyay, R. Detection of Methyl Salicylate in Black Tea Using a Quartz Crystal Microbalance Sensor. IEEE Sens. J. 2016, 16, 5160–5166. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, J.; Wang, Q.; Wang, L.; Shang, Y.; Jiang, Y.; Yuan, H. Volatolomics-Assisted Characterization of the Key Odorants in Green Off-Flavor Black Tea and Their Dynamic Changes during Processing. Food Chem. X 2024, 22, 101432. [Google Scholar] [CrossRef]
- Flaig, M.; Qi, S.; Wei, G.; Yang, X.; Schieberle, P. Characterization of the Key Odorants in a High-Grade Chinese Green Tea Beverage (Camellia sinensis; Jingshan Cha) by Means of the Sensomics Approach and Elucidation of Odorant Changes in Tea Leaves Caused by the Tea Manufacturing Process. J. Agric. Food Chem. 2020, 68, 5168–5179. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, D.; Mi, X.; Yu, S.; Jing, T.; An, Y. Widely Targeted Metabolomics and SPME-GC-MS Analysis Revealed the Quality Characteristics of Non-Volatile/Volatile Compounds in Zheng’an Bai Tea. Front. Nutr. 2024, 11, 1484257. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Xie, J.; Deng, Y.; Zhu, J.; Xie, Z.; Yuan, H.; Jiang, Y. Uncovering the Dynamic Alterations of Volatile Components in Sweet and Floral Aroma Black Tea during Processing. Foods 2024, 13, 728. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Li, W.-X.; Zhang, Y.-H.; Yan, H.; Guo, L.-Y.; Zhang, Y.; Lv, H.-P.; Zhou, L.-H.; Lin, Z.; Wu, W.-L.; et al. Insight into Volatile Metabolites and Key Odorants in Black Teas Processed from Jianghua Kucha Tea Germplasm (Camellia sinensis Var. Assamica Cv. Jianghua). Food Chem. 2025, 464, 141794. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Liu, Y.; Liu, Y.; Dong, C.; Lin, Z.; Teng, J. Black Tea Aroma Formation during the Fermentation Period. Food Chem. 2022, 374, 131640. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-T.; Zheng, X.; Li, S. Tea Aroma Formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Li, L.; Zan, J.; Chen, W.; Zong, X.; Yuan, H.; Jiang, Y.; Zhu, H. Maillard Reaction Inducing Amino Acids Degradation Can Adjust the Flavour Characteristic of Black Tea. Food Res. Int. 2025, 201, 115685. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, P.; Li, Z.; Wang, Y.; Liu, Y.; Zhu, Y.; Fu, H. Re-Rolling Treatment in the Fermentation Process Improves the Aroma Quality of Black Tea. Foods 2023, 12, 3702. [Google Scholar] [CrossRef]
- Xiao, Z.; Shen, T.; Ke, Q.; Shen, X.; Yang, E.; Sun, Z.; Niu, Y.; Zhu, J. Identification of Characteristic Aroma Compounds of Longjing Tea and Their Molecular Mechanisms of Interaction with Olfactory Receptors Using Molecular Docking. Eur. Food Res. Technol. 2024, 250, 1363–1378. [Google Scholar] [CrossRef]
- Tong, T.; Wang, Y.; Kang, S.-G.; Huang, K. Ectopic Odorant Receptor Responding to Flavor Compounds: Versatile Roles in Health and Disease. Pharmaceutics 2021, 13, 1314. [Google Scholar] [CrossRef]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-Transmembrane Receptors. Nat. Rev. Mol. Cell Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef]
- Floriano, W.B. Making Sense of Olfaction through Predictions of the 3-D Structure and Function of Olfactory Receptors. Chem. Senses 2004, 29, 269–290. [Google Scholar] [CrossRef]
