Volvariella volvacea Polypeptide Mitigates Alcohol-Induced Liver Injury: A Multi-Omics Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. VVFP Extraction and Purification
2.3. Animal Experiments
2.4. The Determination of Drunkenness and Sobering Time
2.5. Liver Index Detection
2.6. Transcriptome Analysis
2.7. Metabonomics Analysis
2.8. Multi-Omics Joint Analysis
2.9. Statistical Analysis of Data
3. Results
3.1. Ethanol Metabolism Modulation by VVFP
3.2. Hepatoprotective Efficacy Assessment
3.3. Transcriptomic Profiling of Hepatic Responses
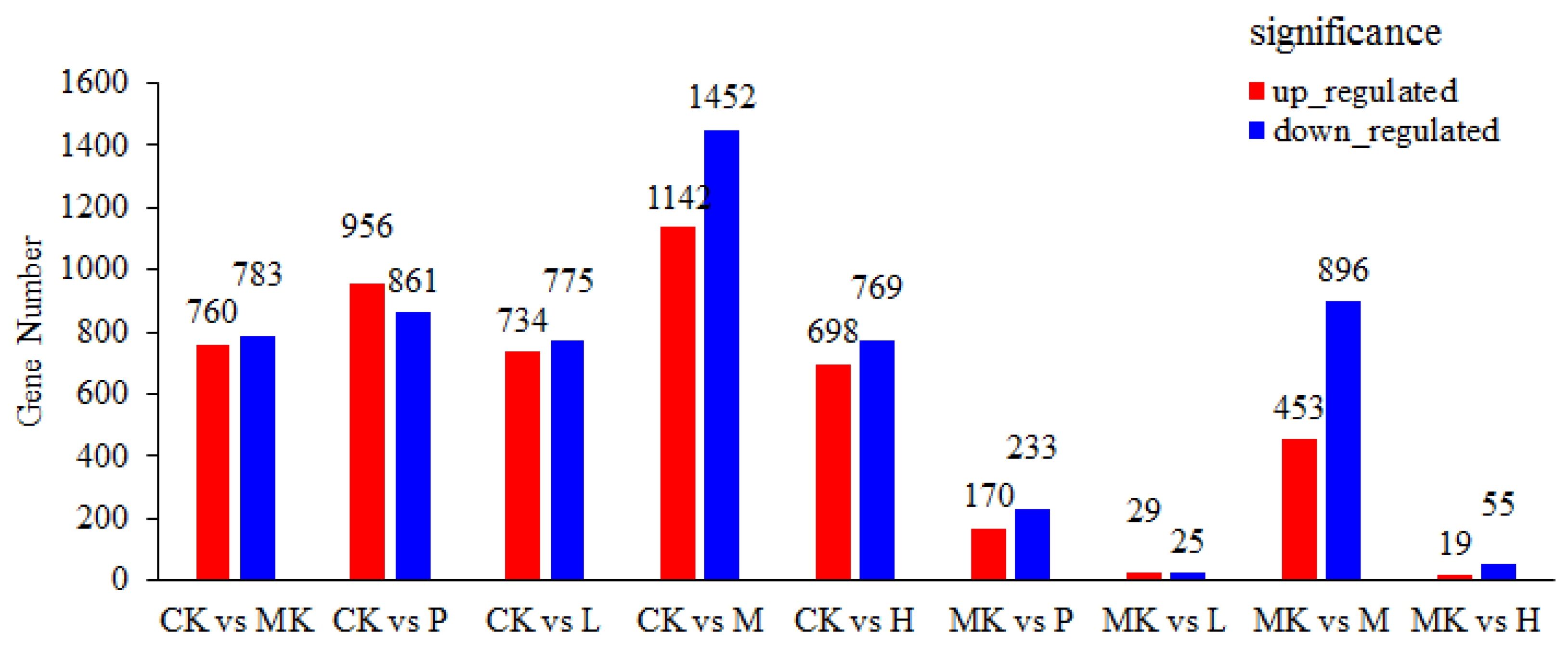
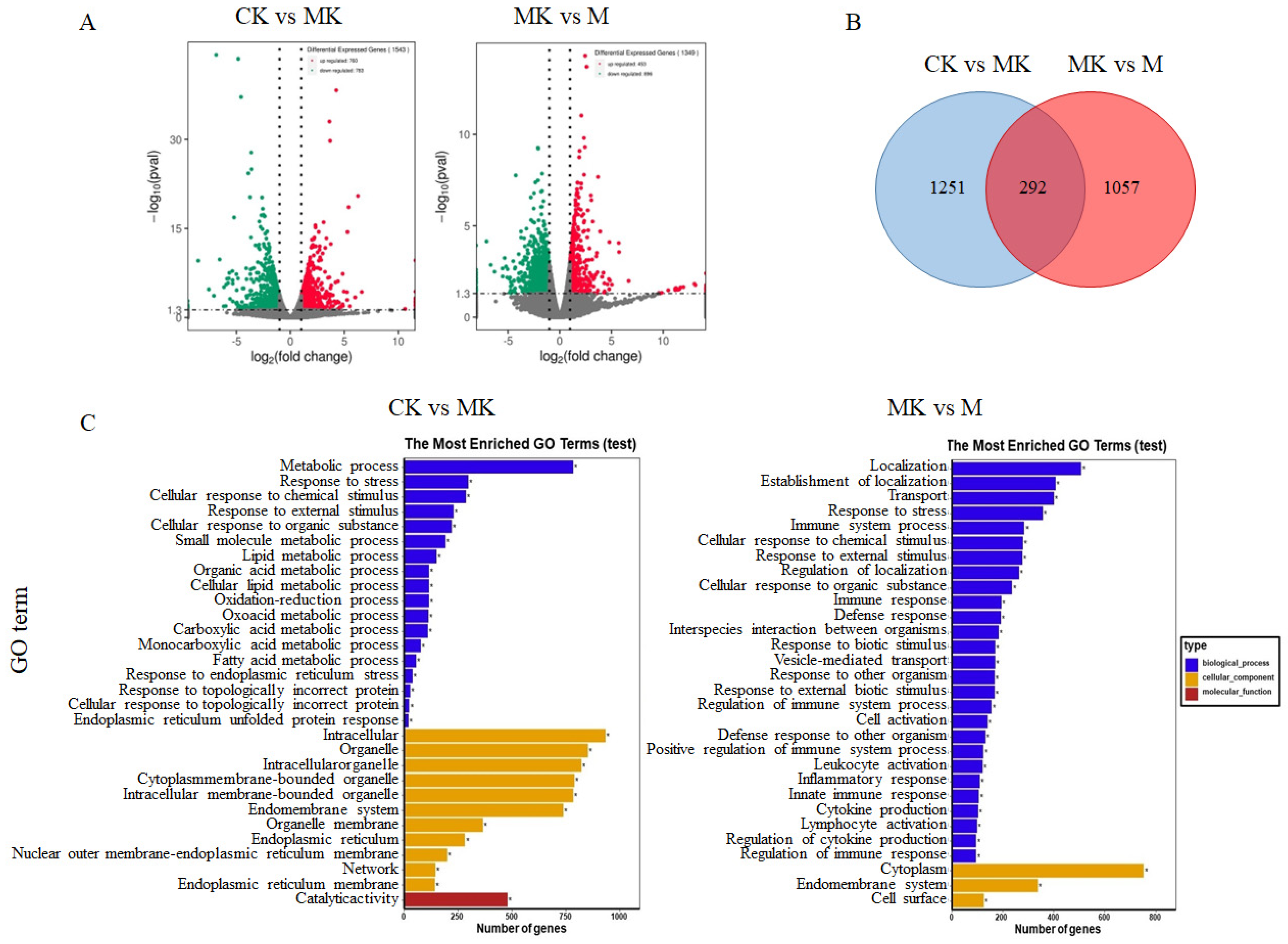
3.3.1. Identification of Differentially Expressed Genes (DEGs)
3.3.2. GO Function Annotation and Enrichment Analysis
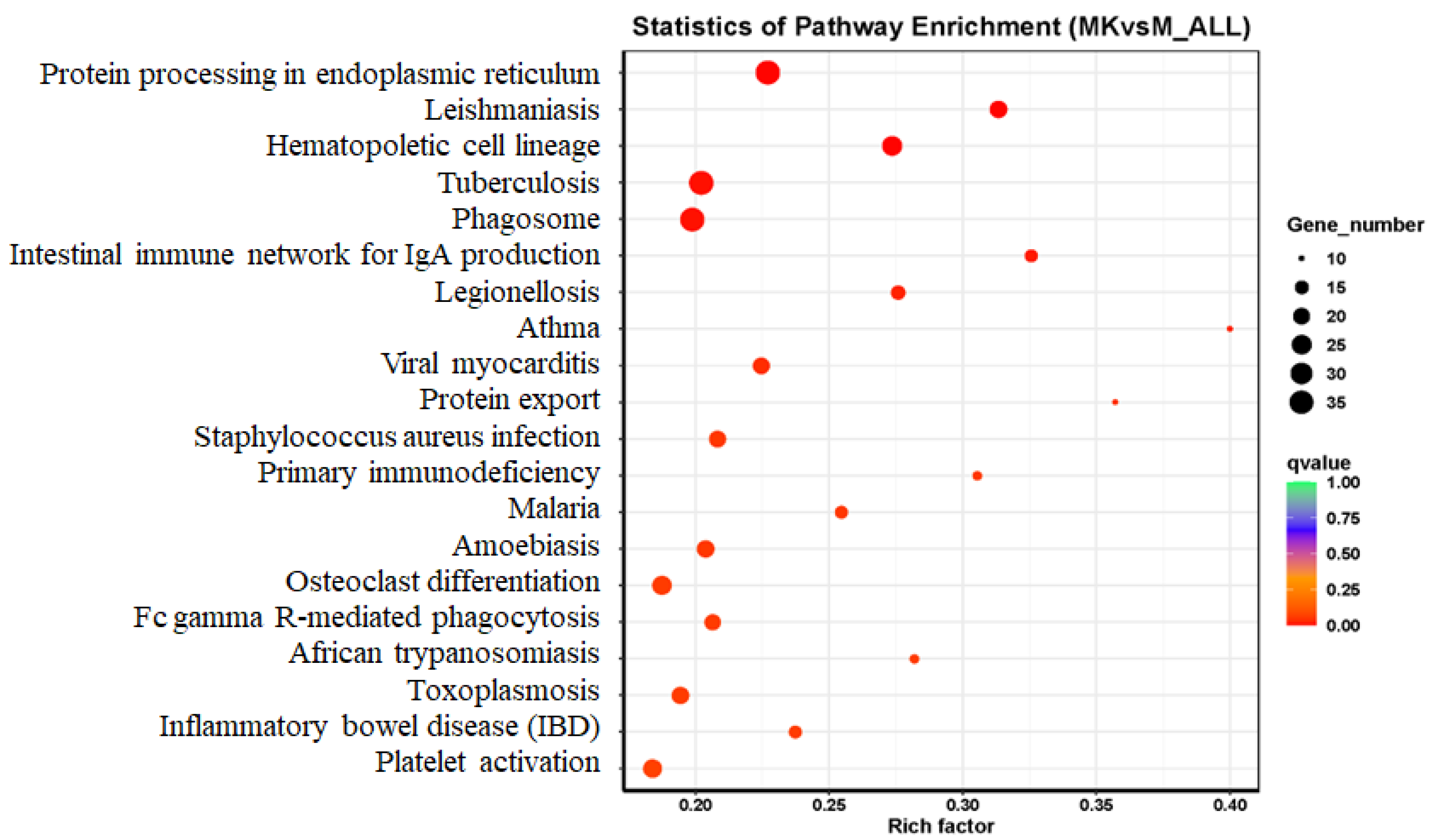
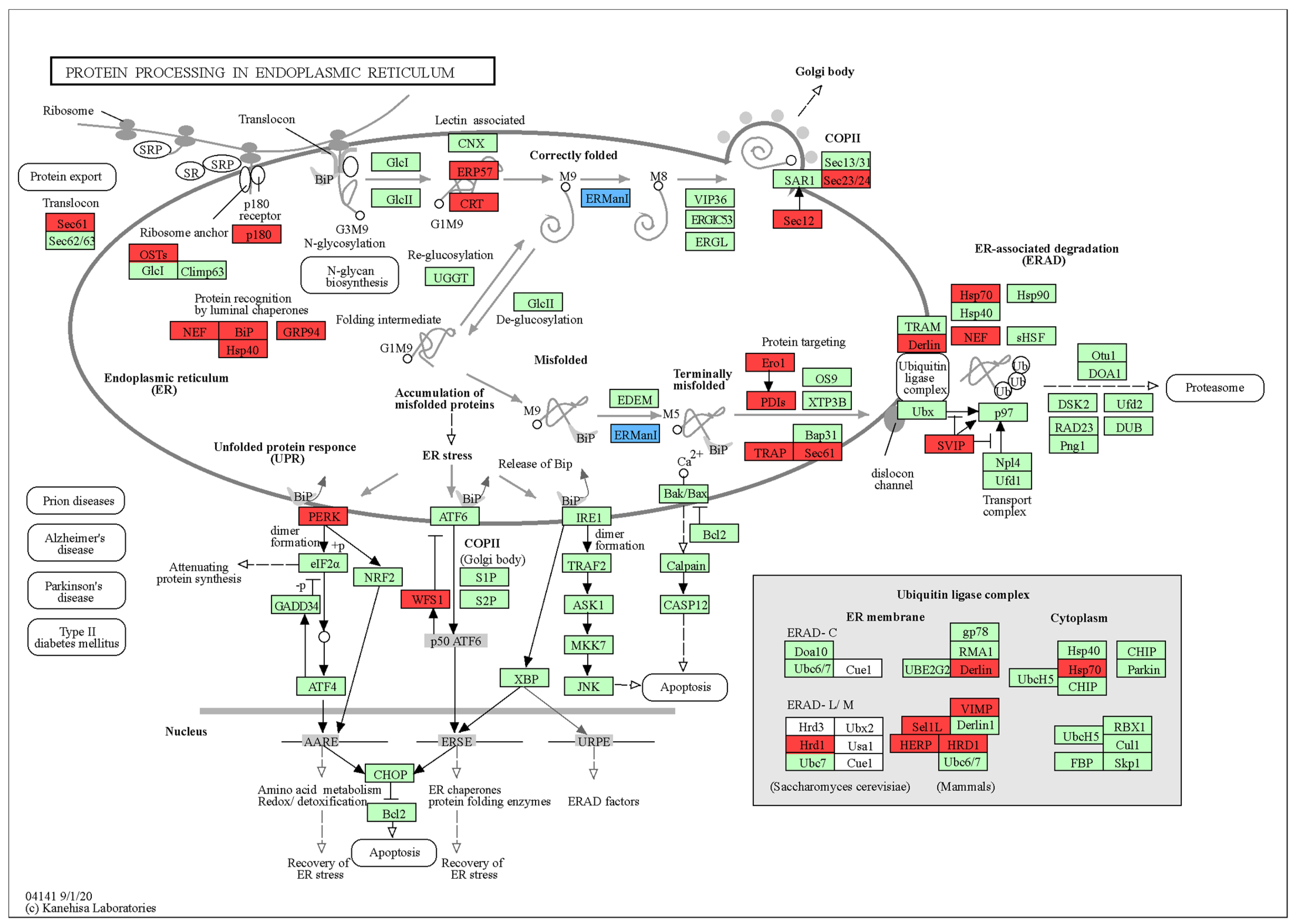
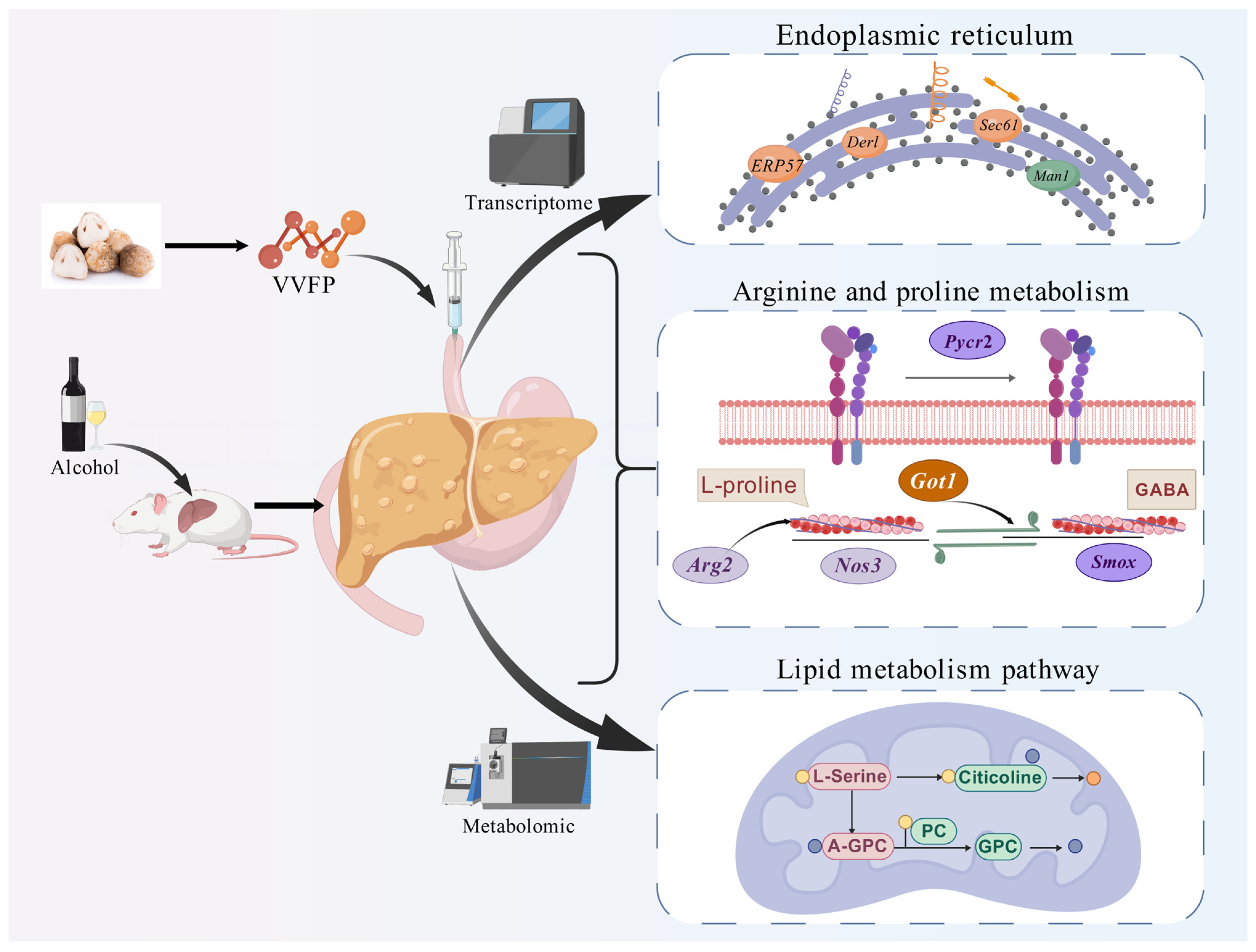
3.3.3. Pathway Enrichment and Mechanistic Insights
3.4. Metabonomics Analysis of Mouse Liver
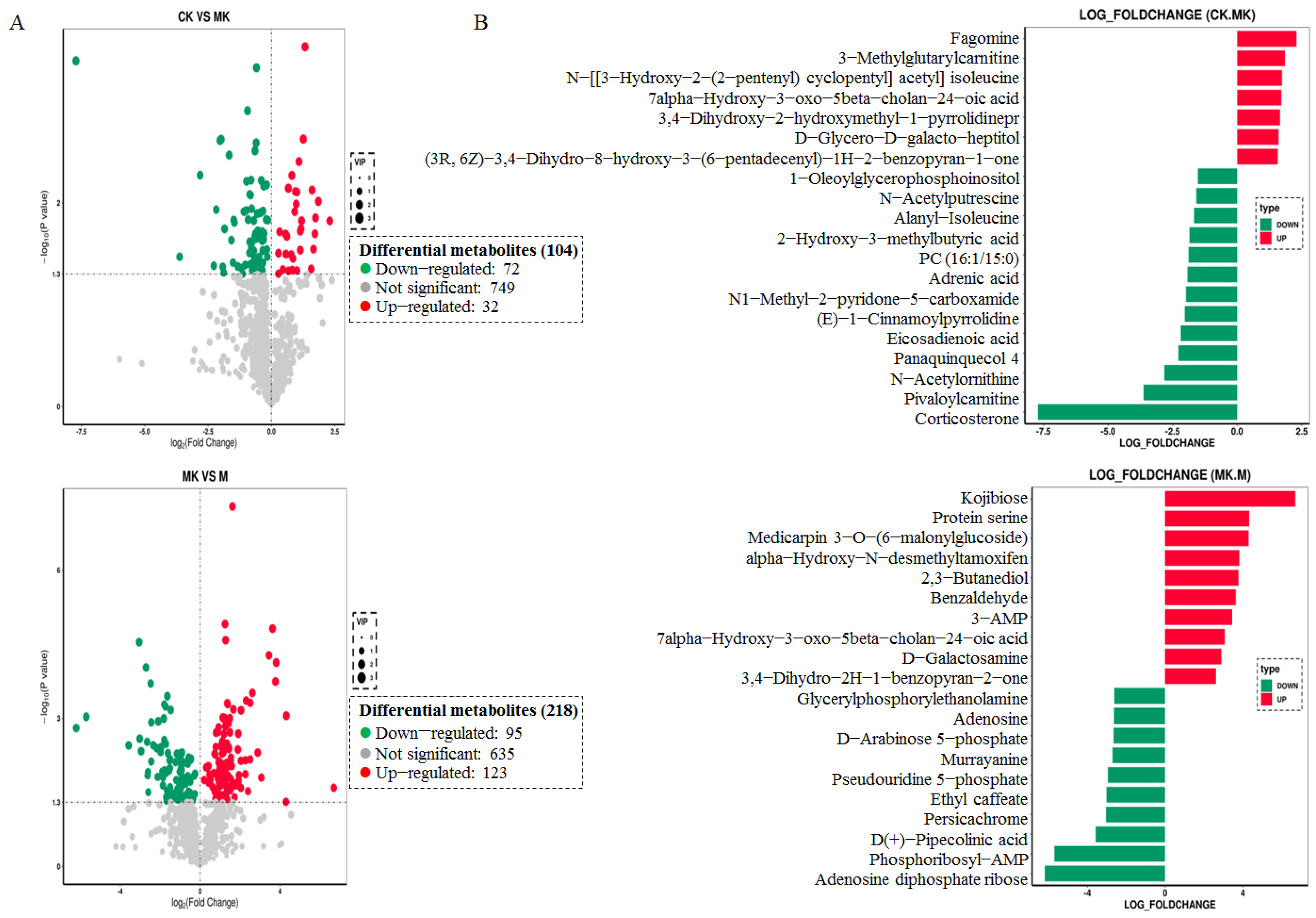
3.4.1. Screening of Differential Metabolites
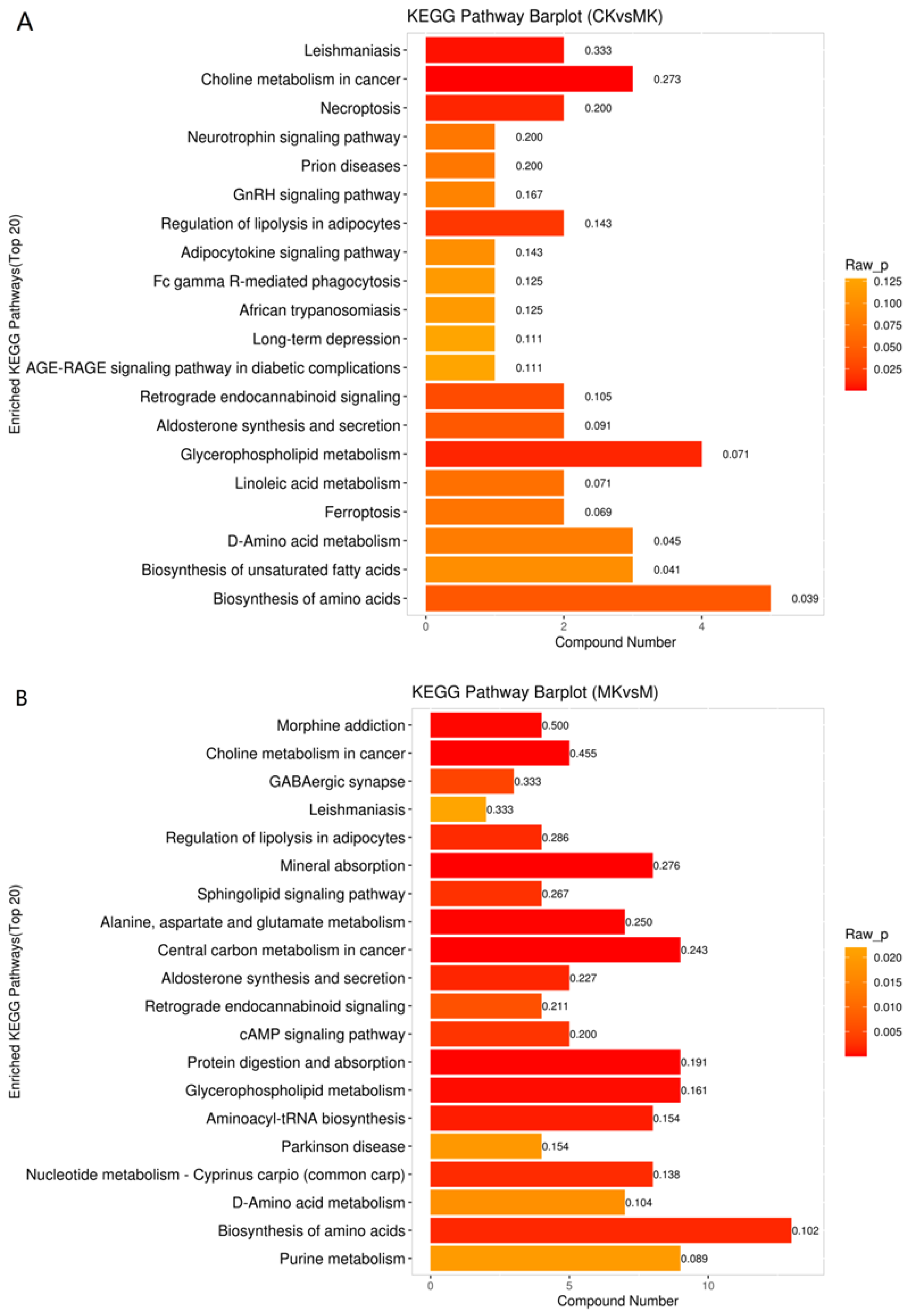
3.4.2. KEGG Enrichment Analysis of Differential Metabolites
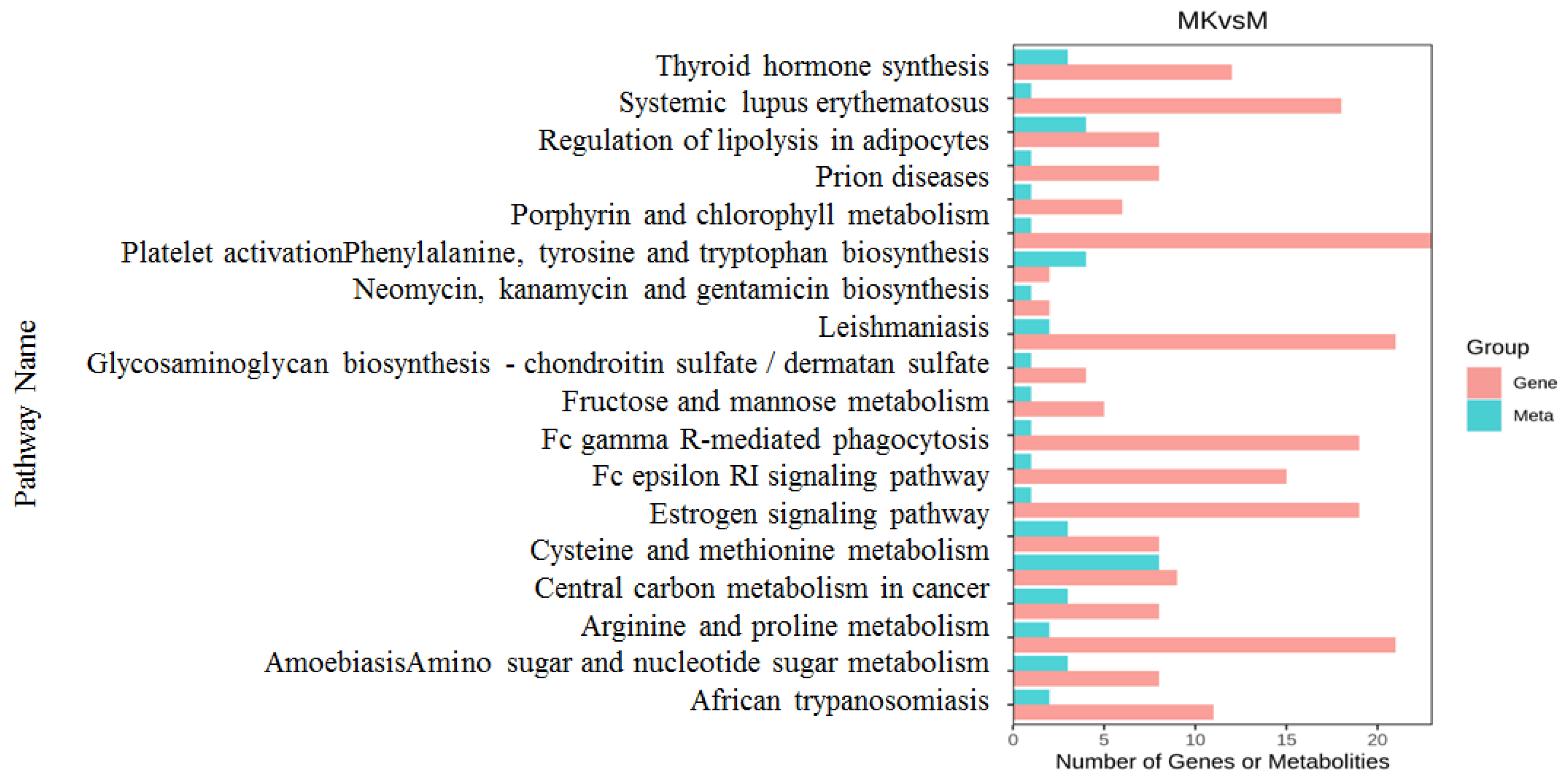
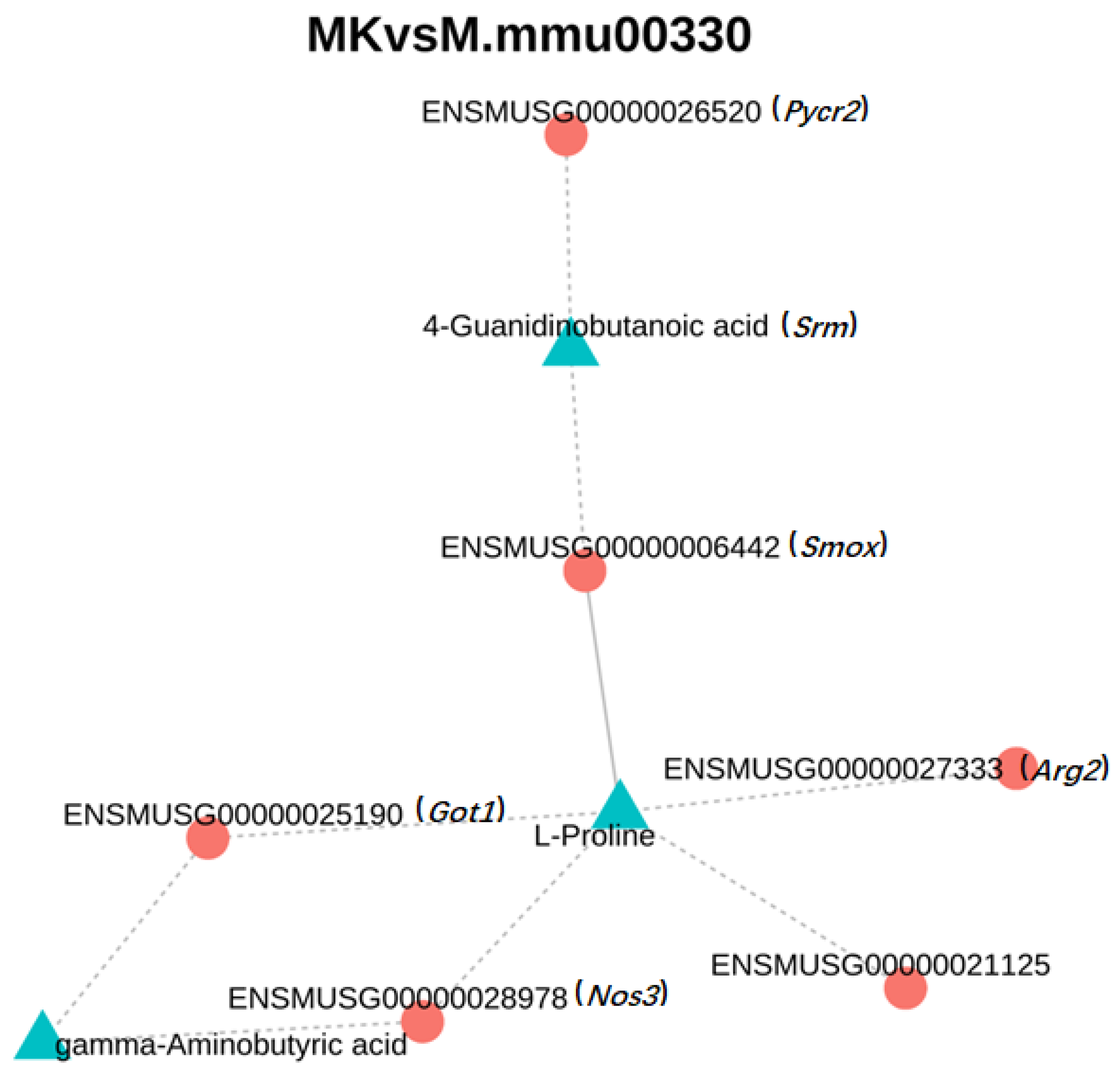
3.5. Combined Transcriptome and Metabolomics Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abeyrathne, E.D.N.S.; Wijesekara, T.; Ahn, D.U. Effect of Bioactive Peptides on Gut Microbiota and Their Relations to Human Health. Foods 2024, 13, 1853. [Google Scholar] [CrossRef]
