Evaluating the Potential for Different Fabrics to Protect Grapes from Contamination by Smoke
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fabric Coverings
2.2. Smoke Exposure Trials
2.2.1. Trial 1: Evaluation of Different Fabrics During Single Smoke Exposure
2.2.2. Trial 2: Evaluation of Different Fabrics During Repeated Smoke Exposure
2.2.3. Trial 3: Evaluation of Fabric Re-Usability
2.2.4. Trial 4: Evaluation of Reinforced Activated Carbon Fibre Cloth
2.3. Compositional Analysis of Grapes
2.4. Physical Testing of Fabrics
2.5. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Different Fabrics During Single Smoke Exposure
3.2. Evaluation of Different Fabrics During Repeated Smoke Exposure
3.3. Evaluation of Fabric Re-Usability
3.3.1. Desorption of Volatile Phenols from Smoke-Exposed Fabrics
3.3.2. Performance of Different Smoke-Exposed Fabrics After Washing
3.4. Evaluation of Reinforced Activated Carbon Fibre Cloth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Activated carbon fibre |
| ANOVA | Analysis of variance |
| GC-MS | Gas chromatography-mass spectrometry |
| HPLC-MS/MS | High-performance liquid chromatography-tandem mass spectrometry |
| SIDA | Stable isotope dilution assay |
| VOCs | Volatile organic compounds |
| VPs | Volatile phenols |
References
- Stavins, R.N.; Catena, L.; Forrestel, E.; Rivail, J.-B.; Sahn, M.; Sumner, D.A. Global climate change and wine production: Industry and academic perspectives. Harv. Data Sci. Rev. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust. J. Grape Wine Res. 2009, 15, 228–237. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.A.; Pardon, K.H.; Jeffery, D.W.; Herderich, M.J. Investigation into the formation of guaiacol conjugates in berries and leaves of grapevine Vitis vinifera L. Cv. Cabernet Sauvignon using stable isotope tracers combined with HPLC-MS and MS/MS analysis. J. Agric. Food Chem. 2010, 58, 2076–2081. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.A.; Parker, M.; Pardon, K.H.; Black, C.A.; Herderich, M.J.; Jeffery, D.W. Glycosylation of smoke derived volatile phenols in grapes as a consequence of grapevine exposure to bushfire smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar] [CrossRef]
- Dungey, K.A.; Hayasaka, Y.; Wilkinson, K.L. Quantitative analysis of glycoconjugate precursors of guaiacol in smoke-affected grapes using liquid chromatography-tandem mass spectrometry based stable isotope dilution analysis. Food Chem. 2011, 126, 801–806. [Google Scholar] [CrossRef]
- Noestheden, M.; Dennis, E.G.; Romero-Montalvo, E.; DiLabio, G.A.; Zandberg, W.F. Detailed characterization of glycosylated sensory-active volatile phenols in smoke-exposed grapes and wine. Food Chem. 2018, 259, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.; Ristic, R.; Capone, D.; Puglisi, C.; Pagay, V.; Culbert, J.; Jiang, W.; Herderich, M.; Tuke, J.; Wilkinson, K. Uptake and glycosylation of smoke-derived volatile phenols by Cabernet Sauvignon grapes and their subsequent fate during winemaking. Molecules 2020, 25, 3720. [Google Scholar] [CrossRef]
- Kennison, K.R.; Gibberd, M.R.; Pollnitz, A.P.; Wilkinson, K.L. Smoke-derived taint in wine: The release of smoke-derived volatile phenols during fermentation of Merlot juice following grapevine exposure to smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.A.; Hayasaka, Y.; Fudge, A.L.; Wilkinson, K.L. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- Kelly, D.; Zerihun, A.; Hayasaka, Y.; Gibberd, M. Winemaking practice affects the extraction of smoke-borne phenols from grapes into wines. Aust. J. Grape Wine Res. 2014, 20, 386–393. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wine. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Krstic, M.P.; Johnson, D.L.; Herderich, M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Favell, J.W.; Wilkinson, K.L.; Zigg, I.; Lyons, S.M.; Ristic, R.; Puglisi, C.J.; Wilkes, E.; Taylor, R.; Kelly, D.; Howell, G.; et al. Correlating sensory assessment of smoke-tainted wines with inter-laboratory study consensus values for volatile phenols. Molecules 2022, 27, 4892. [Google Scholar] [CrossRef] [PubMed]
- Bilogrevic, E.; Jiang, W.W.; Culbert, J.; Francis, L.; Herderich, M.; Parker, M. Consumer response to wine made from smoke-affected grapes. OENO One 2023, 57, 417–430. [Google Scholar] [CrossRef]
- Australian Wine Research Institute. Annual Report; Høj, P., Pretorius, I., Blair, R.J., Eds.; The Australian Wine Research Institute: Urrbrae, SA, Australia, 2003. [Google Scholar]
- Simos, C. The implications of smoke taint and management practices. Aust. Vitic. Jan./Feb. 2008, 77–80. Available online: https://www.awri.com.au/wp-content/uploads/simos_smoke_taint_2008.pdf (accessed on 23 March 2025).