- Fuchs, T.; Glusman, G.; Horn-Saban, S.; Lancet, D.; Pilpel, Y. The Human Olfactory Subgenome: From Sequence to Structure and Evolution. Hum. Genet. 2001, 108, 1–13. [Google Scholar] [CrossRef]
- Mombaerts, P. Seven-Transmembrane Proteins as Odorant and Chemosensory Receptors. Science 1999, 286, 707–711. [Google Scholar] [CrossRef]
- Wachowiak, M.; Shipley, M.T. Coding and Synaptic Processing of Sensory Information in the Glomerular Layer of the Olfactory Bulb. Semin. Cell Dev. Biol. 2006, 17, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, L.; Li, P.; Pu, D.; Fu, Y.; Zheng, R.; Xi, H.; Qiao, K.; Wang, D.; Sun, B.; et al. Molecular Mechanisms of Caramel-like Odorant-Olfactory Receptor Interactions Based on a Computational Chemistry Approach. Food Res. Int. 2023, 171, 113063. [Google Scholar] [CrossRef] [PubMed]
- Katada, S.; Hirokawa, T.; Oka, Y.; Suwa, M.; Touhara, K. Structural Basis for a Broad But Selective Ligand Spectrum of a Mouse Olfactory Receptor: Mapping the Odorant-Binding Site. J. Neurosci. 2005, 25, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
| Taste | Standards (g/L) | Scores | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Umami | Monosodium glutamate | 0 | 0.16 | 0.32 | 0.48 | 0.64 | 0.8 |
| Sweetness | Sucrose | 0 | 2 | 4 | 6 | 8 | 10 |
| Astringency | EGCG | 0 | 0.2 | 0.4 | 0.6 | 0.8 | 1 |
| Bitterness | Caffeine | 0 | 0.3 | 0.6 | 0.9 | 1.2 | 1.5 |
| ID | Compounds | m/z | VIP (LAB) | VIP (Taste) |
|---|---|---|---|---|
| pos_870 | Pro-Gln | 226.12 | 1.34 | <1 |
| pos_1524 | Histidylleucine | 268.15 | 2.38 | 2.16 |
| pos_1716 | 2,6-Digalloylglucose | 502.12 | <1 | 1.26 |
| pos_1813 | Delphinidin 3-caffeoylglucoside | 666.09 | <1 | 1.05 |
| pos_2297 | 1,6-Digalloyl-β-D-glucopyranose | 467.08 | <1 | 1.38 |
| pos_2892 | Kaempferol 3-o-rhamninoside | 741.22 | 1.05 | 1.11 |
| pos_3107 | Indole-3-acetyl-L-aspartic acid | 291.1 | 1.27 | 1.4 |
| pos_3142 | Kaempferol 7-(6″-galloylglucoside) | 601.12 | 2.4 | 1.81 |
| pos_3914 | Delphinidin 3-(6-p-coumaroylgalactoside) | 611.14 | 2.18 | 1.54 |
| pos_4371 | Glu-Leu-His-Glu | 527.25 | 1.31 | <1 |
| pos_5630 | Propyl gallate | 177.05 | 1.08 | 1.1 |
| pos_7437 | Gly-Ala-Pro-Met-Phe | 620.32 | 1.31 | 1.01 |
| pos_8384 | Gly-Ser-Pro-Met-Phe | 636.32 | 1.68 | 1.06 |
| pos_9659 | Artemetin | 389.12 | 1.34 | 1.33 |
| pos_9964 | Neotheaflavin 3-gallate | 734.17 | 1.59 | 1.92 |
| pos_9969 | Biflorin | 359.08 | 1.05 | 1.23 |
| pos_10356 | Schinifoline | 283.14 | 2.14 | 2.88 |
| pos_10419 | (-)-Epigallocatechin 3-cinnamate | 437.12 | <1 | 1.01 |
| pos_11115 | Ile-His-Ala-Glu | 469.24 | 1.62 | 1.21 |
| pos_11116 | Glu-Phe-Arg | 451.23 | 1.57 | 1.22 |
| pos_11262 | His-His-His | 412.19 | 1.03 | 1.47 |
| pos_12140 | 2′-C-Methylmyricetin 3-rhamnoside 5′-gallate | 595.11 | 3.12 | 3.99 |
| pos_12770 | Quercetin 3-arabinoside 7-glucoside | 601.12 | 1.89 | 1.82 |
| pos_12874 | (-)-Epigallocatechin 3,4′-di-gallate | 611.11 | 1.45 | 1.63 |
| pos_12943 | Kaempferol 3-α-L-arabinofuranoside | 441.08 | <1 | 1.08 |
| pos_13329 | Pretyrosine | 192.07 | <1 | 1.04 |
| pos_13370 | 1,2,6-Trigalloylglucose | 654.13 | <1 | 1.37 |
| pos_13602 | Val-Trp | 321.19 | <1 | 1.02 |
| pos_13657 | Trans-p-Coumaroyl β-D-glucopyranoside | 344.13 | <1 | 1.35 |