- Ichim, N.; Marín, F.; Orenes-Piñero, E. Potential Impact of Bioactive Peptides from Foods in the Treatment of Hypertension. Mol. Nutr. Food Res. 2024, 68, e2400084. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Olatunji, O.J.; Karaca, A.C.; Lee, C.C.; Jafari, S.M. Anti-Obesity and Anti-Diabetic Bioactive Peptides: A Comprehensive Review of Their Sources, Properties, and Techno-Functional Challenges. Food Res. Int. 2024, 187, 114427. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Gharehbeglou, P.; Sarabandi, K. Insights into Enzymatic Hydrolysis: Exploring Effects on Antioxidant and Functional Properties of Bioactive Peptides from Chlorella Proteins. J. Agric. Food Res. 2024, 16, 101129. [Google Scholar] [CrossRef]
- Hu, M.; Du, Y.; Li, W.; Zong, X.; Du, W.; Sun, H.; Liu, H.; Zhao, K.; Li, J.; Farooq, M.Z.; et al. Interplay of Food-Derived Bioactive Peptides with Gut Microbiota: Implications for Health and Disease Management. Mol. Nutr. Food Res. 2024, 68, e2400251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Z.; Cai, H.; Wang, L.; Hu, C.; Li, D.; Chen, Y.; Kang, Y.; Li, L. Controlled Moisture Permeability of Thermoplastic Starch/Polylactic Acid/Poly Butylene Adipate-co-Terephthalate Film for the Autolysis of Straw Mushroom Volvariella volvacea. Food Chem. 2022, 373, 131409. [Google Scholar] [CrossRef]
- Lian, L.; Gu, F.; Du, M.; Lin, Y.; Chang, H.; Wang, J. The Combination of High Oxygen and Nanocomposite Packaging Alleviated Quality Deterioration by Promoting Antioxidant Capacity and Phenylpropane Metabolism in Volvariella volvacea. Food Chem. 2024, 439, 138092. [Google Scholar] [CrossRef]
- Shao, K.D.; Mao, P.W.; Li, Q.Z.; Li, L.D.J.; Wang, Y.L.; Zhou, X.W. Characterization of a Novel Fungal Immunomodulatory Protein, FIP-SJ75 Shuffled from Ganoderma lucidum, Flammulina velutipes and Volvariella volvacea. Food Agric. Immunol. 2019, 30, 1253–1270. [Google Scholar] [CrossRef]