- Whiting, J.; Krstic, M.P. Understanding the Sensitivity to Timing and Management Options to Mitigate the Negative Impacts of Bush Fire Smoke on Grape and Wine Quality—Scoping Study; Department of Primary Industries: Knoxfield, VIC, Australia, 2007; Available online: https://www.wineaustralia.com/getmedia/528c4aa3-e868-4b32-b1cb-b2c5128dfd1f/Smoke_Taint_Final_Report_2007 (accessed on 23 March 2025).
- Oberholster, A.; Wen, Y.; Suarez, S.D.; Erdmann, J.; Giradello, R.C.; Rumbaugh, A.; Neupane, B.; Brennemand, C.; Cantu, A.; Heymann, H. Investigation of different winemaking protocols to mitigate smoke taint character in wine. Molecules 2022, 27, 1732. [Google Scholar] [CrossRef]
- Mirabelli-Montan, Y.A.; Marangon, M.; Graça, A.; Mayr Marangon, C.M.; Wilkinson, K.L. Techniques for mitigating the effects of smoke taint while maintaining quality in wine production: A review. Molecules 2021, 26, 1672. [Google Scholar] [CrossRef]
- Fudge, A.L.; Ristic, R.; Wollan, D.; Wilkinson, K.L. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Fudge, A.L.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K.L. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- Culbert, J.A.; Jiang, W.; Bilogrevic, E.; Likos, D.; Francis, I.L.; Krstic, M.P.; Herderich, M.J. Compositional changes in smoke-affected grape juice as a consequence of activated carbon treatment and the impact on phenolic compounds and smoke flavor in wine. J. Agric. Food Chem. 2021, 69, 10246–10259. [Google Scholar] [CrossRef]
- Puglisi, C.; Ristic, R.; Saint, J.; Wilkinson, K. Evaluation of spinning cone column distillation as a strategy for remediation of smoke taint in juice and wine. Molecules 2022, 27, 8096. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Ristic, R.; Puglisi, C.; Wang, X.; Muhlack, R.; Baars, S.; Herderich, M.J.; Wilkinson, K.L. Amelioration of smoke taint in wine via addition of molecularly imprinted polymers during or after fermentation. J. Agric. Food Chem. 2024, 72, 18121–18131. [Google Scholar] [CrossRef]
- Huo, Y.; Ristic, R.; Wollan, D.; Angela, S.; Muhlack, R.; Herderich, M.; Wilkinson, K. Optimizing the use of membrane filtration for the amelioration of smoke tainted wine. Food Chem. 2025, 479, 143704. [Google Scholar] [CrossRef] [PubMed]
- van der Hulst, L.; Munguia, P.; Culbert, J.A.; Ford, C.M.; Burton, R.A.; Wilkinson, K.L. Accumulation of volatile phenol glycoconjugates in grapes following grapevine exposure to smoke and potential mitigation of smoke taint by foliar application of kaolin. Planta 2019, 249, 941–952. [Google Scholar] [CrossRef]
