Could a Mediterranean Diet Modulate Alzheimer’s Disease Progression? The Role of Gut Microbiota and Metabolite Signatures in Neurodegeneration
Abstract
1. Introduction
2. Microbiome Signatures in Alzheimer’s Disease
2.1. Gut Microbiota Dysbiosis in Alzheimer’s Disease
2.2. Gut Microbiota-Derived Metabolites and Neurodegeneration: Molecular Mechanisms of SCFAs, Tryptophan, and Bile Acid Pathways
2.2.1. SCFAs: Modulation of Neuroinflammation, BBB Integrity, and Amyloid-β Clearance
2.2.2. Tryptophan Metabolism: A Balance Between Neuroprotection and Neurotoxicity
2.2.3. Bile Acids and the Gut–Liver–Brain Axis
2.3. Molecular Crosstalk Between Gut Microbes and Neuroinflammatory Pathways
3. Inflammatory and Neuroimmune Modulation by Gut Microbiota
3.1. Gut Microbiota and the Blood–Brain Barrier (BBB)
3.2. Chronic Inflammation as a Driving Force of Neurodegeneration
4. Role of Diet in Modulating Gut Microbiota and Neurodegeneration
4.1. Dietary Patterns and Their Effects on the Gut–Brain Axis
4.2. Bioactive Dietary Compounds with Neuroprotective Potential
4.3. Clinical Relevance: Can Diet-Based Modulation of Gut Microbiota Slow Alzheimer’s Progression?
4.4. Molecular Pathways Linking Diet to Neuroinflammation
5. Mechanistic Insights: Gut Microbiota in Amyloid-β and Tau Pathology
5.1. Dysbiosis and Tau Hyperphosphorylation
5.2. Neurotransmitter Metabolism by Gut Microbiota
5.3. Role of the Gut Microbiota in Misfolded Protein Clearance
5.4. Molecular Targets of Microbial Metabolites in Neuroprotection
6. Future Directions: Microbiome-Based Therapeutic Interventions
6.1. Probiotics, Prebiotics, and Postbiotics for Neuroprotection
6.2. Fecal Microbiota Transplantation (FMT) as a Potential Therapy
6.3. Personalized Microbiome-Based Dietary Strategies
6.4. Emerging Therapies Modulating the Gut–Brain Link
7. Complications, Limitations, and Future Research Priorities
7.1. Gaps in Understanding the Gut–Brain Axis in Alzheimer’s
7.2. Variability in Microbiome Response to Dietary Interventions
7.3. Integrating Microbiome Science with Precision Medicine
7.4. Comparative Analysis of Microbiome-Based vs. Conventional Therapies
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| SCFAs | Short-chain fatty acids |
| BBB | Blood–brain barrier |
| FMT | Fecal microbiota transplantation |
| LPS | Lipopolysaccharides |
| NMDA | N-methyl-D-aspartate |
| FXR | Farnesoid X receptor |
| TGR5 | G-protein receptor 5 |
| CNS | Central nervous system |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-alpha |
| NF-κB | Nuclear factor kappa B |
| TLR4 | Toll-like receptor 4 |
| HDACs | Histone deacetylases |
| GPCRs | G-protein-coupled receptors |
| PD | Parkinson’s disease |
| AhR | Aryl hydrocarbon receptor |
| GABA | Gamma-aminobutyric acid |
| ROS | Reactive oxygen species |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| Aβ | Amyloid-β |
| GSK-3β | Glycogen synthase kinase 3 beta |
| CDK5 | Cyclin-dependent kinase 5 |
| FOS | Fructooligosaccharides |
References
- Wang, S.; Jiang, Y.; Yang, A.; Meng, F.; Zhang, J. The Expanding Burden of Neurodegenerative Diseases: An Unmet Medical and Social Need. Aging Dis. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Lastuka, A.; Bliss, E.; Breshock, M.R.; Iannucci, V.C.; Sogge, W.; Taylor, K.V.; Pedroza, P.; Dieleman, J.L. Societal Costs of Dementia: 204 Countries, 2000–2019. J. Alzheimer’s Dis. 2024, 101, 277–292. [Google Scholar] [CrossRef]
- Economic Burden of Alzheimer Disease and Managed Care Considerations. Am. J. Manag. Care 2020, 26 (Suppl. S8), S177–S183. [CrossRef]
- Lohiya, A.; Dhaniwala, N.; Dudhekar, U.; Goyal, S.; Patel, S.K. A Comprehensive Review of Treatment Strategies for Early Avascular Necrosis. Cureus 2023, 15, e50510. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The Gut Microbiota-Immune-Brain Axis: Therapeutic Implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Chandra, S.; Sisodia, S.S.; Vassar, R.J. The Gut Microbiome in Alzheimer’s Disease: What We Know and What Remains to Be Explored. Mol. Neurodegener. 2023, 18, 9. [Google Scholar] [CrossRef]
- Qian, X.; Xie, R.; Liu, X.; Chen, S.; Tang, H. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252. [Google Scholar] [CrossRef]
- Ullah, R.; Park, T.J.; Huang, X.; Kim, M.O. Abnormal Amyloid Beta Metabolism in Systemic Abnormalities and Alzheimer’s Pathology: Insights and Therapeutic Approaches from Periphery. Ageing Res. Rev. 2021, 71, 101451. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liang, J.; Hu, N.; He, N.; Liu, B.; Liu, G.; Qin, Y. The Gut Microbiota Modulates Neuroinflammation in Alzheimer’s Disease: Elucidating Crucial Factors and Mechanistic Underpinnings. CNS Neurosci. Ther. 2024, 30, e70091. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Carrión, N.; Cabrera, C.; Heredia, L.; Marquès, M.; Forcadell-Ferreres, E.; Pino, M.; Zaragoza, J.; Moral, A.; Cavallé, L.; et al. Gut Microbiota Alterations in Alzheimer’s Disease: Relation with Cognitive Impairment and Mediterranean Lifestyle. Microorganisms 2024, 12, 2046. [Google Scholar] [CrossRef]
- Khatoon, S.; Kalam, N.; Rashid, S.; Bano, G. Effects of Gut Microbiota on Neurodegenerative Diseases. Front. Aging Neurosci. 2023, 15, 1145241. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Koutsokostas, C.; Merkouris, E.; Goulas, A.; Aidinopoulou, K.; Sini, N.; Dimaras, T.; Tsiptsios, D.; Mueller, C.; Nystazaki, M.; Tsamakis, K. Gut Microbes Associated with Neurodegenerative Disorders: A Comprehensive Review of the Literature. Microorganisms 2024, 12, 1735. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, R.; Tu, Q. Gut Microbial Involvement in Alzheimer’s Disease Pathogenesis. Aging 2021, 13, 13359–13371. [Google Scholar] [CrossRef]
- Missiego-Beltrán, J.; Beltrán-Velasco, A.I. The Role of Microbial Metabolites in the Progression of Neurodegenerative Diseases—Therapeutic Approaches: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 10041. [Google Scholar] [CrossRef]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut Microbiota-Derived Metabolites and Their Importance in Neurological Disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate Producers, “The Sentinel of Gut”: Their Intestinal Significance with and beyond Butyrate, and Prospective Use as Microbial Therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, D.; Wu, C.; Wu, Z.; Zhang, W.; Chen, S.; Zhao, X.; Wu, S. Role of Histone Deacetylase Inhibitors in Non-Neoplastic Diseases. Heliyon 2024, 10, e33997. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut Microbiota Dysbiosis: The Potential Mechanisms by Which Alcohol Disrupts Gut and Brain Functions. Front. Microbiol. 2022, 13, 916765. [Google Scholar] [CrossRef]
- Mossad, O.; Erny, D. The Microbiota–Microglia Axis in Central Nervous System Disorders. Brain Pathol. 2020, 30, 1159–1177. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef]
- Shaw, C.; Hess, M.; Weimer, B.C. Microbial-Derived Tryptophan Metabolites and Their Role in Neurological Disease: Anthranilic Acid and Anthranilic Acid Derivatives. Microorganisms 2023, 11, 1825. [Google Scholar] [CrossRef]
- Meier, T.B.; Savitz, J. The Kynurenine Pathway in Traumatic Brain Injury: Implications for Psychiatric Outcomes. Biol. Psychiatry 2022, 91, 449–458. [Google Scholar] [CrossRef]