| pos_13662 | Phe-Glu | 295.13 | 1.01 | 1.09 |
| pos_13667 | Leu-Hyp | 227.14 | 1.14 | 1.22 |
| pos_14125 | Ile-Thr-Trp | 401.22 | 1.07 | <1 |
| pos_14959 | Asn-Pro | 271.14 | 2.52 | 1.26 |
| pos_15431 | Citrulline | 158.09 | 1.31 | <1 |
| pos_15692 | Peonidin 3-rutinoside | 627.22 | 1.1 | <1 |
| neg_1790 | Phe-Thr | 265.12 | <1 | 1.04 |
| neg_2521 | Feruloylquinic Acid | 367.1 | 1.22 | <1 |
| neg_2957 | Quercetin 3-O-xylosyl-glucuronide | 631.1 | 3.3 | 2.64 |
| neg_2985 | 1,2,4-Trigalloyl-β-D-glucopyranose | 635.09 | <1 | 1.29 |
| neg_3696 | Vitexin | 431.1 | <1 | 1.07 |
| neg_3758 | Myricitrin | 927.19 | 1.08 | 1.36 |
| neg_3960 | 4-Hydroxybenzoyl glucose | 281.07 | 1.06 | <1 |
| neg_3990 | Gly-Phe | 203.08 | <1 | 1.16 |
| neg_3991 | N-acetyl-l-tryptophan | 245.09 | <1 | 1.12 |
| neg_4461 | 3-(4-Hydroxybenzoyl)epicatechin | 455.1 | 1.15 | 1.07 |
| neg_4541 | Catechin 7-glucoside | 497.13 | 1.05 | 1.03 |
| neg_4626 | Trans-3-Hydroxycinnamate | 163.04 | <1 | 1.07 |
| neg_4999 | Prunin 6″-O-gallate | 585.13 | 1.96 | 1.49 |
| neg_5195 | Hexyl glucoside | 245.14 | 1.3 | 1.05 |
| neg_5379 | Quercetin 3-(3-p-coumaroylglucoside) | 609.13 | 1.42 | <1 |
| neg_5542 | Theaflavate B | 699.14 | 1.32 | 1.52 |
| neg_5653 | Caryatin glucoside | 551.14 | 1.19 | 1.36 |
| neg_5737 | (1S,2S,4R,5S,7S)-2,5,7-Fenchanetriol 2-O-b-D-glucoside | 347.17 | 1.27 | 1.17 |
| neg_5859 | Luteolin 7-O-(2-apiosyl-4-glucosyl-6-malonyl)-glucoside | 849.17 | 1.77 | 1.94 |
| neg_6221 | Ligustroside | 569.19 | 2.27 | 1.36 |
| neg_6234 | Ononin | 429.12 | 1.66 | 2.27 |
| neg_6659 | Formononetin 7-(6″-methylmalonylglucoside) | 551.12 | 2.28 | 1.83 |
| neg_9096 | Lys-Trp-Lys | 459.28 | 1.13 | <1 |
| neg_9708 | Canavalioside | 605.28 | <1 | 1.19 |
| neg_11484 | Arg-Val-Lys | 400.27 | 1.94 | 1.41 |
| neg_12563 | 6-Acetyl-D-glucose | 221.07 | 1.28 | <1 |
| neg_13036 | 3′,4,4′-Trihydroxypulvinone | 311.06 | 1.85 | 1.76 |
| neg_13240 | 7-Hydroxybutylidenephthalide 7-(6-malonylglucoside) | 451.13 | 1.33 | <1 |
| neg_13437 | Lys-Asp-Glu-Leu | 524.24 | <1 | 1.04 |
| neg_13708 | Taxifolin 3-α-L-arabinofuranoside | 435.09 | <1 | 1.36 |
| neg_14965 | Quercetin 3-O-glucuronide | 477.07 | 1.63 | 2.69 |
| neg_15158 | 3,5-Digalloylepicatechin | 593.09 | 1.09 | 1.36 |
| neg_16109 | 6′’-(4-Hydroxycinnamoyl)astragalin 4′-glucoside | 737.17 | 1.02 | 1.68 |
| neg_16123 | (S)-Mandelic acid O-β-D-Glucopyranoside | 295.08 | <1 | 1.33 |
| neg_16316 | Epigallocatechin 3,5-digallate | 609.09 | 1.2 | 1.16 |
| neg_16336 | 1,2,4,6-tetragalloylglucose | 787.1 | 1.15 | 1.36 |
| neg_16411 | (-)-Epigallocatechin 3-(4-methyl-gallate) | 471.09 | 1.21 | 1.03 |
| neg_16511 | Quercetin 3-(2-galloylglucoside) | 615.1 | 1.79 | 1.2 |
| neg_16755 | Feruloyl quinic acid | 367.1 | 1.08 | <1 |
| neg_17083 | Carthamidin 6,7-diglucoside | 611.16 | <1 | 1.01 |
| neg_18318 | Asp-Val | 231.1 | 1.22 | <1 |
| pos_11536 | Theaflavin-3-gallate | 717.15 | 1.39 | 1.33 |
| neg_1932 | Theacitrin A | 759.12 | 1.61 | 1.29 |
| neg_2903 | Theacitrin C | 911.13 | 1.68 | 1.72 |
| pos_12345 | Theanine | 174.2 | 2.47 | 3.36 |
| pos_12346 | EGC | 457.08 | 3.41 | 3.11 |
| pos_12347 | GC | 305.07 | 2.21 | 2.9 |
| pos_12348 | C | 289.06 | 3.6 | 3.63 |
| pos_12349 | GCG | 487.08 | 2.56 | 1.95 |