- Rogiers, S.; Fahey, D.; Holzapfel, B. Mitigating sunburn, dehydration and smoke taint in the vineyard: Is there a role for sunscreens, antitranspirants and film forming barriers? Acta Hortic. 2020, 1274, 71–78. [Google Scholar] [CrossRef]
- Favell, J.W.; Noestheden, M.; Lyons, S.M.; Zandberg, W.F. Development and evaluation of a vineyard-based strategy to mitigate smoke-taint in wine grapes. J. Agric. Food Chem. 2019, 67, 14137–14142. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Jung, J.; Garcia, L.; DeShields, J.B.; Cerrato, D.C.; Penner, M.H.; Tomasino, E.; Levin, A.D.; Zhao, Y. Impact of functional spray coatings on smoke volatile phenol compounds and Pinot noir grape growth. J. Food Sci. 2023, 88, 367–380. [Google Scholar] [CrossRef]
- Wilkinson, K.L.; Ristic, R.; Szeto, C.; Capone, D.L.; Yu, L.; Losic, D. Novel use of activated carbon fabric to mitigate smoke taint in grapes and wine. Aust. J. Grape Wine Res. 2022, 28, 500–507. [Google Scholar] [CrossRef]
- Szeto, C.; Ristic, R.; Wilkinson, K. Thinking inside the box: A novel approach to smoke taint mitigation trials. Molecules 2022, 27, 1667. [Google Scholar] [CrossRef]
- Chen, J.Y. Activated Carbon Fiber and Textiles; Woodhead Publishing: Sawston, UK, 2017; pp. 3–20. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Singh, V.V.; Sathe, M.; Thakare, V.B.; Singh, B. Activated carbon fabric: An adsorbent material for chemical protective clothing. Def. Sci. J. 2018, 16, 83–90. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Murphy, L.R.; Penn, C.J.; Boddu, V.M.; Sanders, L.L. Atrazine removal from water by activated charcoal cloths. Int. Soil Water Conserv. Res. 2020, 8, 205–212. [Google Scholar] [CrossRef]
- Kim, J.G.; Bai, B.C. A chemical safety assessment of lyocell-based activated carbon fiber with a high surface area through the evaluation of HCl gas adsorption and electrochemical properties. Separations 2024, 11, 79. [Google Scholar] [CrossRef]
- Huidobro, A.; Pastor, A.C.; Rodríguez-Reinoso, F. Preparation of activated carbon cloth from viscous rayon: Part IV. Chemical activation. Carbon 2001, 39, 389–398. [Google Scholar] [CrossRef]
- Oliveira, M.; Teles, J.; Barbosa, P.; Olazabal, F.; Queiroz, J. Shading of the fruit zone to reduce grape yield and quality losses caused by sunburn. OENO One 2014, 48, 179–187. [Google Scholar] [CrossRef]
- Micciché, D.; de Rosas, M.I.; Ferro, M.V.; Di Lorenzo, R.; Puccio, S.; Pisciotta, A. Effects of artificial canopy shading on vegetative growth and ripening processes of cv. Nero d’Avola (Vitis vinifera L.). Front. Plant Sci. 2023, 14, 1210574. [Google Scholar] [CrossRef] [PubMed]