- Jiang, X.; Pei, W.; Zhao, L.; Ma, N.; Fang, D.; Zhong, L.; Hu, Q. Separation, Identification and Taste Characteristics of Umami Peptides from Straw Mushroom. Food Sci. 2022, 43, 235–242. [Google Scholar] [CrossRef]
- Sangthong, S.; Pintathong, P.; Pongsua, P.; Jirarat, A.; Chaiwut, P. Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities, and Cosmetic Efficacy. J. Fungi 2022, 8, 572. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Y.; Zha, L.; Chen, M.; Yu, P.; Dong, Q.; Suwantong, O.; Zhao, Y. Novel antioxidant peptides from hydrolyzed proteins of Volvariella volvacea: Purification, characterization and protective effects on H2O2-induced human skin fibroblasts. Food Biosci. 2025, 64, 105825. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, X.; Huang, H.; Zeng, H.; Hu, Y.; Xie, B.; Jiang, Y.; Chen, B. Extraction Technology and Antioxidant Activities of Polypeptides from Fruiting Bodies of Volvariella volvacea. Mycosystema 2023, 42, 584–596. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Luo, X.; Liu, P.; Lin, X.; Deng, Y.; Jiang, Y.; Chen, B. Purification and Structural Identification of Polypeptides from Fruiting Bodies of Volvariella volvacea. Mycosystema 2023, 42, 1775–1784. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, H.; Chen, L.; Luo, X.; Chu, L.; Jiang, Y.; Chen, B. Preventive Effect of Volvariella volvacea Fruit Body Polypeptides on Acute Alcoholic Liver Injury in Mice and Its Influence on Intestinal Microflora. Food Sci. 2024, 45, 135–143. [Google Scholar] [CrossRef]
- Putri, G.H.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing High-Throughput Sequencing Data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Y.C.; Yang, Y.Y.; Lu, T.; Liu, Y.; Lin, S. Advances in Endoplasmic Reticulum Stress in Liver Diseases. Chin. Gen. Pract. 2024, 27, 2679–2684. [Google Scholar] [CrossRef]