| No. | CAS | RI | BY | LJ | ML | YS | CF | JK | |
|---|---|---|---|---|---|---|---|---|---|
| Alcoholic compounds | |||||||||
| 1 | 1576-87-0 | trans-2-Pentenal | 1228 | 4.53 ± 0.25 c | 9.06 ± 0.36 a | 4.62 ± 0.48 c | 2.83 ± 0.31 d | 6.75 ± 0.27 b | 8.55 ± 0.91 a |
| 2 | 10340-23-5 | cis-3-Nonen-1-ol | 1565 | 0.87 ± 0.1 a | 0.79 ± 0.09 a | 0.57 ± 0.09 b | 0 | 0 | 0 |
| 3 | 106-25-2 | Nerol | 1255 | 0 | 0 | 0 | 2.21 ± 0.17 b | 1.53 ± 0.05 c | 4.61 ± 0.34 a |
| 4 | 96-76-4 | 2,4-Di-tert-butylphenol | 1518 | 0.59 ± 0.02 a | 0.46 ± 0.07 b | 0 | 0.45 ± 0.06 b | 0 | 0 |
| 5 | 40716-66-3 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | 1175 | 0.61 ± 0.02 b | 2.39 ± 0.16 a | 0 | 0 | 0 | 0 |
| 6 | 77-53-2 | Cedrol | 1600 | 1.15 ± 0.14 a | 0.24 ± 0.03 b | 0 | 0 | 0 | 0 |
| 7 | 481-34-5 | α-Cadinol | 1190 | 0.52 ± 0.02 b | 0 | 0 | 0 | 0.61 ± 0.04 a | 0 |
| 8 | 111-70-6 | 1-Heptanol | 973 | 0 | 0 | 43.94 ± 0.77 b | 31.13 ± 0.93 d | 39.96 ± 2.65 c | 57.29 ± 1.94 a |
| 9 | 3391-86-4 | 1-Octen-3-ol | 982 | 0 | 7.07 ± 0.11 c | 5.61 ± 0.22 c | 12.64 ± 0.1 b | 11.46 ± 0.32 b | 73.38 ± 2.26 a |
| 10 | 100-51-6 | Benzyl alcohol | 1037 | 29.76 ± 0.43 d | 40.44 ± 0.48 c | 12.75 ± 0.51 e | 80.5 ± 1.03 a | 62.52 ± 2.36 b | 62.68 ± 3.93 b |
| 11 | 78-70-6 | Linalool | 1102 | 28.17 ± 1.07 c | 30.11 ± 1.16 c | 30.96 ± 3.37 c | 69.32 ± 0.75 a | 50.72 ± 1.83 b | 72.86 ± 3.46 a |
| 12 | 60-12-8 | Phenylethyl alcohol | 1116 | 0 | 99.26 ± 1.77 c | 76.94 ± 5.46 d | 43.3 ± 3.16 e | 176.01 ± 6.33 a | 142.75 ± 2.36 b |
| 13 | 106-24-1 | Geraniol | 1256 | 61.37 ± 3.33 b | 70.05 ± 5.61 b | 146.44 ± 14.06 a | 13.36 ± 0.94 c | 8.55 ± 1.43 c | 11.18 ± 0.37 c |
| Aldehydic compounds | |||||||||
| 14 | 111-71-7 | Heptanal | 903 | 2.46 ± 0.25 b | 1.22 ± 0.08 d | 3.21 ± 0.27 a | 0 | 1.49 ± 0.15 d | 1.97 ± 0.1 c |
| 15 | 824-22-6 | 4-methylindan | 803 | 0 | 0 | 3.99 ± 0.24 b | 3.77 ± 0.23 b | 3.99 ± 0.21 b | 4.66 ± 0.26 a |
| 16 | 55722-59-3 | 3,6-Octadienal, 3,7-dimethyl- | 855 | 0 | 0 | 2.44 ± 0.25 d | 3.71 ± 0.26 c | 5.6 ± 0.11 a | 4.46 ± 0.42 b |
| 17 | 116-26-7 | Safranal | 930 | 0.98 ± 0.11 c | 1.02 ± 0.07 c | 2.06 ± 0.13 a | 0.44 ± 0.04 d | 0.56 ± 0.03 d | 1.35 ± 0.08 b |
| 18 | 112-31-2 | Decyl aldehyde | 1206 | 0.52 ± 0.03 c | 0 | 0 | 1.1 ± 0.14 b | 1.34 ± 0.15 a | 1.13 ± 0.09 b |
| 19 | 15764-16-6 | 2,4-Dimethylbenzaldehyde | 1150 | 0.52 ± 0.03 c | 0 | 0 | 1.1 ± 0.14 b | 1.34 ± 0.15 a | 1.13 ± 0.09 b |
| 20 | 5910-87-2 | 2,4-Nonadienal, (E,E)- | 1214 | 0.83 ± 0.09 b | 0.93 ± 0.13 b | 1.3 ± 0.16 a | 0 | 0 | 0 |
| 21 | 432-25-7 | Pentadeuterio--cyclocitral | 1260 | 0 | 0.67 ± 0.08 c | 1 ± 0.07 b | 2.25 ± 0.21 a | 0.67 ± 0.07 c | 0 |
| 22 | 106-26-3 | Neral | 1290 | 0 | 0 | 0 | 1.19 ± 0.13 c | 2.36 ± 0.13 b | 3 ± 0.15 a |
| 23 | 26643-91-4 | 4-methyl-2-phenyl-1-pentenal | 1315 | 0 | 1.96 ± 0.17 a | 1.94 ± 0.18 a | 1.29 ± 0.03 c | 1.57 ± 0.14 b | 1.74 ± 0.12 ab |
| 24 | 13019-16-4 | (E)-2-butyloct-2-enal | 1040 | 0.98 ± 0.09 d | 1.69 ± 0.12 c | 2.88 ± 0.26 b | 3.34 ± 0.27 a | 3.27 ± 0.2 a | 2 ± 0.2 c |
| 25 | 21834-92-4 | Cocal | 960 | 7.84 ± 0.75 a | 1.12 ± 0.03 c | 0.87 ± 0.13 c | 0 | 0 | 2.31 ± 0.42 b |
| 26 | 6728-26-3 | 2-Hexenal, (E)- | 960 | 7.05 ± 0.15 a | 7.02 ± 0.62 a | 6.95 ± 0.25 a | 6.13 ± 0.4 b | 5.21 ± 0.1 c | 7.18 ± 0.33 a |