- Pallotti, L.; Silvestroni, O.; Dottori, E.; Lattanzi, T.; Lanari, V. Effects of shading nets as a form of adaptation to climate change on grapes production: A review. OENO One 2023, 57, 7414. [Google Scholar] [CrossRef]
- Pollnitz, A.P.; Pardon, K.H.; Sykes, M.; Sefton, M.A. The effects of sample preparation and gas chromatograph injection techniques on the accuracy of measuring guaiacol, 4-methylguaiacol and other volatile oak compounds in oak extracts by stable isotope dilution analyses. J. Agric. Food Chem. 2004, 52, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, Y.; Parker, M.; Baldock, G.A.; Pardon, K.H.; Black, C.A.; Jeffery, D.W.; Herderich, M.J. Assessing the impact of smoke exposure in grapes: Development and validation of an HPLC-MS/MS method for the quantitative analysis of smoke-derived phenolic glycosides in grapes and wine. J. Agric. Food Chem. 2013, 61, 25–33. [Google Scholar] [CrossRef]
- Standards Australia. Available online: https://store.standards.org.au/product/as-2001-2-34-1990 (accessed on 4 March 2025).
- Standards Australia. Available online: https://store.standards.org.au/product/as-2001-2-3-1-2001 (accessed on 4 March 2025).
- Noble, R.E. Environmental tobacco smoke uptake by clothing fabrics. Sci. Total Environ. 2000, 262, 1–3. [Google Scholar] [CrossRef]
- Edebali, O.; Goellner, A.; Stiborek, M.; Šimek, Z.; Muz, M.; Vrana, B.; Melymuk, L. Characterizing the distribution of aromatic amines between polyester, cotton and wool textiles and air. Environ. Sci. Process. Impacts 2025, 27, 1054–1062. [Google Scholar] [CrossRef]
- Saini, A.; Okeme, J.O.; Mark Parnis, J.; McQueen, R.H.; Diamond, M.L. From air to clothing: Characterizing the accumulation of semi-volatile organic compounds to fabrics in indoor environments. Indoor Air. 2017, 27, 631–641. [Google Scholar] [CrossRef]
- Borujeni, E.T.; Taghmaian, K.; Naddafi, K.; Hassanvand, M.S.; Naderi, M. Identification and determination of the volatile organics of third-hand smoke from different cigarettes and clothing fabrics. J. Environ. Health Sci. Eng. 2022, 20, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; Ristic, R.; McNamara, I.; Loveys, B.; Jiang, W.W.; Krstic, M. Evaluating the potential for smoke from stubble burning to taint grapes and wine. Molecules 2021, 26, 7540. [Google Scholar] [CrossRef] [PubMed]
- Pozuelos, G.; Jacob, P.; Schick, S.F.; Omaiye, E.E.; Talbot, P. Adhesion and removal of thirdhand smoke from indoor fabrics: A method for rapid assessment and identification of chemical repositories. Int. J. Environ. Res. Public Health 2021, 18, 3592. [Google Scholar] [CrossRef]