- Tien, S.; Zhou, H.; Zhou, Q.; Liu, H.; Wu, B.; Guo, Y. PTTG1 Alleviates Acute Alcoholic Liver Injury by Inhibiting Endoplasmic Reticulum Stress-Induced Hepatocyte Pyroptosis. Liver Int. 2023, 43, 840–854. [Google Scholar] [CrossRef]
- Insausti-Urkia, N.; Solsona-Vilarrasa, E.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Sphingomyelinases and Liver Diseases. Biomolecules 2020, 10, 1497. [Google Scholar] [CrossRef]
- Huang, J.J.; Geng, Q.L.; Li, A.Q.; Qin, Y.D.; Qi, N. Study on Alleviating Alcoholic Fatty Liver with Milk-Derived Pentapeptide PGPIP and Its Mechanism. Acta Univ. Med. Anhui 2023, 58, 1813–1818. [Google Scholar] [CrossRef]
- Lu, J.F.; Liu, S.Y.; Xing, S.P.; Fang, B.; Tan, S.; Zhang, H.; Lu, T.T.; Zhu, D.; Lin, J. Mechanism of Action of 4-Hydroxy-2(3H)-Benzoxazolone through Lipid Metabolism and Endoplasmic Reticulum Stress Pathway in the Treatment of Alcoholic Fatty Liver in Rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 2023, 34, 581–590. [Google Scholar] [CrossRef]
- Meena, A.S.; Shukla, P.K.; Rao, R.; Canelas, C.; Pierre, J.F.; Rao, R. TRPV6 Deficiency Attenuates Stress and Corticosterone-Mediated Exacerbation of Alcohol-Induced Gut Barrier Dysfunction and Systemic Inflammation. Front. Immunol. 2023, 14, 1093584. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Wu, Y.; Li, Y.; Xiang, L.; Cheng, B.; Hu, Y.; Jiang, X.; Wang, Z.; Wu, S.; Si, L.; et al. The Important Role of Glycerophospholipid Metabolism in the Protective Effects of Polyphenol-Enriched Tartary Buckwheat Extract against Alcoholic Liver Disease. Food Funct. 2022, 13, 10415–10425. [Google Scholar] [CrossRef]