| 27 | 4313-03-5 | 2,4-Heptadienal, (E,E)- | 1012 | 56.73 ± 0.35 a | 36.21 ± 1.24 c | 28.1 ± 1.02 e | 13.28 ± 0.98 f | 31.73 ± 0.42 d | 47.73 ± 3.58 b |
| 28 | 122-78-1 | Benzeneacetaldehyde | 1044 | 0 | 3.66 ± 0.21 c | 0 | 25.71 ± 1.49 a | 21.11 ± 0.05 b | 25.58 ± 1.25 a |
| 29 | 141-27-5 | 2,6-Octadienal, 3,7-dimethyl-, (E)- | 1271 | 0 | 0 | 4.67 ± 0.22 c | 10.02 ± 0.74 b | 12.74 ± 1.37 a | 12.54 ± 0.48 a |
| 30 | 5392-40-5 | Citral | 1240 | 2.92 ± 0.16 e | 3.02 ± 0.26 e | 5.22 ± 0.1 d | 8.22 ± 0.29 b | 7.35 ± 0.14 c | 15.75 ± 0.48 a |
| 31 | 66-25-1 | Hexanal | 803 | 30.8 ± 0.94 e | 51.32 ± 0.9 b | 61.51 ± 1.07 a | 44.69 ± 2.28 c | 39.09 ± 4.35 d | 64.09 ± 1 a |
| 32 | 505-57-7 | 2-Hexenal | 855 | 0 | 0 | 0 | 7.82 ± 0.49 c | 8.65 ± 0.48 b | 22.92 ± 0.55 a |
| 33 | 100-52-7 | Benzaldehyde | 960 | 69.34 ± 1.31 d | 86.06 ± 4.94 c | 89.08 ± 0.61 c | 104.93 ± 2.9 b | 108.54 ± 3.67 b | 135.77 ± 5.8 a |
| 34 | 124-13-0 | Octanal | 1003 | 9.68 ± 0.88 b | 14.89 ± 1.03 a | 10.45 ± 0.56 b | 0 | 0 | 0 |
| Acid esters | |||||||||
| 35 | 106-70-7 | Hexanoic acid, methyl ester | 928 | 3.63 ± 0.23 c | 4.1 ± 0.19 c | 3.71 ± 0.51 c | 5.84 ± 0.69 b | 6.02 ± 0.74 b | 9.67 ± 0.48 a |
| 36 | 16491-36-4 | 3-hexenylbutyrate | 1187 | 2.78 ± 0.18 a | 2.22 ± 0.13 b | 2.12 ± 0.15 b | 2.97 ± 0.21 a | 2.67 ± 0.12 a | 2.07 ± 0.19 b |
| 37 | 35154-45-1 | cis-3-Hexenyl isovalerate | 1150 | 4.28 ± 0.25 a | 2.89 ± 0.15 c | 3.36 ± 0.3 b | 2.57 ± 0.32 cd | 2.43 ± 0.15 d | 3.55 ± 0.21 b |
| 38 | 1189-09-9 | E-Methylgeranate | 1380 | 0 | 1.29 ± 0.11 a | 0.79 ± 0.07 b | 0 | 1.28 ± 0.1 a | 0 |
| 39 | 104-61-0 | Nonanolactone | 1363 | 1.7 ± 0.12 a | 1.33 ± 0.07 b | 1.29 ± 0.07 b | 1.36 ± 0.12 b | 0 | 0 |
| 40 | 31501-11-8 | cis-3-Hexenyl hexanoate | 1120 | 0.53 ± 0.06 b | 1 ± 0.11 a | 0.91 ± 0.1 a | 0 | 0 | 0 |
| 41 | 6378-65-0 | Hexyl hexanoate | 1194 | 0.66 ± 0.08 d | 0 | 1.42 ± 0.11 c | 1.57 ± 0.03 b | 1.79 ± 0.09 a | 1.65 ± 0.1 ab |
| 42 | 53398-86-0 | trans-2-Hexenyl Hexanoate | 1250 | 0.24 ± 0.08 c | 0.42 ± 0.07 a | 0.34 ± 0.01 b | 0.24 ± 0.01 c | 0.24 ± 0.04 c | 0 |
| 43 | 103-52-6 | Phenylethyl butyrate | 1170 | 0.82 ± 0.06 d | 1.19 ± 0.13 a | 1.14 ± 0.1 ab | 0.9 ± 0.08 cd | 1.02 ± 0.06 bc | 1.15 ± 0.07 ab |
| 44 | 17092-92-1 | Dihydroactinidiolide | 1360 | 0.42 ± 0.04 c | 2.18 ± 0.11 b | 2.78 ± 0.07 a | 0 | 0 | 0 |
| 45 | 84-66-2 | Diethyl Phthalate | 1560 | 2.45 ± 0.06 a | 0 | 1.06 ± 0.06 c | 0.54 ± 0.05 d | 1.48 ± 0.02 b | 0.59 ± 0.05 d |
| 46 | 1211-29-6 | Methyl jasmonate | 1681 | 0 | 0 | 0.87 ± 0.02 b | 0.39 ± 0.01 d | 1.21 ± 0.08 a | 0.65 ± 0.1 c |
| 47 | 124-06-1 | Ethyl myristate | 1599 | 0.36 ± 0.03 b | 0.11 ± 0.01 e | 0.25 ± 0.01 c | 0.19 ± 0.01 d | 0.22 ± 0.02 c | 0.4 ± 0.01 a |
| 48 | 112-39-0 | Hexadecanoic acid, methyl ester | 1796 | 0 | 0 | 0 | 0.24 ± 0.02 a | 0.11 ± 0.02 b | 0.05 ± 0.01 c |
| 49 | 84-74-2 | Dibutyl phthalate | 2000+ | 0.49 ± 0.09 b | 0.58 ± 0.05 a | 0.55 ± 0.05 ab | 0 | 0 | 0 |
| 50 | 111-61-5 | Ethyl stearate | 1994 | 0 | 0 | 0 | 3.9 ± 0.53 b | 5.6 ± 0.76 a | 5.75 ± 0.89 a |
| 51 | 628-97-7 | Ethyl Palmitate | 2193 | 32.47 ± 1.48 c | 43.94 ± 1.66 a | 34.95 ± 0.94 b | 26.79 ± 1.54 d | 28.85 ± 1.22 d | 31.92 ± 1.1 c |