- Nongnuch, W.; Suwanruji, P.; Setthayanond, J. Colour properties of cigarette smoke-exposed cotton and silk fabrics and their nicotine release. Ind. Textila 2018, 69, 328–333. [Google Scholar] [CrossRef]
- Kayar, M.; Boztoprak, Y.; Nallbani, B.G. Cigarette smoke uptake by different woven fabrics: Analysis of mechanical and colour properties. Color. Technol. 2024, 140, 585–597. [Google Scholar] [CrossRef]
- Wang, C.; Liang, Y.; Yao, W.; Bergendahl, J.; Liu, S. Indoor Fabric as an Adsorptive Reservoir for Volatile Organic Compounds in Wildfire Smoke: A Preliminary Study. In Proceedings of the 5th International Conference on Building Energy and Environment, Montreal, QC, Canada, 25–29 July 2022. [Google Scholar] [CrossRef]
- Culbert, J.A.; Krstic, M.P.; Herderich, M.J. Development and utilization of a model system to evaluate the potential of surface coatings for protecting grapes from volatile phenols implicated in smoke taint. Molecules 2021, 26, 5197. [Google Scholar] [CrossRef]
- Culbert, J.A.; Jiang, W.; Ristic, R.; Puglisi, C.J.; Nixon, E.; Shi, H.; Wilkinson, K.L. Glycosylation of volatile phenols in grapes following pre-harvest (on-vine) vs. post-harvest (off-vine) exposure to smoke. Molecules 2021, 26, 5277. [Google Scholar] [CrossRef]
- Petrick, L.; Destaillats, H.; Zouev, I.; Sabach, S.; Dubowski, Y. Sorption, desorption, and surface oxidative fate of nicotine. Phys. Chem. Chem. Phys. 2010, 12, 10356–10364. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J. The physical processes and chemical transformations of third-hand smoke in indoor environments and its health effects: A review. Atmosphere 2025, 16, 370. [Google Scholar] [CrossRef]
- Jasińska, I. Industrial washing conditions as factor that influence the cellulose structure and mechanical strength of bed linens. Sci. Rep. 2023, 13, 12214. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; He, J.Z.; Lu, Y.H.; Jiang, S.M.; Wang, M. Interaction effects of washing and abrasion on thermal protective performance of flame-retardant fabrics. Int. J. Occup. Saf. Ergon. 2021, 27, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Won, A.Y.; Yun, C. The effects of laundering on the protective performance of firefighter clothing. Fibers Polym. 2021, 22, 3232–3239. [Google Scholar] [CrossRef]
- González, C.V.; Prieto, J.A.; Mazza, C.; Jeréz, D.N.; Biruk, L.N.; Jofré, M.F.; Giordano, C.V. Grapevine morphological shade acclimation is mediated by light quality whereas hydraulic shade acclimation is mediated by light intensity. Plant Sci. 2021, 307, 110893. [Google Scholar] [CrossRef]