- Wu, K.J.; Liu, P.P.; Chen, M.Y.; Zhou, M.X.; Liu, X.; Yang, Q.; Xu, L.; Gong, Z. The Hepatoprotective Effect of Leonurine Hydrochloride against Alcoholic Liver Disease Based on Transcriptomic and Metabolomic Analysis. Front. Nutr. 2022, 9, 904557. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.Y.; Meng, G.L.; Wu, L.Y.; Hao, J.B.; Cheng, B.; Fu, J.; Wu, X. Preventive Effects of the Ganoderma lingzhi Fruit-Body Polysaccharides on the Acute Alcoholic Injury of Mice Liver Based on Metabonomics Analysis. Mycosystema 2021, 40, 2376–2389. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, P.; Yao, X.; Cao, H.; Zhu, H.; Wang, Q.; Liu, Y.; Fang, M.; Wu, Y.; Gong, Z. Hepatoprotective Effects of Peach Gum Polysaccharides against Alcoholic Liver Injury: Moderation of Oxidative Stress and Promotion of Lipid Metabolism. Front. Nutr. 2024, 10, 1325450. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Cai, Z.; Yang, Q.; Liu, J.; Yu, Y.; Pan, F.; Zhang, T. Soybean Meal Peptide Gly-Thr-Tyr-Trp Could Protect Mice from Acute Alcoholic Liver Damage: A Study of Protein-Protein Interaction and Proteomic Analysis. Food Chem. 2024, 451, 139337. [Google Scholar] [CrossRef]
- Cui, K.; Wu, W.W.; Diao, Q.Y. Application and Research Progress on Transcriptomics. Biotechnol. Bull. 2019, 35, 1–9. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Chen, S.Y.; Deng, X.W.; Deng, W.F.; Liu, G.; Chen, Y.J.; Cao, Y. Oenothein B Ameliorates Hepatic Injury in Alcoholic Liver Disease Mice by Improving Oxidative Stress and Inflammation and Modulating the Gut Microbiota. Front. Nutr. 2022, 9, 1053718. [Google Scholar] [CrossRef]