- Uceda, S.; Echeverry-Alzate, V.; Reiriz-Rojas, M.; Martínez-Miguel, E.; Pérez-Curiel, A.; Gómez-Senent, S.; Beltrán-Velasco, A.I. Gut Microbial Metabolome and Dysbiosis in Neurodegenerative Diseases: Psychobiotics and Fecal Microbiota Transplantation as a Therapeutic Approach—A Comprehensive Narrative Review. Int. J. Mol. Sci. 2023, 24, 13294. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.; Faisal, Z.; Irfan, R.; Shah, Y.A.; Batool, S.A.; Zahid, T.; Zulfiqar, A.; Fatima, A.; Jahan, Q.; Tariq, H.; et al. Exploring the serotonin-probiotics-gut Health Axis: A Review of Current Evidence and Potential Mechanisms. Food Sci. Nutr. 2024, 12, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Kruczkowska, W.; Gałęziewska, J.; Wanke, K.; Kałuzińska-Kołat, Ż.; Aleksandrowicz, M.; Kontek, R. Alzheimer’s Disease as Type 3 Diabetes: Understanding the Link and Implications. Int. J. Mol. Sci. 2024, 25, 11955. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining Roles: A Paradigm Shift in Tryptophan–Kynurenine Metabolism for Innovative Clinical Applications. Int. J. Mol. Sci. 2024, 25, 12767. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, S.; He, Y.; Xu, M.; Qiao, X.; Zhu, Y.; Wu, W. Kynurenine Pathway Metabolites as Biomarkers in Alzheimer’s Disease. Dis. Mark. 2022, 2022, 9484217. [Google Scholar] [CrossRef]
- Sabahat, S.E.; Saqib, M.; Talib, M.; Shaikh, T.G.; Khan, T.; Kailash, S.J. Bile Acid Modulation by Gut Microbiota: A Bridge to Understanding Cognitive Health. Ann. Med. Surg. 2024, 86, 5410–5415. [Google Scholar] [CrossRef]
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef]
- Jia, M.; Fan, Y.; Ma, Q.; Yang, D.; Wang, Y.; He, X.; Zhao, B.; Zhan, X.; Qi, Z.; Ren, Y.; et al. Gut Microbiota Dysbiosis Promotes Cognitive Impairment via Bile Acid Metabolism in Major Depressive Disorder. Transl. Psychiatry 2024, 14, 503. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Suleiman Khoury, Z.; Sohail, F.; Wang, J.; Mendoza, M.; Raake, M.; Tahoor Silat, M.; Reddy Bathinapatta, M.; Sadeghzadegan, A.; Meghana, P.; Paul, J. Neuroinflammation: A Critical Factor in Neurodegenerative Disorders. Cureus 2024, 16, e62310. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Shahini, A. Role of Interleukin-6-Mediated Inflammation in the Pathogenesis of Inflammatory Bowel Disease: Focus on the Available Therapeutic Approaches and Gut Microbiome. J. Cell Commun. Signal. 2023, 17, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in Neuroinflammation and Synaptic Dysfunction: A Focus on Alzheimer’s Disease. Mol. Neurodegener. 2022, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells 2022, 12, 54. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-ΚB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Xie, L.; Wu, Q.; Li, K.; Khan, M.A.S.; Zhang, A.; Sinha, B.; Li, S.; Chang, S.L.; Brody, D.L.; Grinstaff, M.W.; et al. Tryptophan Metabolism in Alzheimer’s Disease with the Involvement of Microglia and Astrocyte Crosstalk and Gut-Brain Axis. Aging Dis. 2024, 15, 2168. [Google Scholar] [CrossRef]
- Park, K.J.; Gao, Y. Gut-Brain Axis and Neurodegeneration: Mechanisms and Therapeutic Potentials. Front. Neurosci. 2024, 18, 1481390. [Google Scholar] [CrossRef]
- Peng, X.; Luo, Z.; He, S.; Zhang, L.; Li, Y. Blood-Brain Barrier Disruption by Lipopolysaccharide and Sepsis-Associated Encephalopathy. Front. Cell. Infect. Microbiol. 2021, 11, 768108. [Google Scholar] [CrossRef]
- Li, S. Modulation of Immunity by Tryptophan Microbial Metabolites. Front. Nutr. 2023, 10, 1209613. [Google Scholar] [CrossRef]
- Keitel, V.; Stindt, J.; Häussinger, D. Bile Acid-Activated Receptors: GPBAR1 (TGR5) and Other G Protein-Coupled Receptors; Springer: New York, NY, USA, 2019; pp. 19–49. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Luz Latorre-Moratalla, M.; Sánchez-Pérez, S.; Teresa Veciana-Nogués, M.; del Carmen Vidal-Carou, M. Histamine and Other Biogenic Amines in Food. From Scombroid Poisoning to Histamine Intolerance. In Biogenic Amines; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Anderson, G.; Carbone, A.; Mazzoccoli, G. Tryptophan Metabolites and Aryl Hydrocarbon Receptor in Severe Acute Respiratory Syndrome, Coronavirus-2 (SARS-CoV-2) Pathophysiology. Int. J. Mol. Sci. 2021, 22, 1597. [Google Scholar] [CrossRef]
- Fashogbon, R.O.; Samson, O.J.; Awotundun, T.A.; Olanbiwoninu, A.A.; Adebayo-Tayo, B.C. Microbial Gamma-Aminobutyric Acid Synthesis: A Promising Approach for Functional Food and Pharmaceutical Applications. Lett. Appl. Microbiol. 2024, 77, ovae122. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, O.A.; Isaguliants, M.G.; Hyvonen, M.T.; Keinanen, T.A.; Tunitskaya, V.L.; Vepsalainen, J.; Alhonen, L.; Kochetkov, S.N.; Ivanov, A.V. Chemically Induced Oxidative Stress Increases Polyamine Levels by Activating the Transcription of Ornithine Decarboxylase and Spermidine/Spermine-N1-Acetyltransferase in Human Hepatoma HUH7 Cells. Biochimie 2012, 94, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Merchant, K.S.; Zlotnik, A.; Boyko, M. Gut Microbiome Modulation of Glutamate Dynamics: Implications for Brain Health and Neurotoxicity. Nutrients 2024, 16, 4405. [Google Scholar] [CrossRef]
- Ciupei, D.; Colişar, A.; Leopold, L.; Stănilă, A.; Diaconeasa, Z.M. Polyphenols: From Classification to Therapeutic Potential and Bioavailability. Foods 2024, 13, 4131. [Google Scholar] [CrossRef]
- Sarb, O.-F.; Sarb, A.-D.; Iacobescu, M.; Vlad, I.-M.; Milaciu, M.-V.; Ciurmarnean, L.; Vacaras, V.; Tantau, A.-I. From Gut to Brain: Uncovering Potential Serum Biomarkers Connecting Inflammatory Bowel Diseases to Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 5676. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The Barrier and Interface Mechanisms of the Brain Barrier, and Brain Drug Delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The Impact of Microbiota-Derived Short-Chain Fatty Acids on Macrophage Activities in Disease: Mechanisms and Therapeutic Potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Liu, M.; Peng, R.; Tian, C.; Shi, J.; Ma, J.; Shi, R.; Qi, X.; Zhao, R.; Guan, H. Effects of the Gut Microbiota and Its Metabolite Short-Chain Fatty Acids on Endometriosis. Front. Cell. Infect. Microbiol. 2024, 14, 1373004. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Velasco, A.I.; Clemente-Suárez, V.J. Impact of Peripheral Inflammation on Blood–Brain Barrier Dysfunction and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 2440. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-Brain Axis: A Cutting-Edge Approach to Target Neurological Disorders and Potential Synbiotic Application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors Influencing the Blood-Brain Barrier Permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. Mechanisms and Emerging Regulators of Neuroinflammation: Exploring New Therapeutic Strategies for Neurological Disorders. Curr. Issues Mol. Biol. 2024, 47, 8. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Han, J.; Wei, W.; Chen, F. Blood-Brain Barrier Dysfunction and Alzheimer’s Disease: Associations, Pathogenic Mechanisms, and Therapeutic Potential. Front. Aging Neurosci. 2023, 15, 1258640. [Google Scholar] [CrossRef]
- Rob, M.; Yousef, M.; Lakshmanan, A.P.; Mahboob, A.; Terranegra, A.; Chaari, A. Microbial Signatures and Therapeutic Strategies in Neurodegenerative Diseases. Biomed. Pharmacother. 2025, 184, 117905. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Balkhi, S.; Bilato, G.; Mortara, L. Gut Microbiota and Immune System Dynamics in Parkinson’s and Alzheimer’s Diseases. Int. J. Mol. Sci. 2024, 25, 12164. [Google Scholar] [CrossRef]