- González, C.V.; Jeréz, D.N.; Jofré, M.F.; Guevara, A.; Prieto, J.; Mazza, C.; Williams, L.E.; Giordano, C.V. Blue light attenuation mediates morphological and architectural acclimation of Vitis vinifera cv. Malbec to shade and increases light capture. Environ. Exp. Bot. 2019, 157, 112–120. [Google Scholar] [CrossRef]
- Ghiglieno, I.; Mattivi, F.; Cola, G.; Trionfini, D.; Perenzoni, D.; Simonetto, A.; Gilioli, G.; Valenti, L. The effects of leaf removal and artificial shading on the composition of Chardonnay and Pinot noir grapes. OENO One 2020, 54, 761–777. [Google Scholar] [CrossRef]
- Reta, K.; Netzer, Y.; Lazarovitch, N.; Fait, A. Canopy management practices in warm environment vineyards to improve grape yield and quality in a changing climate. A review A vademecum to vine canopy management under the challenge of global warming. Sci. Hortic. 2025, 341, 113998. [Google Scholar] [CrossRef]
- Austin, C.N.; Wilcox, W.F. Effects of sunlight exposure on grapevine powdery mildew development. Phytopathology 2012, 102, 857–866. [Google Scholar] [CrossRef]
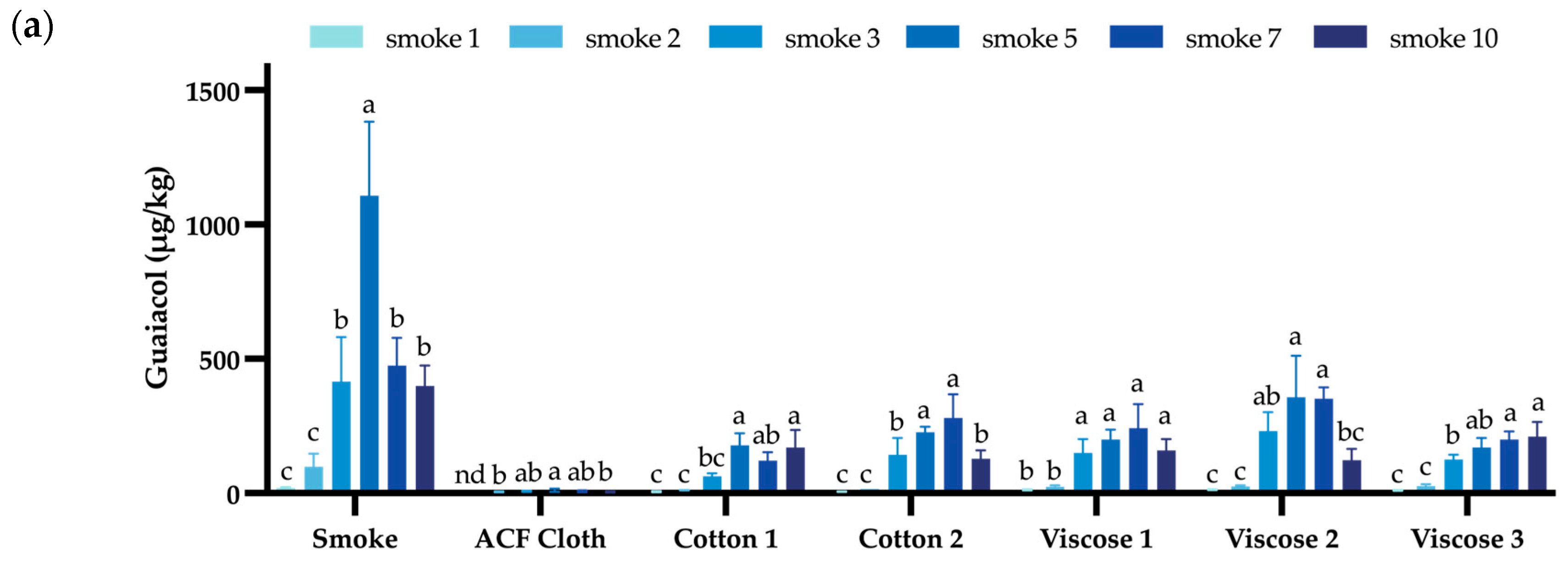
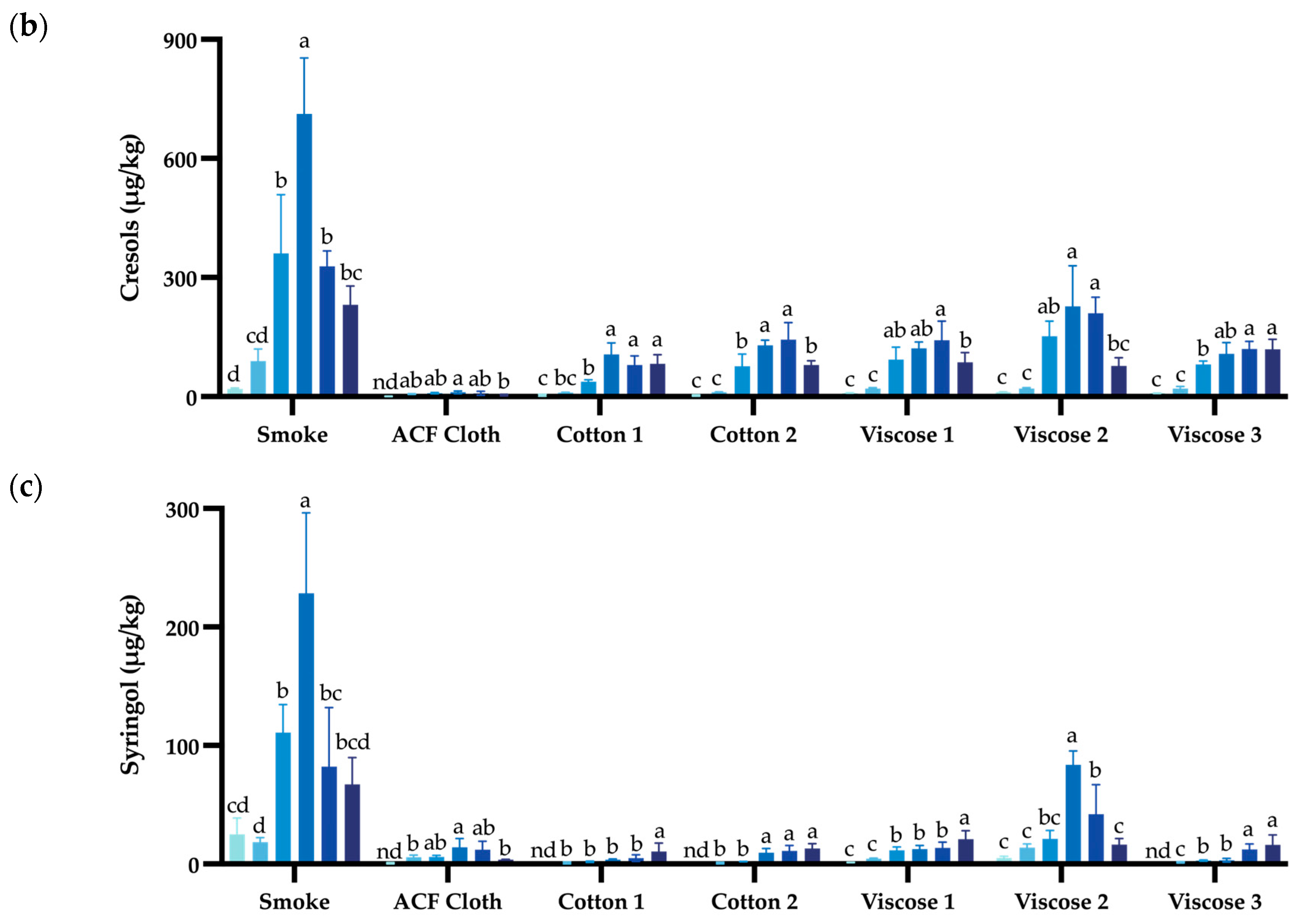
| Guaiacol | 4-Methyl Guaiacol | o-Cresol | m-Cresol | p-Cresol | Syringol | 4-Methyl Syringol | |
|---|---|---|---|---|---|---|---|
| control | nd | nd | nd | nd | nd | nd | nd |
| smoke | 16.0 ± 0.1 a | 5.0 ± 0.1 a | 7.5 ± 0.5 a | 7.0 ± 0.1 a | 2.0 ± 0.1 | 36.0 ± 0.1 a | 7.0 ± 0.1 |
| ACF cloth | 4.3 ± 1.2 c | nd | 1.7 ± 0.3 c | 1.3 ± 0.7 c | nd | nd | nd |
| polyester | 14.3 ± 2.6 ab | 3.7 ± 0.7 b | 6.0 ± 1.2 ab | 4.0 ± 0.6 b | 1.0 ± 0.6 | 5.0 ± 0.6 b | nd |
| polypropylene | 10.7 ± 1.2 b | 2.3 ± 0.3 bc | 5.3 ± 0.3 b | 3.7 ± 0.7 b | 1.0 ± 0.6 | 6.0 ± 1.0 b | nd |
| cotton | 5.3 ± 0.7 c | 1.3 ± 0.3 c | 2.7 ± 0.3 c | 1.3 ± 0.3 c | nd | nd | nd |
| viscose | 5.0 ± 0.1 c | 1.3 ± 0.3 c | 2.0 ± 0.1 c | 1.0 ± 0.1 c | nd | nd | nd |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.165 | <0.001 | na |
| Thickness (mm) | Air Permeability 1 (cm3/cm2.s) | Max. Force Length (N/50 mm) | Max. Force 2 Width (N/50 mm) | Elongation at Max. Force 2 Length (%) | Elongation at Max. Force 2 Width (%) | |
|---|---|---|---|---|---|---|
| ACF cloth * | 0.332 | 44.9 and 42.4 | 15 | 10 | 1.4 | 8.5 |
| polyester * | 0.064 | 30.6 and 31.0 | 450 | 470 | 28.5 | 30.5 |
| polypropylene * | 0.225 | >680 | 58 | na | 119 | na |
| cotton 1 * | 0.415 | 22.4 and 22.7 | 690 | 300 | 19.0 | 15.5 |
| cotton 2 | 0.182 | 18.1 and 17.7 | 720 | 300 | 11.0 | 13.5 |
| viscose 1 * | 0.215 | 37.2 and 37.4 | 340 | 390 | 34.0 | 30.5 |