- Pan, Z.; Guo, J.; Tang, K.; Chen, Y.; Gong, X.; Chen, Y.; Zhong, Y.; Xiao, X.; Duan, S.; Cui, T.; et al. Ginsenoside Rc Modulates SIRT6-NRF2 Interaction to Alleviate Alcoholic Liver Disease. J. Agric. Food Chem. 2022, 70, 14220–14234. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, N.; Tan, H.Y.; Chueng, F.; Zhang, Z.J.; Yuen, M.F.; Feng, Y. Modulation of Gut Microbiota Mediates Berberine-Induced Expansion of Immuno-Suppressive Cells to Against Alcoholic Liver Disease. Clin. Transl. Med. 2020, 10, e112. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, X.; Tang, N.; Zhang, X.; Cheng, Y. A Novel Health Care Function of Acetic Acid Bacteria: Its Effect on Immune System Disorders during Alcohol Intake. China Brew. 2022, 41, 19–24. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Tsai, P.J.; Chen, P.H.; Ye, M.; Guo, J.; Su, Z. Mutual Interaction between Endoplasmic Reticulum and Mitochondria in Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2020, 19, 72. [Google Scholar] [CrossRef]
- Lipiński, P.; Cielecka-Kuszyk, J.; Czarnowska, E.; Bogdańska, A.; Socha, P.; Tylki-Szymańska, A. Congenital Disorders of Glycosylation in Children-Histopathological and Ultrastructural Changes in the Liver. Pediatr. Neonatol. 2021, 62, 278–283. [Google Scholar] [CrossRef]
- Fernando, D.H.; Forbes, J.M.; Angus, P.W.; Herath, C.B. Development and Progression of Non-Alcoholic Fatty Liver Disease: The Role of Advanced Glycation End Products. Int. J. Mol. Sci. 2019, 20, 5037. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Sharma, S.; Durgappa, M.; Gupta, N.; Raj, R.; Kumar, A.; Sharma, P.N.; Krishna, V.P.; Kumar, R.V.; Guleria, A.; et al. Serum Metabolic Disturbances Associated with Acute-on-Chronic Liver Failure in Patients with Underlying Alcoholic Liver Diseases: An Elaborative NMR-Based Metabolomics Study. J. Pharm. Bioallied Sci. 2021, 13, 276–282. [Google Scholar] [CrossRef]