- Yoo, J.; Groer, M.; Dutra, S.; Sarkar, A.; McSkimming, D. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The Role of Neuroinflammation in Neurodegenerative Diseases: Current Understanding and Future Therapeutic Targets. Front. Aging Neurosci. 2024, 16, 1347987. [Google Scholar] [CrossRef]
- Xu, G.; Dong, F.; Su, L.; Tan, Z.-X.; Lei, M.; Li, L.; Wen, D.; Zhang, F. The Role and Therapeutic Potential of Nuclear Factor ΚB (NF-ΚB) in Ischemic Stroke. Biomed. Pharmacother. 2024, 171, 116140. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-ΚB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Popescu, C.; Munteanu, C.; Anghelescu, A.; Ciobanu, V.; Spînu, A.; Andone, I.; Mandu, M.; Bistriceanu, R.; Băilă, M.; Postoiu, R.-L.; et al. Novelties on Neuroinflammation in Alzheimer’s Disease–Focus on Gut and Oral Microbiota Involvement. Int. J. Mol. Sci. 2024, 25, 11272. [Google Scholar] [CrossRef]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-Derived Low-Grade Endotoxaemia, Atherothrombosis and Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. The Crucial Role of the Blood–Brain Barrier in Neurodegenerative Diseases: Mechanisms of Disruption and Therapeutic Implications. J. Clin. Med. 2025, 14, 386. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and Molecular Mechanisms of the Blood–Brain Barrier Dysfunction in Neurodegenerative Diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef]
- Che Mohd Nassir, C.M.N.; Che Ramli, M.D.; Mohamad Ghazali, M.; Jaffer, U.; Abdul Hamid, H.; Mehat, M.Z.; Hein, Z.M. The Microbiota–Gut–Brain Axis: Key Mechanisms Driving Glymphopathy and Cerebral Small Vessel Disease. Life 2024, 15, 3. [Google Scholar] [CrossRef]
- Clerici, L.; Bottari, D.; Bottari, B. Gut Microbiome, Diet and Depression: Literature Review of Microbiological, Nutritional and Neuroscientific Aspects. Curr. Nutr. Rep. 2025, 14, 30. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Severino, A.; Tohumcu, E.; Tamai, L.; Dargenio, P.; Porcari, S.; Rondinella, D.; Venturini, I.; Maida, M.; Gasbarrini, A.; Cammarota, G.; et al. The Microbiome-Driven Impact of Western Diet in the Development of Noncommunicable Chronic Disorders. Best Pract. Res. Clin. Gastroenterol. 2024, 72, 101923. [Google Scholar] [CrossRef]
- Song, M.; Bai, Y.; Song, F. High-Fat Diet and Neuroinflammation: The Role of Mitochondria. Pharmacol. Res. 2025, 212, 107615. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Akpoghelie, P.O.; Mafe, A.N.; Isoje, E.F.; Igbuku, U.A.; Yousif, E.; Zainulabdeen, K.; Jikah, A.N.; Owheruo, J.O.; et al. An Overview of the Nutritional Composition, Bioactivities and Applications of Chinese Yam (Dioscoreas Oppositae). Ecol. Front. 2025, in press. [CrossRef]
- Mafe, A.N.; Edo, G.I.; Akpoghelie, P.O.; Yousif, E.; Gaaz, T.S.; Opiti, R.A.; Onyibe, P.N.; Owheruo, J.O.; Isoje, E.F.; Igbuku, U.A.; et al. Pepper Soup: A Cultural and Culinary Exploration of a Traditional Nigerian Dish, with a Focus on Health Benefits and Antimicrobial Activity. Int. J. Gastron. Food Sci. 2024, 38, 101036. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Di Bella, G.; Cusumano, C.; Parisi, A.; Tagliaferri, F.; Ciriminna, S.; Barbagallo, M. Mediterranean Diet in the Management and Prevention of Obesity. Exp. Gerontol. 2023, 174, 112121. [Google Scholar] [CrossRef]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef]
- Picone, P.; Girgenti, A.; Buttacavoli, M.; Nuzzo, D. Enriching the Mediterranean Diet Could Nourish the Brain More Effectively. Front. Nutr. 2024, 11, 1489489. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, M.; Li, W.; Fang, R.; Tang, C.; Wang, Q. Diet and Physical Activity Influence the Composition of Gut Microbiota, Benefit on Alzheimer’s Disease. Food Sci. Hum. Wellness 2024, 13, 541–555. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Montalvo Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- Kang, G.G.; Trevaskis, N.L.; Murphy, A.J.; Febbraio, M.A. Diet-Induced Gut Dysbiosis and Inflammation: Key Drivers of Obesity-Driven NASH. iScience 2023, 26, 105905. [Google Scholar] [CrossRef]
- Lotz, S.K.; Blackhurst, B.M.; Reagin, K.L.; Funk, K.E. Microbial Infections Are a Risk Factor for Neurodegenerative Diseases. Front. Cell. Neurosci. 2021, 15, 691136. [Google Scholar] [CrossRef]
- Młynarska, E.; Jakubowska, P.; Frąk, W.; Gajewska, A.; Sornowska, J.; Skwira, S.; Wasiak, J.; Rysz, J.; Franczyk, B. Associations of Microbiota and Nutrition with Cognitive Impairment in Diseases. Nutrients 2024, 16, 3570. [Google Scholar] [CrossRef]
- Park, G.; Kadyan, S.; Hochuli, N.; Pollak, J.; Wang, B.; Salazar, G.; Chakrabarty, P.; Efron, P.; Sheffler, J.; Nagpal, R. A Modified Mediterranean-Style Diet Enhances Brain Function via Specific Gut-Microbiome-Brain Mechanisms. Gut Microbes 2024, 16, 2323752. [Google Scholar] [CrossRef]
- Hyży, A.; Rozenek, H.; Gondek, E.; Jaworski, M. Effect of Antioxidants on the Gut Microbiome Profile and Brain Functions: A Review of Randomized Controlled Trial Studies. Foods 2025, 14, 176. [Google Scholar] [CrossRef]
- Mafe, A.N.; Iruoghene Edo, G.; Akpoghelie, P.O.; Gaaz, T.S.; Yousif, E.; Zainulabdeen, K.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; Garba, Y.; et al. Probiotics and Food Bioactives: Unraveling Their Impact on Gut Microbiome, Inflammation, and Metabolic Health. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Mursal, M.; Kumar, A.; Hasan, S.M.; Hussain, S.; Singh, K.; Kushwaha, S.P.; Arif, M.; Kumar Singh, R.; Singh, D.; Mohammad, A.; et al. Role of Natural Bioactive Compounds in the Management of Neurodegenerative Disorders. Intell. Pharm. 2024, 2, 102–113. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Thapa, R.; Moglad, E.; Afzal, M.; Gupta, G.; Bhat, A.A.; Hassan almalki, W.; Kazmi, I.; Alzarea, S.I.; Pant, K.; Singh, T.G.; et al. The Role of Sirtuin 1 in Ageing and Neurodegenerative Disease: A Molecular Perspective. Ageing Res. Rev. 2024, 102, 102545. [Google Scholar] [CrossRef]
- Lagoa, R.; Rajan, L.; Violante, C.; Babiaka, S.B.; Marques-da-Silva, D.; Kapoor, B.; Reis, F.; Atanasov, A.G. Application of Curcuminoids in Inflammatory, Neurodegenerative and Aging Conditions—Pharmacological Potential and Bioengineering Approaches to Improve Efficiency. Biotechnol. Adv. 2025, 82, 108568. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef] [PubMed]
- Zinkow, A.; Grodzicki, W.; Czerwińska, M.; Dziendzikowska, K. Molecular Mechanisms Linking Omega-3 Fatty Acids and the Gut–Brain Axis. Molecules 2024, 30, 71. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 Fatty Acids Eicosapentaenoic (EPA) and Docosahexaenoic (DHA) as Modulatory and Anti-Inflammatory Agents in Noncommunicable Diet-Related Diseases—Reports from the Last 10 Years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rohilla, A.; Ahire, J.J. Omega-3 Fatty Acids and the Gut Microbiome: A New Frontier in Cardiovascular Disease Prevention. Discov. Med. 2025, 2, 53. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Majeed, O.S.; Gaaz, T.S.; Akpoghelie, P.O.; Isoje, E.F.; Igbuku, U.A.; Owheruo, J.O.; Opiti, R.A.; Garba, Y.; et al. A Review on Probiotics and Dietary Bioactives: Insights on Metabolic Well-Being, Gut Microbiota, and Inflammatory Responses. Food Chem. Adv. 2025, 6, 100919. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols—Polyphenols as an Element of Diet Therapy in Depressive Disorders. Int. J. Mol. Sci. 2023, 24, 2258. [Google Scholar] [CrossRef]