| viscose 2 | 0.182 | >680 | 100 | na | 44.0 | na |
| viscose 3 | 0.250 | 23.5 and 23.2 | 520 | 320 | 16.0 | 29.5 |
| ACF (single backing) | 0.345 | 46.2 and 45.6 | 30 | 14 | 6.4 | 16.0 |
| ACF (double backing) | 0.457 | 33.0 and 33.6 | 57 | 22 | 8.0 | 17.0 |
| Guaiacol | 4-Methyl Guaiacol | o-Cresol | m-Cresol | p-Cresol | Syringol | 4-Methyl Syringol | |
|---|---|---|---|---|---|---|---|
| ACF cloth * | 35.7 ± 10.9 b | 6.6 ± 2.0 | 11.7 ± 2.5 c | 11.4 ± 3.0 b | 12.2 ± 3.2 b | 52.4 ± 20.9 a | 3.0 ± 0.7 |
| cotton 1 * | 269 ± 178 a | 21.2 ± 13.6 | 146 ± 97.9 a | 74.9 ± 37.2 a | 54.0 ± 36.4 a | 25.3 ± 10.1 b | 3.5 ± 0.9 |
| cotton 2 | 130 ± 75.1 ab | 11.2 ± 4.9 | 89.6 ±45.1 abc | 43.6 ± 18.1 ab | 42.4 ± 15.8 ab | 29.6 ± 19.9 ab | 4.2 ± 1.8 |
| viscose 1 * | 277 ± 83.7 a | 15.1 ± 5.2 | 132 ± 43.6 ab | 67.4 ± 18.0 a | 36.7 ± 10.2 ab | 15.3 ± 6.6 b | 3.5 ±1.0 |
| viscose 2 | 52.9 ± 6.2 b | 4.3 ± 0.4 | 26.7 ± 4.8 bc | 20.8 ± 3.1 b | 12.0 ± 0.8 b | 17.8 ± 7.4 b | 4.4 ± 1.3 |
| viscose 3 | 227 ± 195 ab | 13.7 ± 9.8 | 118 ± 102 abc | 53.0 ± 38.6 ab | 36.0 ± 22.3 ab | 13.9 ± 4.0 b | 3.3 ± 0.9 |
| p | 0.087 | 0.158 | 0.093 | 0.042 | 0.090 | 0.042 | 0.619 |
| Guaiacol Glycosides | 4-Methyl Guaiacol Glycosides | Phenol Glycosides | Cresol Glycosides | Syringol Glycosides | 4-Methyl Syringol Glycosides | |
|---|---|---|---|---|---|---|
| control | 24 ± 0.1 c | 2.2 ± 0 c | 9.0 ± 0.2 c | 12.5 ± 0.3 b | 6.7 ± 0.1 c | 2.3 ± 0 c |
| ACF cloth * | 227 ± 52 bc | 37 ± 10.0 c | 71 ± 8 c | 107 ± 24.8 b | 44 ± 12.3 c | 5.3 ± 1.4 c |
| cotton 1 * | 1926 ± 542 a | 482 ± 246 a | 320 ± 49.2 a | 978 ± 264 a | 191 ± 31.3 b | 20 ± 5.6 b |
| cotton 2 | 1375 ± 296 a | 381 ± 129 a | 257 ± 68.1 ab | 923 ± 272 a | 352 ± 81.4 a | 37 ± 7 a |
| viscose 1 * | 1811 ± 205 a | 337 ± 83 ab | 315 ± 29.9 a | 844 ± 87.7 a | 255 ± 13.4 b | 40 ± 0.9 a |
| viscose 2 | 715 ± 41 b | 114 ± 7.5 bc | 186 ± 48 b | 334 ± 19.2 b | 239 ± 30.5 b | 33 ± 1.4 a |
| viscose 3 | 1811 ± 601 a | 363 ± 176 a | 224 ± 58.2 b | 834 ± 272 a | 216 ± 88.5 b | 32 ± 16.3 ab |
| p | <0.0001 | 0.003 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Guaiacol | 4-Methyl Guaiacol | o-Cresol | m-Cresol | p-Cresol | Syringol | 4-Methyl Syringol | |
|---|---|---|---|---|---|---|---|
| control | nd | nd | nd | nd | nd | nd | nd |
| control * | nd | nd | nd | nd | nd | nd | nd |
| smoke | 18.7 ± 2.4 | 2.6 ± 0.2 | 7.0 ± 1.3 | 6.4 ± 0.8 | 5.8 ± 0.4 | 25.1 ± 13.7 | 9.7 ± 3.0 |
| smoke * | 18.4 ± 2.8 | 3.4 ± 0.2 | 7.5 ± 0.6 | 5.5 ± 0.3 | 6.2 ± 0.4 | 56.9 ± 18.5 | 16.2 ± 3.7 |
| p | 0.811 | 0.003 | 0.579 | 0.263 | 0.297 | 0.090 | 0.096 |