| 52 | 119-36-8 | Methyl salicylate | 1194 | 88.31 ± 2.43 d | 115.04 ± 12.11 c | 53 ± 3.87 e | 252.19 ± 7.88 a | 104.62 ± 3.29 c | 170.28 ± 2.63 b |
| 53 | 112-05-0 | Nonanoic acid | 1311 | 0 | 0 | 0 | 0.97 ± 0.15 a | 0.57 ± 0.04 c | 0.83 ± 0.09 b |
| 54 | 459-80-3 | Geranic acid | 1170 | 0 | 0.22 ± 0.03 c | 0 | 0.5 ± 0.02 a | 0.34 ± 0.01 b | 0.22 ± 0.03 c |
| 55 | 57-10-3 | Palmitic acid | 1994 | 1.15 ± 0.1 b | 0 | 1.91 ± 0.08 a | 0 | 0.23 ± 0.02 c | 0 |
| Hydrocarbons | |||||||||
| 56 | 17301-23-4 | Undecane, 2,6-dimethyl- | 1332 | 4.21 ± 0.23 bc | 4.4 ± 0.25 b | 2.86 ± 0.15 d | 3.84 ± 0.21 c | 4.12 ± 0.22 bc | 4.91 ± 0.46 a |
| 57 | 91-57-6 | 2-Methylnaphthalene | 1315 | 0.63 ± 0.07 b | 0.81 ± 0.06 a | 0.84 ± 0.13 a | 0 | 0 | 0.9 ± 0.08 a |
| 58 | 35599-77-0 | 1-Iodotridecane | 1400 | 2.15 ± 0.15 b | 4.19 ± 0.13 a | 1.26 ± 0.07 c | 0 | 0.29 ± 0.02 e | 0.91 ± 0.16 d |
| 59 | 294-62-2 | Cyclododecane | 1420 | 3.47 ± 0.1 b | 3.5 ± 0.2 b | 2.72 ± 0.08 d | 3.09 ± 0.06 c | 2.88 ± 0.03 cd | 4.05 ± 0.25 a |
| 60 | 544-77-4 | Hexadecyl iodide | 1400 | 4.73 ± 0.19 b | 0 | 0.18 ± 0.02 d | 0 | 6.03 ± 0.13 a | 0.47 ± 0.05 c |
| 61 | 629-99-2 | Pentacosane | 1600 | 2.66 ± 0.11 a | 0.71 ± 0.08 c | 0 | 0.75 ± 0.07 c | 1.63 ± 0.15 b | 0.69 ± 0.08 c |
| 62 | 1560-92-5 | Hexadecane, 2-methyl- | 1800 | 0.45 ± 0.03 c | 2.18 ± 0.11 b | 2.78 ± 0.07 a | 0 | 0 | 0 |
| 63 | 27458-90-8 | Tert-Dodecyl Disulfide | 2000 | 1.11 ± 0.11 bc | 1.22 ± 0.1 b | 2.72 ± 0.18 a | 0 | 0.99 ± 0.14 c | 0 |
| 64 | 18435-45-5 | 1-Nonadecene | 2200 | 1.86 ± 0.05 a | 1.51 ± 0.04 b | 0 | 0 | 1.55 ± 0.1 b | 0 |
| 65 | 544-76-3 | Hexadecane | 1200 | 0.74 ± 0.06 c | 3.5 ± 0.09 a | 0.97 ± 0.07 b | 0.41 ± 0.06 d | 0.68 ± 0.04 c | 0.23 ± 0.03 e |
| 66 | 7390-81-0 | 1,2-Epoxyoctadecane | 1500 | 0 | 0 | 0 | 0 | 0.94 ± 0.06 a | 0.62 ± 0.06 b |
| 67 | 3075-84-1 | 2,2′,5,4′-Tetramethylbiphenyl | 1700 | 0.82 ± 0.1 b | 0.27 ± 0.02 c | 1.55 ± 0.1 a | 0.26 ± 0.02 c | 0.28 ± 0.02 c | 0.21 ± 0.02 c |
| 68 | 629-94-7 | Heneicosane | 1900 | 6.6 ± 0.29 a | 0 | 6.42 ± 0.25 a | 0 | 0 | 0 |
| 69 | 112-88-9 | 1-Octadecene | 1100 | 0 | 0.19 ± 0.02 c | 0.44 ± 0.03 b | 0 | 0.46 ± 0.02 b | 0.78 ± 0.13 a |
| Alkenic compounds | |||||||||
| 70 | 5989-27-5 | D-Limonene | 1028 | 3.63 ± 0.23 c | 4.1 ± 0.19 c | 3.71 ± 0.51 c | 5.84 ± 0.69 b | 6.02 ± 0.74 b | 9.67 ± 0.48 a |
| 71 | 535-77-3 | M-cymene | 1024 | 2.01 ± 0.12 b | 4 ± 0.13 a | 2 ± 0.08 b | 1.98 ± 0.11 b | 0 | 0 |
| 72 | 7216-56-0 | 2,4,6-Octatriene, 2,6-dimethyl-, (E,Z)- | 1415 | 1.47 ± 0.09 c | 1.19 ± 0.11 d | 0.79 ± 0.06 e | 4.6 ± 0.29 a | 0 | 1.97 ± 0.16 b |
| 73 | 99-86-5 | α-Terpinene | 1016 | 5.98 ± 0.33 a | 3.05 ± 0.18 c | 2.14 ± 0.14 d | 1.73 ± 0.12 d | 2.81 ± 0.43 c | 4.81 ± 0.31 b |
| 74 | 17699-14-8 | (-)-α-cubebene | 1349 | 2.33 ± 0.18 a | 0 | 0 | 0 | 0.38 ± 0.04 c | 1.57 ± 0.07 b |
| 75 | 475-20-7 | Longifolene | 1380 | 0 | 0 | 1.59 ± 0.08 a | 0.17 ± 0.02 c | 0.28 ± 0.06 c | 0.69 ± 0.13 b |
| 76 | 469-61-4 | (-)-α-cedrene | 1410 | 0.7 ± 0.15 d | 0.89 ± 0.1 cd | 0 | 2.72 ± 0.11 a | 0.96 ± 0.11 c | 1.42 ± 0.11 b |
| 77 | 21391-99-1 | α-calacorene | 1405 | 0.29 ± 0.01 e | 0.81 ± 0.06 c | 0.54 ± 0.05 d | 1.22 ± 0.06 a | 1.08 ± 0.07 b | 0.12 ± 0.02 f |
| 78 | 3856-25-5 | Copaene | 1420 | 0 | 0 | 0 | 4.32 ± 0.14 c | 10.03 ± 0.83 b | 13.13 ± 0.39 a |