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. JAMA 2021, 326, 165–176. [Google Scholar] [CrossRef]
- Pan, J.H.; Lee, K.Y.; Kim, J.H.; Shin, H.; Lee, J.H.; Kim, Y.J. Prunus mume Sieb. et Zucc. Fruit Ameliorates Alcoholic Liver Injury in Mice by Inhibiting Apoptosis and Inflammation through Oxidative Stress. J. Funct. Foods 2016, 25, 135–148. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, H.; Liang, J.; Sun, C.; Wang, D.; Chen, K.; Zhao, J.; Ji, S.; Ma, C.; Ye, X.; et al. Hepatoprotective Potential of Four Fruit Extracts Rich in Different Structural Flavonoids against Alcohol-Induced Liver Injury via Gut Microbiota-Liver Axis. Food Chem. 2024, 460, 140460. [Google Scholar] [CrossRef]
- Wang, S.; Sui, S.; Liu, Z.; Peng, C.; Liu, J.; Luo, D.; Fan, X.; Liu, C.; Lu, W.-Y. Protective Roles of Hepatic Gamma-Aminobutyric Acid Signaling in Acute Ethanol Exposure-Induced Liver Injury. J. Appl. Toxicol. 2018, 38, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Park, N.H.; Lee, S.J.; Mechesso, A.F.; Boby, N.; Yixian, Q.; Yoon, W.K.; Lee, S.-P.; Lee, J.-S.; Park, S.-C. Hepatoprotective Effects of Gamma-Aminobutyric Acid-Enriched Fermented Hovenia dulcis Extract on Ethanol-Induced Liver Injury in Mice. BMC Complement. Med. Ther. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Dose (mg/kg) | Intoxication Latency (min) | Sobriety Recovery (min) |
|---|---|---|---|
| Control | - | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Model | - | 9.333 ± 0.471 ** | 216.667 ± 3.859 ** |
| Positive | 200 | 32.667 ± 1.700 **## | 96.667 ± 4.110 **## |
| L-VVFP | 100 | 23.667 ± 1.247 **## | 139.667 ± 3.300 **## |
| M-VVFP | 200 | 69.333 ± 2.625 **## | 75.667 ± 4.190 **## |