- Dissanayaka, D.M.S.; Jayasena, V.; Rainey-Smith, S.R.; Martins, R.N.; Fernando, W.M.A.D.B. The Role of Diet and Gut Microbiota in Alzheimer’s Disease. Nutrients 2024, 16, 412. [Google Scholar] [CrossRef]
- Siervo, M.; Shannon, O.M.; Llewellyn, D.J.; Stephan, B.C.; Fontana, L. Mediterranean Diet and Cognitive Function: From Methodology to Mechanisms of Action. Free Radic. Biol. Med. 2021, 176, 105–117. [Google Scholar] [CrossRef]
- Soldán, M.; Argalášová, Ľ.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Yavari, M.; Kalupahana, N.S.; Harris, B.N.; Ramalingam, L.; Zu, Y.; Kahathuduwa, C.N.; Moustaid-Moussa, N. Mechanisms Linking Obesity, Insulin Resistance, and Alzheimer’s Disease: Effects of Polyphenols and Omega-3 Polyunsaturated Fatty Acids. Nutrients 2025, 17, 1203. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-García, A.M.; Villarino, M.; Arias, N. A Systematic Review and Meta-analysis of Basal Microbiota and Cognitive Function in Alzheimer’s Disease: A Potential Target for Treatment or a Contributor to Disease Progression? Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2024, 16, e70057. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Malviya, R.; Sundram, S. Nutritional Neurology: Unraveling Cellular Mechanisms of Natural Supplements in Brain Health. Hum. Nutr. Metab. 2024, 35, 200232. [Google Scholar] [CrossRef]
- Stein, R.A.; Riber, L. Epigenetic Effects of Short-Chain Fatty Acids from the Large Intestine on Host Cells. microLife 2023, 4, uqad032. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Cheong, C.S.Y.; Verma, K.; Tencomnao, T.; Brimson, J.M.; Prasansuklab, A. Role of Epigenetic Modulation in Neurodegenerative Diseases: Implications of Phytochemical Interventions. Antioxidants 2024, 13, 606. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Gorantla, V.R.; Mahalakshmi, A.M.; Ghanekar, R.K.; Yang, J.; Sakharkar, M.K.; Chidambaram, S.B. Polyphenols, Autophagy and Neurodegenerative Diseases: A Review. Biomolecules 2023, 13, 1196. [Google Scholar] [CrossRef]
- Gómez-Virgilio, L.; Silva-Lucero, M.-C.; Flores-Morelos, D.-S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.-M.; Zacapala-Gómez, A.-E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Lin, X.; Liu, W.; Hu, X.; Liu, Z.; Wang, F.; Wang, J. The Role of Polyphenols in Modulating Mitophagy: Implications for Therapeutic Interventions. Pharmacol. Res. 2024, 207, 107324. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Xing, Z.; Peng, C.; Li, D. Autophagy in Neuroinflammation: A Focus on Epigenetic Regulation. Aging Dis. 2024, 15, 739. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of Inflammatory Microglia by Dietary Fiber and Short-Chain Fatty Acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Guo, M.-S.; Zhang, Y.; Yu, L.; Wu, J.-M.; Tang, Y.; Ai, W.; Zhu, F.-D.; Law, B.Y.-K.; Chen, Q.; et al. Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities. Oxid. Med. Cell. Longev. 2022, 2022, 5288698. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaur, P.; Kumar, M.; Shafi, S.; Upadhyay, P.K.; Tiwari, A.; Tiwari, V.; Rangra, N.K.; Thirunavukkarasu, V.; Kumari, S.; et al. The Role of Phytochemicals in Modulating the Gut Microbiota: Implications for Health and Disease. Med. Microecol. 2025, 24, 100125. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediators Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Vinderola, G.; Cotter, P.D.; Freitas, M.; Gueimonde, M.; Holscher, H.D.; Ruas-Madiedo, P.; Salminen, S.; Swanson, K.S.; Sanders, M.E.; Cifelli, C.J. Fermented Foods: A Perspective on Their Role in Delivering Biotics. Front. Microbiol. 2023, 14, 1196239. [Google Scholar] [CrossRef]
- Acevedo-Román, A.; Pagán-Zayas, N.; Velázquez-Rivera, L.I.; Torres-Ventura, A.C.; Godoy-Vitorino, F. Insights into Gut Dysbiosis: Inflammatory Diseases, Obesity, and Restoration Approaches. Int. J. Mol. Sci. 2024, 25, 9715. [Google Scholar] [CrossRef]
- Naliyadhara, N.; Kumar, A.; Kumar Gangwar, S.; Nair Devanarayanan, T.; Hegde, M.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A. Interplay of Dietary Antioxidants and Gut Microbiome in Human Health: What Has Been Learnt Thus Far? J. Funct. Foods 2023, 100, 105365. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Chassaing, B.; Langella, P. Exploring the Interaction and Impact of Probiotic and Commensal Bacteria on Vitamins, Minerals and Short Chain Fatty Acids Metabolism. Microb. Cell Fact. 2024, 23, 172. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Wu, S.; Bhat, Z.; Gounder, R.; Mohamed Ahmed, I.; Al-Juhaimi, F.; Ding, Y.; Bekhit, A. Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Frausto, D.M.; Forsyth, C.B.; Keshavarzian, A.; Voigt, R.M. Dietary Regulation of Gut-Brain Axis in Alzheimer’s Disease: Importance of Microbiota Metabolites. Front. Neurosci. 2021, 15, 736814. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.; Shin, S.J.; Park, Y.H.; Nam, Y.; Kim, C.; Lee, K.; Kim, S.-M.; Jung, I.D.; Yang, H.D.; et al. Gram-Negative Bacteria and Their Lipopolysaccharides in Alzheimer’s Disease: Pathologic Roles and Therapeutic Implications. Transl. Neurodegener. 2021, 10, 49. [Google Scholar] [CrossRef]
- Ma, B.; Barathan, M.; Ng, M.H.; Law, J.X. Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials. Int. J. Mol. Sci. 2025, 26, 3148. [Google Scholar] [CrossRef]
- Sowmiya, S.; Dhivya, L.S.; Praveen, R.; Harikrishnan, N.; Singh, A. Exploring the Potential of Probiotics in Alzheimer’s Disease and Gut Dysbiosis. IBRO Neurosci. Rep. 2024, 17, 441–455. [Google Scholar] [CrossRef]
- Dhami, M.; Raj, K.; Singh, S. Relevance of Gut Microbiota to Alzheimer’s Disease (AD): Potential Effects of Probiotic in Management of AD. Aging Health Res. 2023, 3, 100128. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Nunzi, E.; Pariano, M.; Costantini, C.; Garaci, E.; Puccetti, P.; Romani, L. Host–Microbe Serotonin Metabolism. Trends Endocrinol. Metab. 2025, 36, 83–95. [Google Scholar] [CrossRef]
- Qu, S.; Yu, Z.; Zhou, Y.; Wang, S.; Jia, M.; Chen, T.; Zhang, X. Gut Microbiota Modulates Neurotransmitter and Gut-Brain Signaling. Microbiol. Res. 2024, 287, 127858. [Google Scholar] [CrossRef]
- Salim, S.; Ahmad, F.; Banu, A.; Mohammad, F. Gut Microbiome and Parkinson’s Disease: Perspective on Pathogenesis and Treatment. J. Adv. Res. 2023, 50, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S. Gut Microbiota Neurotransmitters: Influence on Risk and Outcome of Ischemic Stroke. Neural Regen. Res. 2022, 18, 1707–1708. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From Gut Dysbiosis to Altered Brain Function and Mental Illness: Mechanisms and Pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, S.; Xu, C.; Zhou, X.; Lian, X.; He, L.; Li, K. Gut Microbiota, Pathogenic Proteins and Neurodegenerative Diseases. Front. Microbiol. 2022, 13, 959856. [Google Scholar] [CrossRef]
- Ajmal, M.R. Protein Misfolding and Aggregation in Proteinopathies: Causes, Mechanism and Cellular Response. Diseases 2023, 11, 30. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, E.-K.; Chey, Y.; Song, M.-J.; Jang, H.H. Targeted Protein Degradation: Principles and Applications of the Proteasome. Cells 2023, 12, 1846. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, L.; Li, J.; Yao, C.; Wang, G.; Mi, J.; Yu, Y.; Ding, L.; Zhao, Y.; Yan, G.; et al. New Insights into the Interplay between Autophagy, Gut Microbiota and Insulin Resistance in Metabolic Syndrome. Biomed. Pharmacother. 2024, 176, 116807. [Google Scholar] [CrossRef]