| 79 | 123-35-3 | Myrcene | 991 | 0 | 0 | 0 | 5.89 ± 0.19 b | 5.43 ± 0.16 b | 13.64 ± 0.73 a |
| 80 | 13877-91-3 | .beta.-Ocimene | 1036 | 50.86 ± 0.38 d | 66.04 ± 2.13 d | 102.92 ± 5.76 c | 108.22 ± 5.41 c | 237.53 ± 15.83 b | 266.83 ± 11.41 a |
| Ketones compounds | |||||||||
| 81 | 14309-57-0 | 3-Nonen-2-one | 1013 | 1.02 ± 0.18 c | 1.68 ± 0.13 a | 1.36 ± 0.1 b | 1 ± 0.17 c | 0.89 ± 0.1 c | 1.68 ± 0.17 a |
| 82 | 127-41-3 | α-Ionone | 1422 | 0 | 0 | 1.08 ± 0.06 b | 1.76 ± 0.16 a | 0 | 0 |
| 83 | 6901-97-9 | 1-cyclohexene | 1480 | 0 | 0 | 0.19 ± 0.01 d | 0.67 ± 0.03 c | 0.83 ± 0.05 b | 1.04 ± 0.06 a |
| 84 | 689-67-8 | Geranylacetone | 1453 | 0.33 ± 0.03 b | 0.59 ± 0.06 a | 0.59 ± 0.03 a | 0 | 0 | 0 |
| 85 | 79-77-6 | β-Lonone | 1480 | 0 | 0.59 ± 0.05 d | 3.51 ± 0.07 b | 0.82 ± 0.09 c | 4.06 ± 0.22 a | 0.77 ± 0.09 cd |
| 86 | 127-41-3 | α-Ionone | 1422 | 1.55 ± 0.05 a | 0 | 0 | 0.68 ± 0.1 b | 0.82 ± 0.12 b | 0.69 ± 0.13 b |
| 87 | 110-93-0 | 5-Hepten-2-one, 6-methyl- | 887 | 15.64 ± 0.39 e | 0 | 20.19 ± 0.98 d | 32.57 ± 0.32 b | 29 ± 0.15 c | 38.45 ± 1.35 a |
| 88 | 488-10-8 | Jasmone | 1404 | 21.38 ± 0.44 b | 1.1 ± 0.12 c | 23.62 ± 1.04 a | 0 | 0 | 0 |
| 89 | 110-43-0 | 2-Heptanone | 887 | 60.78 ± 0.22 b | 64.17 ± 1.6 a | 27.22 ± 0.38 f | 42.83 ± 2.23 c | 37.39 ± 1.05 d | 30.94 ± 2.87 e |
| 90 | 38284-27-4 | 3,5-Octadien-2-one | 1041 | 40.02 ± 1.63 c | 78.81 ± 1.66 a | 74.61 ± 1.92 b | 0 | 0 | 0 |
| Others | |||||||||
| 91 | 75-18-3 | Dimethyl sulfide | <600 | 0 | 0 | 0 | 4.05 ± 0.19 b | 3.83 ± 0.28 b | 6.32 ± 0.45 a |
| Compounds | Threshold | OAVS | Odor Quality | |||||
|---|---|---|---|---|---|---|---|---|
| BY | LJ | ML | YS | CF | JK | |||
| 1-Heptanol | 0.76 | 0 | 0 | 57.81 | 40.96 | 52.58 | 75.38 | Fresh, fatty, citrus-like |
| 1-Octen-3-ol | 1 | 0 | 7.07 | 5.61 | 12.64 | 11.46 | 73.38 | Mushroom, lavender, rose |
| 2,4-Heptadienal, (E,E)- | 0.057 | 995.32 | 635.32 | 493.04 | 233.04 | 556.67 | 837.31 | Fatty, green |
| Benzeneacetaldehyde | 4 | 0 | 0.91 | 0 | 6.43 | 5.28 | 6.4 | Floral, daffodil |
| β-Ocimene | 2 | 25.43 | 33.02 | 51.46 | 54.11 | 118.76 | 133.42 | Floral |
| Linalool | 0.58 | 48.57 | 51.91 | 53.37 | 119.52 | 87.44 | 125.62 | Floral |
| Geraniol | 1.1 | 55.79 | 63.68 | 133.13 | 12.14 | 7.77 | 10.16 | Floral |
| Jasmone | 7 | 3.05 | 0.16 | 3.37 | 0 | 0 | 0 | Woody, herbaceous, jasmine |
| Ethyl Palmitate | 1 | 32.47 | 43.94 | 34.95 | 26.79 | 28.85 | 31.92 | Waxy, fruity, creamy |
| Hexanal | 2.4 | 12.83 | 21.38 | 25.63 | 18.62 | 16.29 | 26.7 | grassy |
| 2-Hexenal | 0.03 | 0 | 0 | 0 | 260.78 | 288.33 | 763.89 | Fruity, grassy |
| Methyl salicylate | 40 | 2.21 | 2.88 | 1.33 | 6.3 | 2.62 | 4.42 | Minty, wintergreen-like |
| 3,5-Octadien-2-one | 0.15 | 266.78 | 525.38 | 497.38 | 0 | 0 | 0 | Fruity, fatty, mushroom |
| Receptor | Building Energy (kcal/mol) | |||||
|---|---|---|---|---|---|---|
| Ligand | OR1A1 | OR1G1 | OR2W1 | OR1D2 | OR52D1 | |
| 1-Octen-3-ol | −4.29 | −4.69 | −4.13 | −3.33 | −3.44 | |
| 1-Heptanol | −3.55 | −4.06 | −3.25 | −3.34 | −3.26 | |
| Benzeneacetaldehyde | −4.52 | −3.26 | −4.54 | −3.26 | −4.14 | |
| β-Ocimene | −5.12 | −5.13 | −5.11 | −4.88 | −4.43 | |
| Linalool | −5.2 | −4.8 | −5.2 | −4.57 | −4.01 | |
| Receptor | Ligand | Hydrophobic Interactions | Hydrogen Bonds |