| cotton 1 | 3.9 ± 1.6 | nd | nd | 1.5 ± 0.3 | 1.0 ± 1.7 | nd | nd |
| cotton 1 * | 7.3 ±3.1 | 1.7 ± 0.3 | 2.4 ± 0.6 | 2.0 ± 0.3 | 3.1 ±0.3 | nd | nd |
| p | 0.131 | na | na | <0.001 | 0.130 | na | na |
| cotton 2 | 3.8 ± 0.5 | nd | nd | 1.8 ± 0.5 | 1.0 ± 1.7 | nd | nd |
| cotton 2 * | 7.2 ± 1.6 | 1.7 ± 0.2 | 2.9 ± 0.5 | 2.5 ± 0.5 | 2.4 ±2.1 | nd | nd |
| p | 0.051 | na | na | 0.282 | 0.332 | na | na |
| viscose 1 | 10.4 ± 1.8 | 1.6 ± 0.1 | 3.2 ± 0.3 | 2.5 ± 0.3 | 3.4 ± 0.1 | 1.3 ± 0.2 | nd |
| viscose 1 * | 12.7 ± 2.8 | 2.6 ± 0.3 | 4.8 ± 0.6 | 2.7 ± 0.4 | 4.4 ± 0.1 | 2.5 ± 0.8 | nd |
| p | 0.321 | 0.025 | 0.070 | 0.330 | 0.033 | 0.099 | na |
| viscose 2 | 11.5 ± 1.0 | 1.8 ± 0.1 | 3.8 ± 0.3 | 3.2 ± 0.5 | 4.1 ± 0.3 | 4.9 ± 1.5 | 2.6 ± 0.3 |
| viscose 2 * | 16.2 ± 2.0 | 3.1 ± 0.3 | 6.2 ± 0.4 | 3.7 ± 0.8 | 4.9 ± 0.1 | 10.7 ± 4.9 | 4.5 ± 2.1 |
| p | 0.035 | 0.020 | 0.003 | 0.116 | 0.045 | 0.253 | 0.293 |
| viscose 3 | 8.8 ± 0.8 | 1.5 ± 0.2 | 2.9 ± 0.1 | 2.7 ± 0.5 | 3.1 ± 0.2 | nd | nd |
| viscose 3 * | 9.5 ± 1.7 | 2.3 ± 0.1 | 4.0 ± 0.9 | 2.5 ± 0.3 | 3.9 ± 0.3 | 2.1 ± 0.3 | nd |
| p | 0.601 | 0.030 | 0.177 | 0.351 | 0.103 | na | na |
| Guaiacol | 4-Methyl Guaiacol | o-Cresol | m-Cresol | p-Cresol | Syringol | 4-Methyl Syringol | |
|---|---|---|---|---|---|---|---|
| control | nd | nd | nd | nd | nd | nd | nd |
| smoke | 18.4 ± 2.8 a | 3.4 ± 0.2 a | 7.5 ± 0.6 a | 5.5 ± 0.3 a | 6.2 ± 0.4 a | 56.9 ± 18.5 a | 16.2 ± 3.7 a |
| ACF cloth | 4.3 ± 1.0 b | 1.3 ± 0.1 b | 0.9 ± 0.1 b | 1.5 ± 0.4 b | 1.1 ± 1.9 b | 7.8 ± 2.7 b | 1.9 ± 0.4 b |
| ACF (single backing) | nd | nd | nd | nd | nd | 1.6 ± 0.2 b | nd |
| ACF (double backing) | nd | nd | nd | nd | nd | 2.1 ± 0.7 b | nd |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.001 | 0.001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Ristic, R.; Wilkinson, K. Evaluating the Potential for Different Fabrics to Protect Grapes from Contamination by Smoke. Foods 2025, 14, 1550. https://doi.org/10.3390/foods14091550
Shi T, Ristic R, Wilkinson K. Evaluating the Potential for Different Fabrics to Protect Grapes from Contamination by Smoke. Foods. 2025; 14(9):1550. https://doi.org/10.3390/foods14091550
Chicago/Turabian StyleShi, Tingting, Renata Ristic, and Kerry Wilkinson. 2025. "Evaluating the Potential for Different Fabrics to Protect Grapes from Contamination by Smoke" Foods 14, no. 9: 1550. https://doi.org/10.3390/foods14091550
APA StyleShi, T., Ristic, R., & Wilkinson, K. (2025). Evaluating the Potential for Different Fabrics to Protect Grapes from Contamination by Smoke. Foods, 14(9), 1550. https://doi.org/10.3390/foods14091550