- Mitra, S.; Munni, Y.A.; Dash, R.; Sadhu, T.; Barua, L.; Islam, M.A.; Chowdhury, D.; Bhattacharjee, D.; Mazumder, K.; Moon, I.S. Gut Microbiota in Autophagy Regulation: New Therapeutic Perspective in Neurodegeneration. Life 2023, 13, 957. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging Role of Gut Microbiota Dysbiosis in Neuroinflammation and Neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Jain, A.; Madkan, S.; Patil, P. The Role of Gut Microbiota in Neurodegenerative Diseases: Current Insights and Therapeutic Implications. Cureus 2023, 15, e47861. [Google Scholar] [CrossRef]
- Vaziri, Y. The Mediterranean Diet: A Powerful Defense against Alzheimer Disease—A Comprehensive Review. Clin. Nutr. ESPEN 2024, 64, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Białecka-Dębek, A.; Granda, D.; Szmidt, M.K.; Zielińska, D. Gut Microbiota, Probiotic Interventions, and Cognitive Function in the Elderly: A Review of Current Knowledge. Nutrients 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; Zivkovic, A.M. The Potential Utility of Prebiotics to Modulate Alzheimer’s Disease: A Review of the Evidence. Microorganisms 2021, 9, 2310. [Google Scholar] [CrossRef]
- Quansah, M.; David, M.A.; Martins, R.; El-Omar, E.; Aliberti, S.M.; Capunzo, M.; Jensen, S.O.; Tayebi, M. The Beneficial Effects of Lactobacillus Strains on Gut Microbiome in Alzheimer’s Disease: A Systematic Review. Healthcare 2025, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Reiriz, M.; Beltrán-Velasco, A.I.; Echeverry-Alzate, V.; Martínez-Miguel, E.; Gómez-Senent, S.; Uceda, S.; Clemente-Suárez, V.J. Bifidobacterium Infantis and Bifidobacterium Breve Improve Symptomatology and Neuronal Damage in Neurodegenerative Disease: A Systematic Review. Nutrients 2025, 17, 391. [Google Scholar] [CrossRef]
- Carrillo, J.Á.; Arcusa, R.; Xandri-Martínez, R.; Cerdá, B.; Zafrilla, P.; Marhuenda, J. Impact of Polyphenol-Rich Nutraceuticals on Cognitive Function and Neuroprotective Biomarkers: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2025, 17, 601. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, L.-Y.; Wen, R.; Yang, N.; Zhang, T.-N. Histone Deacetylases and Their Inhibitors in Inflammatory Diseases. Biomed. Pharmacother. 2024, 179, 117295. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Receptors FXR and TGR5 Signaling in Fatty Liver Diseases and Therapy. Am. J. Physiol. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef]
- Ojha, S.; Patil, N.; Jain, M.; Kole, C.; Kaushik, P. Probiotics for Neurodegenerative Diseases: A Systemic Review. Microorganisms 2023, 11, 1083. [Google Scholar] [CrossRef]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic Significance of Lactobacillus Strains: A Comprehensive Review on Health Impacts, Research Gaps, and Future Prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef]
- Kumar, A.; Sivamaruthi, B.S.; Dey, S.; Kumar, Y.; Malviya, R.; Prajapati, B.G.; Chaiyasut, C. Probiotics as Modulators of Gut-Brain Axis for Cognitive Development. Front. Pharmacol. 2024, 15, 1348297. [Google Scholar] [CrossRef]
- Divyashri, G.; Sadanandan, B.; Chidambara Murthy, K.N.; Shetty, K.; Mamta, K. Neuroprotective Potential of Non-Digestible Oligosaccharides: An Overview of Experimental Evidence. Front. Pharmacol. 2021, 12, 712531. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. Gut Microbiome, Short-Chain Fatty Acids, Alpha-Synuclein, Neuroinflammation, and ROS/RNS: Relevance to Parkinson’s Disease and Therapeutic Implications. Redox Biol. 2024, 71, 103092. [Google Scholar] [CrossRef]
- Qiao, L.; Yang, G.; Wang, P.; Xu, C. The Potential Role of Mitochondria in the Microbiota-Gut-Brain Axis: Implications for Brain Health. Pharmacol. Res. 2024, 209, 107434. [Google Scholar] [CrossRef]
- Guamán, L.P.; Carrera-Pacheco, S.E.; Zúñiga-Miranda, J.; Teran, E.; Erazo, C.; Barba-Ostria, C. The Impact of Bioactive Molecules from Probiotics on Child Health: A Comprehensive Review. Nutrients 2024, 16, 3706. [Google Scholar] [CrossRef]
- Yan, H.; Ren, J.; Liu, G.-H. Fecal Microbiota Transplantation: A New Strategy to Delay Aging. hLife 2023, 1, 8–11. [Google Scholar] [CrossRef]
- Varesi, A.; Pierella, E.; Romeo, M.; Piccini, G.B.; Alfano, C.; Bjørklund, G.; Oppong, A.; Ricevuti, G.; Esposito, C.; Chirumbolo, S.; et al. The Potential Role of Gut Microbiota in Alzheimer’s Disease: From Diagnosis to Treatment. Nutrients 2022, 14, 668. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhang, S.-Y.; Wen, R.; Zhang, T.-N.; Yang, N. Role of Histone Deacetylases and Their Inhibitors in Neurological Diseases. Pharmacol. Res. 2024, 208, 107410. [Google Scholar] [CrossRef]
- Zhang, S.; Zhan, L.; Li, X.; Yang, Z.; Luo, Y.; Zhao, H. Preclinical and Clinical Progress for HDAC as a Putative Target for Epigenetic Remodeling and Functionality of Immune Cells. Int. J. Biol. Sci. 2021, 17, 3381–3400. [Google Scholar] [CrossRef]
- Xiang, W.; Xiang, H.; Wang, J.; Jiang, Y.; Pan, C.; Ji, B.; Zhang, A. Fecal Microbiota Transplantation: A Novel Strategy for Treating Alzheimer’s Disease. Front. Microbiol. 2023, 14, 1281233. [Google Scholar] [CrossRef]
- Novelle, M.G.; Naranjo-Martínez, B.; López-Cánovas, J.L.; Díaz-Ruiz, A. Fecal Microbiota Transplantation, a Tool to Transfer Healthy Longevity. Ageing Res. Rev. 2025, 103, 102585. [Google Scholar] [CrossRef]
- Tian, H.; Wang, X.; Fang, Z.; Li, L.; Wu, C.; Bi, D.; Li, N.; Chen, Q.; Qin, H. Fecal Microbiota Transplantation in Clinical Practice: Present Controversies and Future Prospects. hLife 2024, 2, 269–283. [Google Scholar] [CrossRef]
- Zikou, E.; Koliaki, C.; Makrilakis, K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines 2024, 12, 1871. [Google Scholar] [CrossRef]
- Chance, E.A.; Florence, D.; Sardi Abdoul, I. The Effectiveness of Checklists and Error Reporting Systems in Enhancing Patient Safety and Reducing Medical Errors in Hospital Settings: A Narrative Review. Int. J. Nurs. Sci. 2024, 11, 387–398. [Google Scholar] [CrossRef]
- White, S.L.; Rawlinson, W.; Boan, P.; Sheppeard, V.; Wong, G.; Waller, K.; Opdam, H.; Kaldor, J.; Fink, M.; Verran, D.; et al. Infectious Disease Transmission in Solid Organ Transplantation: Donor Evaluation, Recipient Risk, and Outcomes of Transmission. Transplant. Direct 2019, 5, e416. [Google Scholar] [CrossRef]
- Opara, U.C.; Iheanacho, P.N.; Li, H.; Petrucka, P. Facilitating and Limiting Factors of Cultural Norms Influencing Use of Maternal Health Services in Primary Health Care Facilities in Kogi State, Nigeria; a Focused Ethnographic Research on Igala Women. BMC Pregnancy Childbirth 2024, 24, 555. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Masirah M Zain, N.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Bi, D.; Li, L.; Tian, H.; Yin, F.; Zuo, T.; Ianiro, G.; Li, N.; Chen, Q.; et al. Fecal Microbiota Transplantation: Transitioning from Chaos and Controversial Realm to Scientific Precision Era. Sci. Bull. 2025, 70, 970–985. [Google Scholar] [CrossRef]