|---|---|---|---|
| OR1A1 | 1-Octen-3-ol | Met104, Ile105, Gly108, Asn109, Asp180, Phe206, Tyr258 | Tyr178, Tyr276 |
| 1-Heptanol | Phe73, Met104, Ile105, Gly108, Phe206, Asn209, Tyr250, Tyr258 | Tyr178, Tyr276 | |
| Benzeneacetaldehyde | Leu14, Gly16, Glu24, Met81, Asn84, His85, Phe177 | - | |
| β-Ocimene | Phe73, Met104, Ile105, Asn109, Tyr178, Val203, Phe206, Tyr250, Tyr258, Tyr276 | - | |
| Linalool | Phe73, Met104, Ile105, Gly108, Val203, Phe206, Val254, Tyr258 | Asn109, Asn155, Gly202 | |
| OR1G1 | 1-Octen-3-ol | Leu15, Gly16, Phe17, Gln24, Leu27, Met81, Ile85, Gln90, Phe177 | Leu14, Asn84 |
| 1-Heptanol | Leu14, Leu15, Gly16, Gln24, Met81, Asn84, Gln90, Phe177 | Phe17, Ser18 | |
| Benzeneacetaldehyde | Leu14, Gly16, Phe17, Ser18, Gln24, Leu27, Met81, Ile85 | Asn84 | |
| β-Ocimene | Leu14, Leu15, Gly16, Phe17, Ser18, Gln24, Leu27, Asn84, Ile85, Gln88, Ser89, Gln90 | - | |
| Linalool | Leu14, Gly16, Gln24, Asn84, Ile85, Gln88, Ser89, Gln90, Phe177 | Met81 | |
| OR2W1 | 1-Octen-3-ol | Phe12, Thr163, Leu166, Thr168, Leu184 | Tyr94, Leu164 |
| 1-Heptanol | Leu174, His176, Glu180, Ala183, Lys186, Ile187, Lys268 | Asp175, Ile173 | |
| Benzeneacetaldehyde | Tyr104, Met105, Gly108, Ser109, Leu159, Ile206, Ile255 | Asn155 | |
| β-Ocimene | Phe73, Tyr104, Met105, Gly108, Asn155, Gly203, Phe251, Ile255, Tyr259 | - | |
| Linalool | Phe73, Met105, Gly108, Gly203, Ile206, Val207, Ile255, Tyr259, Tyr278 | Tyr104 | |
| OR1D2 | 1-Octen-3-ol | Leu199, Ile200, Gly203, Cys204, Phe207, Leu255, Tyr259 | Tyr182 |
| 1-Heptanol | Asp70, Phe73, Asp111, Leu115, Phe251, Tyr277, Thr281, Pro282 | Ala247, Asn285 | |
| Benzeneacetaldehyde | Leu101, Leu104, Val105, Val108, Tyr155, Glu180, Phe207, Tyr259 | Met181 | |
| β-Ocimene | Val105, Val108, Tyr155, Met181, Leu199, Gly203, Phe207, Tyr259 | - | |
| Linalool | Tyr155, Leu199, Ile200, Gly203, Phe207, Leu255, Tyr259 | Tyr182 | |
| OR52D1 | 1-Octen-3-ol | Glu183, Phe254, Pro257, Ala258, Ser261, Tyr284, Val285, Pro288 | Tyr111, Tyr181 |
| 1-Heptanol | Tyr111, Tyr181, Pro257, Ala258, Ser261, Ala281, Val285, Pro288 | Phe254, Tyr284 | |
| Benzeneacetaldehyde | Asn9, His10, Leu11, Arg167, Arg168, Leu169, Pro170 | Tyr171 | |
| β-Ocimene | Ala206, Leu207, Met210, Gly211, Tyr255, Ala258, Phe259, Phe262 | - | |
| Linalool | Tyr111, Ala112, Phe162, Gly202, Val205, Ala206, Ala209, Met210 | His108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.; Liang, H.; Hu, N.; Lu, J.; Li, C.; Tang, D. Chemical, Sensory Variations in Black Teas from Six Tea Cultivars in Jingshan, China. Foods 2025, 14, 1558. https://doi.org/10.3390/foods14091558
Wu R, Liang H, Hu N, Lu J, Li C, Tang D. Chemical, Sensory Variations in Black Teas from Six Tea Cultivars in Jingshan, China. Foods. 2025; 14(9):1558. https://doi.org/10.3390/foods14091558
Chicago/Turabian StyleWu, Rui, Huiling Liang, Nan Hu, Jiajia Lu, Chunfang Li, and Desong Tang. 2025. "Chemical, Sensory Variations in Black Teas from Six Tea Cultivars in Jingshan, China" Foods 14, no. 9: 1558. https://doi.org/10.3390/foods14091558
APA StyleWu, R., Liang, H., Hu, N., Lu, J., Li, C., & Tang, D. (2025). Chemical, Sensory Variations in Black Teas from Six Tea Cultivars in Jingshan, China. Foods, 14(9), 1558. https://doi.org/10.3390/foods14091558