| H-VVFP | 400 | 51.333 ± 0.943 **## | 88.333 ± 0.471 **## |
| Treatment | Dose (mg/kg) | Liver Weight (g) | Mouse Weight (g) | Liver Index (%) |
|---|---|---|---|---|
| Control | - | 1.573 ± 0.021 | 35.077 ± 0.319 | 4.485 ± 0.034 |
| Model | - | 1.840 ± 0.041 ** | 32.565 ± 0.272 ** | 5.663 ± 0.088 ** |
| Positive | 200 | 1.537 ± 0.009 **## | 34.023 ± 0.411 *# | 4.535 ± 0.054 ## |
| L-VVFP | 100 | 1.613 ± 0.005 **## | 33.448 ± 0.361 **# | 4.825 ± 0.057 **## |
| M-VVFP | 200 | 1.593 ± 0.012 **## | 33.794 ± 0.482 *# | 4.707 ± 0.092 *## |
| H-VVFP | 400 | 1.583 ± 0.021 *## | 33.690 ± 0.436 *# | 4.700 ± 0.075 *## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Chen, J.; Wu, H.; Zhang, F.; Chen, L.; Zhang, W.; Yang, J.; Yuan, L.; Jiang, Y.; Deng, Y. Volvariella volvacea Polypeptide Mitigates Alcohol-Induced Liver Injury: A Multi-Omics Study. Foods 2025, 14, 1557. https://doi.org/10.3390/foods14091557
Chen B, Chen J, Wu H, Zhang F, Chen L, Zhang W, Yang J, Yuan L, Jiang Y, Deng Y. Volvariella volvacea Polypeptide Mitigates Alcohol-Induced Liver Injury: A Multi-Omics Study. Foods. 2025; 14(9):1557. https://doi.org/10.3390/foods14091557
Chicago/Turabian StyleChen, Bingzhi, Juanqin Chen, Huihua Wu, Fangyi Zhang, Lili Chen, Weibin Zhang, Jing Yang, Li Yuan, Yuji Jiang, and Youjin Deng. 2025. "Volvariella volvacea Polypeptide Mitigates Alcohol-Induced Liver Injury: A Multi-Omics Study" Foods 14, no. 9: 1557. https://doi.org/10.3390/foods14091557
APA StyleChen, B., Chen, J., Wu, H., Zhang, F., Chen, L., Zhang, W., Yang, J., Yuan, L., Jiang, Y., & Deng, Y. (2025). Volvariella volvacea Polypeptide Mitigates Alcohol-Induced Liver Injury: A Multi-Omics Study. Foods, 14(9), 1557. https://doi.org/10.3390/foods14091557