- Kamel, M.; Aleya, S.; Alsubih, M.; Aleya, L. Microbiome Dynamics: A Paradigm Shift in Combatting Infectious Diseases. J. Pers. Med. 2024, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Panwar, A.S.; Singh, R.K. Orchestrating the Fecal Microbiota Transplantation: Current Technological Advancements and Potential Biomedical Application. Front. Med. Technol. 2022, 4, 961569. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Ordovás, J.M.; Parks, E.J.; Anderson, C.A.; Barabási, A.-L.; Clinton, S.K.; de la Haye, K.; Duffy, V.B.; Franks, P.W.; Ginexi, E.M.; et al. Research Gaps and Opportunities in Precision Nutrition: An NIH Workshop Report. Am. J. Clin. Nutr. 2022, 116, 1877–1900. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Feng, Y.; Zhao, J.; Chen, W.; Lu, W. Achieving Healthy Aging through Gut Microbiota-Directed Dietary Intervention: Focusing on Microbial Biomarkers and Host Mechanisms. J. Adv. Res. 2025, 68, 179–200. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Randeni, N.; Xu, B. Critical Review of the Cross-Links Between Dietary Components, the Gut Microbiome, and Depression. Int. J. Mol. Sci. 2025, 26, 614. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Li, X.; Yu, J.-T.; Wang, Y.-J. Therapeutics for Neurodegenerative Diseases by Targeting the Gut Microbiome: From Bench to Bedside. Transl. Neurodegener. 2024, 13, 12. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, M.; Meng, J.; Wang, L.; Chen, M. A Review of the Interaction between Diet Composition and Gut Microbiota and Its Impact on Associated Disease. J. Future Foods 2024, 4, 221–232. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The Function of Gut Microbiota in Immune-Related Neurological Disorders: A Review. J. Neuroinflammation 2022, 19, 154. [Google Scholar] [CrossRef]
- Mansuy-Aubert, V.; Ravussin, Y. Short Chain Fatty Acids: The Messengers from down Below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef] [PubMed]
- Mafe, A.N.; Büsselberg, D. Modulation of the Neuro–Cancer Connection by Metabolites of Gut Microbiota. Biomolecules 2025, 15, 270. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Shetty, S.S.; Kumari, N.S. Role of Gut Microbiota Derived Short Chain Fatty Acid Metabolites in Modulating Female Reproductive Health. Hum. Nutr. Metab. 2024, 36, 200256. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Gritsenko, V.A.; Martins, A.C.; Tizabi, Y.; Korobeinikova, T.V.; Paoliello, M.M.B.; Tinkov, A.A. Modulation of Gut Microbiota with Probiotics as a Strategy to Counteract Endogenous and Exogenous Neurotoxicity; Elsevier: Amsterdam, The Netherlands, 2024; pp. 133–176. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Counts, S.E.; Zhou, C.; Hida, H.; Kim, J.-I.; Michikawa, M.; Jung, C.-G. Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer’s Disease. Nutrients 2025, 17, 558. [Google Scholar] [CrossRef]
- Sheng, W.; Ji, G.; Zhang, L. Immunomodulatory Effects of Inulin and Its Intestinal Metabolites. Front. Immunol. 2023, 14, 1224092. [Google Scholar] [CrossRef]
- Mahalak, K.K.; Firrman, J.; Narrowe, A.B.; Hu, W.; Jones, S.M.; Bittinger, K.; Moustafa, A.M.; Liu, L. Fructooligosaccharides (FOS) Differentially Modifies the in Vitro Gut Microbiota in an Age-Dependent Manner. Front. Nutr. 2023, 9, 1058910. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, Y.-J.; Lee, D.-Y.; Kim, K.M.; Yang, S.-J.; Kim, D.S.; Choi, J.-W.; Lee, S.; Kim, D.-H. Lactobacillus Rhamnosus HDB1258 Modulates Gut Microbiota-Mediated Immune Response in Mice with or without Lipopolysaccharide-Induced Systemic Inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef]
- de Oliveira, D.P.; Todorov, S.D.; Fabi, J.P. Exploring the Prebiotic Potentials of Hydrolyzed Pectins: Mechanisms of Action and Gut Microbiota Modulation. Nutrients 2024, 16, 3689. [Google Scholar] [CrossRef]
- Mills, S.; Yang, B.; Smith, G.J.; Stanton, C.; Ross, R.P. Efficacy of Bifidobacterium Longum Alone or in Multi-Strain Probiotic Formulations during Early Life and Beyond. Gut Microbes 2023, 15, 2186098. [Google Scholar] [CrossRef] [PubMed]
- Beteri, B.; Barone, M.; Turroni, S.; Brigidi, P.; Tzortzis, G.; Vulevic, J.; Sekulic, K.; Motei, D.-E.; Costabile, A. Impact of Combined Prebiotic Galacto-Oligosaccharides and Bifidobacterium Breve-Derived Postbiotic on Gut Microbiota and HbA1c in Prediabetic Adults: A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2024, 16, 2205. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.M.; Cho, J.; Lee, C. Gut Microbiota and Alzheimer’s Disease: How to Study and Apply Their Relationship. Int. J. Mol. Sci. 2023, 24, 4047. [Google Scholar] [CrossRef] [PubMed]
- Binda, S.; Tremblay, A.; Iqbal, U.H.; Kassem, O.; Le Barz, M.; Thomas, V.; Bronner, S.; Perrot, T.; Ismail, N.; Parker, J.A. Psychobiotics and the Microbiota–Gut–Brain Axis: Where Do We Go from Here? Microorganisms 2024, 12, 634. [Google Scholar] [CrossRef]
- Larroya, A.; Pantoja, J.; Codoñer-Franch, P.; Cenit, M.C. Towards Tailored Gut Microbiome-Based and Dietary Interventions for Promoting the Development and Maintenance of a Healthy Brain. Front. Pediatr. 2021, 9, 705859. [Google Scholar] [CrossRef]
- Fang, P.; Kazmi, S.A.; Jameson, K.G.; Hsiao, E.Y. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host Microbe 2020, 28, 201–222. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, M.; Wang, P. The Intricate Interplay between Dietary Habits and Cognitive Function: Insights from the Gut-Brain Axis. Front. Nutr. 2025, 12, 1539355. [Google Scholar] [CrossRef]
- Holmes, Z.C.; Villa, M.M.; Durand, H.K.; Jiang, S.; Dallow, E.P.; Petrone, B.L.; Silverman, J.D.; Lin, P.-H.; David, L.A. Microbiota Responses to Different Prebiotics Are Conserved within Individuals and Associated with Habitual Fiber Intake. Microbiome 2022, 10, 114. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, N.; Zhang, H.; Li, H.; Guo, J.; Zhang, Y.; Chen, Y.; Wang, Y.; Shi, N. Resistant Starch and the Gut Microbiome: Exploring Beneficial Interactions and Dietary Impacts. Food Chem. X 2024, 21, 101118. [Google Scholar] [CrossRef]
- Bianchetti, G.; De Maio, F.; Abeltino, A.; Serantoni, C.; Riente, A.; Santarelli, G.; Sanguinetti, M.; Delogu, G.; Martinoli, R.; Barbaresi, S.; et al. Unraveling the Gut Microbiome–Diet Connection: Exploring the Impact of Digital Precision and Personalized Nutrition on Microbiota Composition and Host Physiology. Nutrients 2023, 15, 3931. [Google Scholar] [CrossRef]
- Arafah, A.; Khatoon, S.; Rasool, I.; Khan, A.; Rather, M.A.; Abujabal, K.A.; Faqih, Y.A.H.; Rashid, H.; Rashid, S.M.; Bilal Ahmad, S.; et al. The Future of Precision Medicine in the Cure of Alzheimer’s Disease. Biomedicines 2023, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Singh, S.; Verma, S.; Verma, S.; Rizvi, A.A.; Abbas, M. Targeting the Microbiome to Improve Human Health with the Approach of Personalized Medicine: Latest Aspects and Current Updates. Clin. Nutr. ESPEN 2024, 63, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Di Meco, A.; Vassar, R. Early Detection and Personalized Medicine: Future Strategies Against Alzheimer’s Disease; Elsevier: Amsterdam, The Netherlands, 2021; pp. 157–173. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, G.; Kwok, L.-Y.; Sun, Z. Gut Microbiome-Targeted Therapies for Alzheimer’s Disease. Gut Microbes 2023, 15, 2271613. [Google Scholar] [CrossRef] [PubMed]
- Mafe, A.N.; Büsselberg, D. Microbiome Integrity Enhances the Efficacy and Safety of Anticancer Drug. Biomedicines 2025, 13, 422. [Google Scholar] [CrossRef]
- Ngah, W.Z.W.; Ahmad, H.F.; Ankasha, S.J.; Makpol, S.; Tooyama, I. Dietary Strategies to Mitigate Alzheimer’s Disease: Insights into Antioxidant Vitamin Intake and Supplementation with Microbiota–Gut–Brain Axis Cross-Talk. Antioxidants 2024, 13, 1504. [Google Scholar] [CrossRef]
- Merino del Portillo, M.; Clemente-Suárez, V.J.; Ruisoto, P.; Jimenez, M.; Ramos-Campo, D.J.; Beltran-Velasco, A.I.; Martínez-Guardado, I.; Rubio-Zarapuz, A.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional Modulation of the Gut–Brain Axis: A Comprehensive Review of Dietary Interventions in Depression and Anxiety Management. Metabolites 2024, 14, 549. [Google Scholar] [CrossRef]
- Gulliver, E.L.; Young, R.B.; Chonwerawong, M.; D’Adamo, G.L.; Thomason, T.; Widdop, J.T.; Rutten, E.L.; Rossetto Marcelino, V.; Bryant, R.V.; Costello, S.P.; et al. Review Article: The Future of Microbiome-based Therapeutics. Aliment. Pharmacol. Ther. 2022, 56, 192–208. [Google Scholar] [CrossRef]
- Wasén, C.; Simonsen, E.; Ekwudo, M.N.; Profant, M.R.; Cox, L.M. The Emerging Role of the Microbiome in Alzheimer’s Disease; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–139. [Google Scholar] [CrossRef]
- Pourahmad, R.; Saleki, K.; Zare Gholinejad, M.; Aram, C.; Soltani Farsani, A.; Banazadeh, M.; Tafakhori, A. Exploring the Effect of Gut Microbiome on Alzheimer’s Disease. Biochem. Biophys. Rep. 2024, 39, 101776. [Google Scholar] [CrossRef]
- Ferreiro, A.L.; Choi, J.; Ryou, J.; Newcomer, E.P.; Thompson, R.; Bollinger, R.M.; Hall-Moore, C.; Ndao, I.M.; Sax, L.; Benzinger, T.L.S.; et al. Gut Microbiome Composition May Be an Indicator of Preclinical Alzheimer’s Disease. Sci. Transl. Med. 2023, 15, eabo2984. [Google Scholar] [CrossRef]
- Onisiforou, A.; Charalambous, E.G.; Zanos, P. Shattering the Amyloid Illusion: The Microbial Enigma of Alzheimer’s Disease Pathogenesis—From Gut Microbiota and Viruses to Brain Biofilms. Microorganisms 2025, 13, 90. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Liu, K.; Zhang, H.-L. Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Implications of the Blood-Brain Barrier as an Intervention Target. Mech. Ageing Dev. 2021, 199, 111560. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Lei, D.; Yin, Y.; Xu, R.; Luo, H.; Chen, T.; Liu, M.; Li, X. Frequent Fecal Microbiota Transplantation Improves Cognitive Impairment and Pathological Changes in Alzheimer’s Disease FAD4T Mice via the Microbiota-Gut-Brain Axis. Heliyon 2025, 11, e42925. [Google Scholar] [CrossRef]
- Osama, A.; Anwar, A.M.; Ezzeldin, S.; Ahmed, E.A.; Mahgoub, S.; Ibrahim, O.; Ibrahim, S.A.; Abdelhamid, I.A.; Bakry, U.; Diab, A.A.; et al. Integrative Multi-Omics Analysis of Autism Spectrum Disorder Reveals Unique Microbial Macromolecules Interactions. J. Adv. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
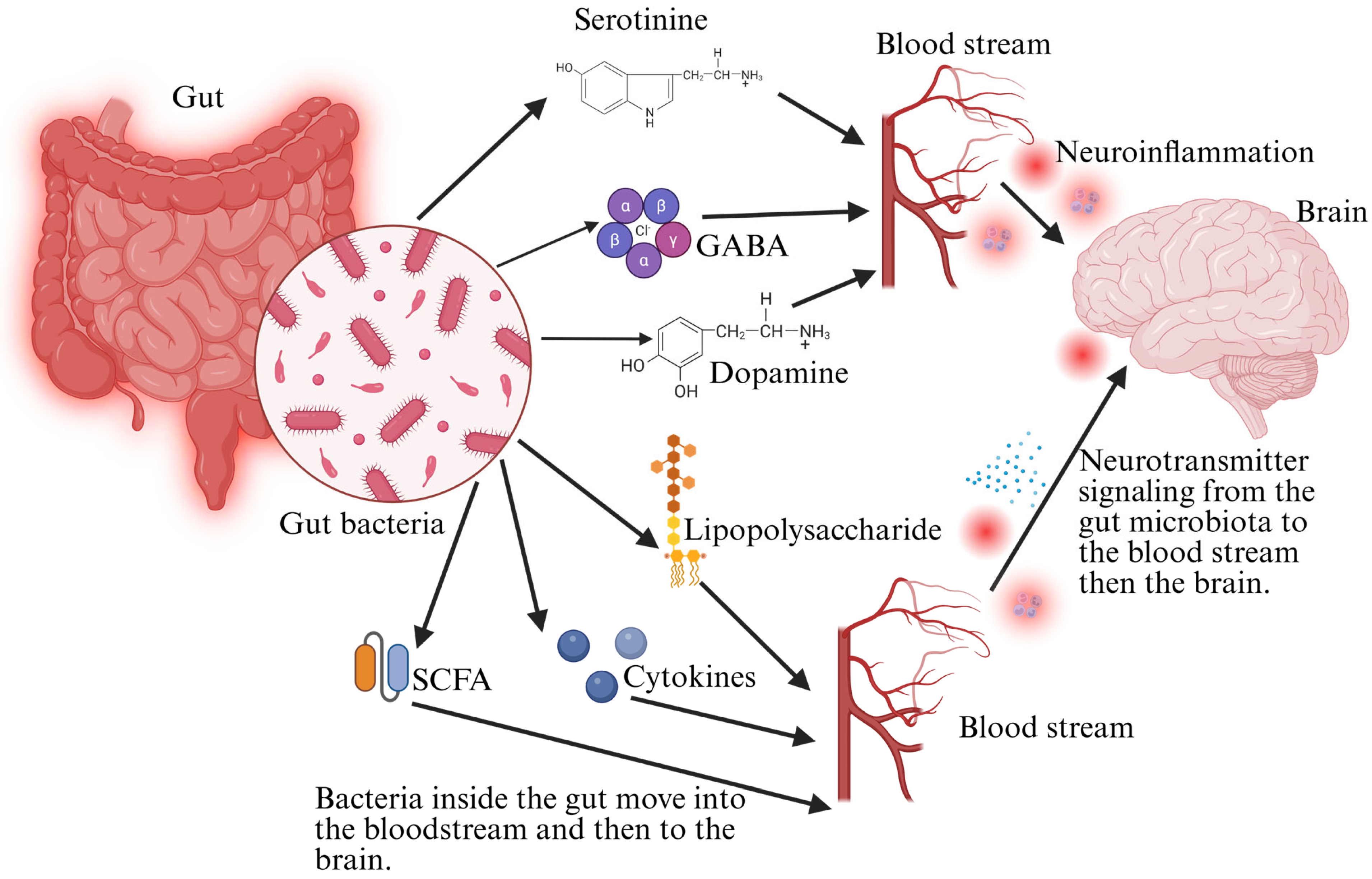



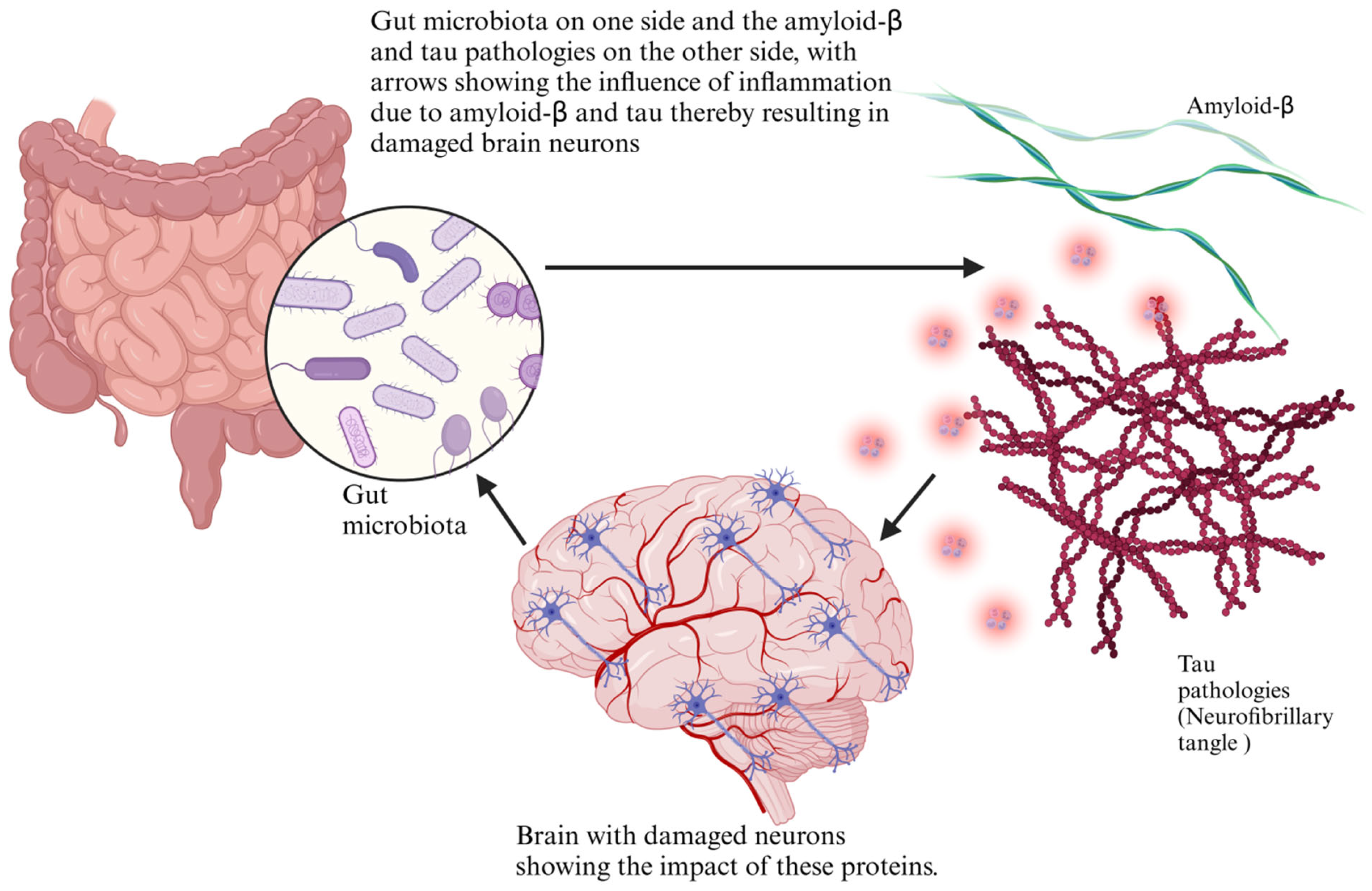
| Microbiota-Derived Metabolite | Source/Pathway | Impact on Neurodegeneration | Mechanism of Action |
|---|---|---|---|
| Short-Chain Fatty Acids (SCFAs) | Breakdown of dietary fibers by intestinal microbes | Neuroprotective; reduces neuroinflammation and enhances BBB integrity | SCFAs, particularly butyrate, regulate histone deacetylases (HDACs), promoting anti-inflammatory gene expression and preventing microglial activation [40]. |
| Lipopolysaccharides (LPS) | Gram-negative bacteria | Promotes neuroinflammation and BBB permeability, exacerbating neurodegeneration | LPS activates TLR4 receptors on microglia and astrocytes, leading to IL-6 and TNF-α production via NF-κB activation [48]. |
| Tryptophan Metabolites (e.g., Indoles) | Tryptophan metabolism by gut bacteria | Modulates immune response; can exert neuroprotective effects | Indoles can inhibit NF-κB activation, reducing neuroinflammation and promoting neuronal survival [49]. |
| Bile Acids | Microbial transformation of bile acids | Modulates microglial activation; impacts inflammation and neuroprotection | Bile acids activate G-protein-coupled receptors (GPCRs) such as TGR5, which can modulate immune cell signaling in the brain [50]. |
| Amines (e.g., Histamine) | Bacterial decarboxylation of amino acids | Can induce neuroinflammation in excess; role in Parkinson’s disease (PD) | Histamine binds to H1 receptors on microglia, triggering pro-inflammatory cytokine release and potentially aggravating neurodegeneration [51]. |
| Aryl Hydrocarbon Receptor Ligands | Metabolites of tryptophan and other aromatic compounds | Modulates immune response and neuroinflammation | These metabolites activate the aryl hydrocarbon receptor (AhR), which influences neuroimmune responses and can suppress inflammation in certain contexts [52]. |
| GABA (Gamma-aminobutyric acid) | Gut microbiota producing GABA | Neuroprotective; regulates neuroinflammation and promotes neuronal survival | GABA modulates GABA receptors on neurons and glial cells, suppressing pro-inflammatory cytokine production and enhancing neuroprotection [53]. |
| Polyamines (e.g., Spermidine) | Bacterial synthesis of polyamines | Protects against oxidative stress and neurodegeneration | Spermidine activates autophagy, reducing cellular stress and promoting neuronal health by mitigating protein aggregation and inflammation [54]. |
| Catechols (e.g., Dopamine) | Gut microbiota influencing dopamine pathways | Potential influence on Parkinson’s disease progression and motor control | Gut-derived dopamine metabolites can impact the brain’s dopaminergic system, influencing neurodegenerative diseases such as Parkinson’s [55]. |
| P-cresol | Bacterial metabolism of aromatic amino acids | Increases oxidative stress, exacerbating neurodegeneration | P-cresol can activate inflammatory pathways and generate reactive oxygen species (ROS), contributing to neuronal damage in neurodegenerative diseases [56]. |
| Amino Acids (e.g., Glutamate) | Bacterial conversion of dietary proteins | Modulates neuronal excitotoxicity; potentially harmful in excess | Excess glutamate from gut microbial metabolism can increase neuronal excitotoxicity and promote neurodegenerative conditions like Alzheimer’s [57]. |
| Polyphenols (e.g., Resveratrol) | Microbial fermentation of polyphenol-rich foods | Antioxidant and anti-inflammatory properties; neuroprotective effects | Resveratrol and other polyphenols can modulate gut microbiota composition and reduce neuroinflammation, promoting brain health and potentially slowing degeneration [58]. |
| Vitamins (e.g., B12, D) | Gut bacteria and fermentation processes | Regulate immune response and neuronal function | Vitamin B12 is essential for nerve function, while Vitamin D modulates immune pathways that influence neuroinflammation and neurodegeneration [59]. |
| Fatty Acids (Omega-3, Omega-6) | Fermentation of dietary fats | Modulate brain inflammation; protect against cognitive decline | Omega-3 fatty acids reduce the production of pro-inflammatory cytokines, whereas an imbalance (e.g., too many omega-6 fatty acids) can promote neuroinflammation [60]. |
| Dietary Component | Effect on Gut Microbiota |
|---|---|
| Polyphenols | Promote the growth of beneficial bacteria (e.g., Bifidobacterium sp., Lactobacillus sp.) and inhibit pathogenic bacteria [125]. |
| Omega-3 Fatty Acids | Enhance gut microbiota diversity, promote anti-inflammatory bacteria, and reduce pro-inflammatory microbial species [126]. |
| Fiber (Prebiotics) | Promote the growth of beneficial gut bacteria, such as Bacteroides, which ferment fiber into SCFAs [127]. |
| Fermented Foods | Introduce probiotics, such as Lactobacillus sp. Bifidobacterium sp., to enhance gut microbiota balance and diversity [128]. |
| Polyunsaturated Fatty Acids | Can alter gut microbiota composition, sometimes promoting the growth of beneficial microbes while reducing harmful bacteria [126]. |
| High-Fat, Low-Fiber Diets | This can lead to dysbiosis, decreasing the diversity of beneficial bacteria and promoting the growth of harmful microbes like Firmicutes [129]. |
| Antioxidants (e.g., Vitamin C, E) | Enhance the growth of beneficial bacteria and reduce gut inflammation, supporting a balanced microbiota [130]. |
| Probiotics | Directly introduce beneficial bacteria that can improve gut health, enhance microbial diversity, and reduce inflammation [131]. |
| High-Sugar Diets | An increased abundance of pathogenic bacteria, such as Firmicutes, and can lead to inflammation and gut dysbiosis [132]. |
| High-Protein Diets | It can alter the gut microbiome by enhancing the prevalence of protein-fermenting microflora, potentially promoting dysbiosis [133]. |
| Study | Intervention | Target Group | Outcome |
|---|---|---|---|
| Analytics Study (2024) | Mediterranean diet | Elderly individuals at risk of Alzheimer’s | Improved cognitive function and reduced neuroinflammation [153]. |
| Omega-3 and Probiotic Trial (2021) | Omega-3 supplementation + probiotics | Alzheimer’s patients with mild cognitive impairment | Improvement in cognitive scores and gut microbiota diversity [154]. |
| Prebiotic Intervention Trial (2021) | Prebiotics (inulin and fructooligosaccharides) | Elderly individuals with early-stage Alzheimer’s | Increased beneficial gut bacteria and reduced amyloid plaques [155]. |
| Gut Microbiota Modulation Study (2025) | High-fiber diet and probiotic supplementation | Adults with early-stage Alzheimer’s disease | Significant improvement in cognitive function and gut health [156]. |
| Bifidobacterium sp. and Cognitive Decline Study (2025) | Bifidobacterium sp. supplementation | Individuals with mild cognitive impairment | Increased gut microbial diversity and improved cognition [157]. |
| Polyphenol-Rich Diet Trial (2025) | Polyphenol-rich foods (e.g., blueberries, green tea) | Adults at risk for Alzheimer’s | Enhancement in cognitive function and reduction of oxidative stress [158]. |
| High-Protein Diet and Neurodegeneration Study (2020) | High-protein, low-carb diet | Alzheimer’s patients with advanced stages | Modulation of gut microbiota towards anti-inflammatory species [133]. |
| Strategy | Probiotic/ Prebiotic Type | Mechanism of Action | Potential Cognitive Benefit |
|---|---|---|---|
| Lactobacillus species | Probiotic | Modulates gut microbiota, reduces inflammation, increases SCFAs | Enhances memory, reduces neuroinflammation [198] |
| Bifidobacterium species | Probiotic | Supports gut barrier integrity, balances gut microbiota | Improves cognitive function, reduces amyloid plaque formation [199] |
| Inulin (prebiotic fiber) | Prebiotic | Stimulates growth of beneficial bacteria, enhances SCFA production | Reduces neuroinflammation, enhances cognitive performance [200] |
| Fructooligosaccharides (FOS) | Prebiotic | Promotes beneficial microbiota, increases SCFAs, reduces endotoxins | Improves synaptic plasticity, reduces cognitive decline [201] |
| Lactobacillus rhamnosus GR1 | Probiotic | Reduces gut permeability, modulates immune response | Improves mood and cognitive functions [202] |
| Prebiotic dietary fiber (e.g., pectin) | Prebiotic | Stimulates beneficial bacteria, enhances microbial diversity | Enhances neuroprotection via gut–brain signaling [203] |
| Bifidobacterium longum | Probiotic | Modulates immune response, enhances short-chain fatty acid production | Reduces neuroinflammation, improves memory and cognitive function [204] |
| Galacto-oligosaccharides (GOS) | Prebiotic | Increases beneficial microbiota, enhances gut barrier function | Modulates gut–brain signaling, promotes neuroprotective effects [205] |
| Research Gap | Description | Future Research Directions |
|---|---|---|
| Longitudinal Microbiome Studies | Limited knowledge of how gut microbiota evolves in Alzheimer’s disease. | Large-scale, long-term microbiome studies should be conducted to identify causal relationships between microbiota shifts and disease development [222]. |
| Microbiome–Drug Interactions | Insufficient knowledge on how the microbiome affects the efficacy of pharmaceutical treatments for Alzheimer’s. | Investigate the interactions between gut microbiota and Alzheimer’s drugs to optimize therapeutic strategies [223]. |
| Microbial Biomarkers for Early Diagnosis | Lack of reliable biomarkers for early detection of Alzheimer’s based on microbiome composition. | Developing microbiome-based diagnostic tools for early detection and monitoring of Alzheimer’s progression [224]. |
| Personalized Diet–Microbiome Interventions | Variability in individual responses to diet-based interventions. | Conduct precision nutrition studies to personalize dietary recommendations based on microbiome profiles for cognitive health [213]. |
| Mechanisms Linking Gut Microbiota to Brain Pathology | Limited conception of the specific microbial metabolites and pathways involved in Alzheimer’s pathology. | Investigate the molecular mechanisms by which microbiome-derived metabolites influence brain inflammation and protein accumulation (e.g., amyloid-β, tau) [225]. |
| Impact of Gut Dysbiosis on Blood–Brain Barrier (BBB) | Inadequate insight on how gut dysbiosis contributes to BBB disruption in Alzheimer’s. | Study the effects of microbiome modulation on BBB integrity and its role in neurodegenerative diseases [226]. |
| Therapeutic Potential of Fecal Microbiota Transplantation (FMT) | Insufficient evidence on the effectiveness of FMT in treating Alzheimer’s. | Conduct clinical trials to evaluate the safety and efficacy of FMT as a therapy for Alzheimer’s [227]. |
| Long-Term Effects of Probiotics and Prebiotics | Uncertainty about the long-term impact of probiotics and prebiotics on Alzheimer’s progression. | Long-term studies should assess probiotic and prebiotic interventions’ sustained benefits and risks in Alzheimer’s patients [156]. |
| Microbiome-Based Personalized Medicine | Lack of comprehensive, personalized treatment options based on microbiome profiling. | Develop personalized treatment strategies using microbiome-based therapies tailored to individual profiles for neurodegenerative diseases [189]. |
| Comprehensive Multi-Omics Approaches | Insufficient integration of multi-omics data to understand the full complexity of the gut–brain axis. | Integrate genomics, metabolomics, and proteomics data to understand better the gut–brain interactions and Alzheimer’s pathology [228]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafe, A.N.; Büsselberg, D. Could a Mediterranean Diet Modulate Alzheimer’s Disease Progression? The Role of Gut Microbiota and Metabolite Signatures in Neurodegeneration. Foods 2025, 14, 1559. https://doi.org/10.3390/foods14091559
Mafe AN, Büsselberg D. Could a Mediterranean Diet Modulate Alzheimer’s Disease Progression? The Role of Gut Microbiota and Metabolite Signatures in Neurodegeneration. Foods. 2025; 14(9):1559. https://doi.org/10.3390/foods14091559
Chicago/Turabian StyleMafe, Alice N., and Dietrich Büsselberg. 2025. "Could a Mediterranean Diet Modulate Alzheimer’s Disease Progression? The Role of Gut Microbiota and Metabolite Signatures in Neurodegeneration" Foods 14, no. 9: 1559. https://doi.org/10.3390/foods14091559
APA StyleMafe, A. N., & Büsselberg, D. (2025). Could a Mediterranean Diet Modulate Alzheimer’s Disease Progression? The Role of Gut Microbiota and Metabolite Signatures in Neurodegeneration. Foods, 14(9), 1559. https://doi.org/10.3390/foods14091559







