Use of the Glycolipopeptid Biosurfactant Produced by Lactiplantibacillus plantarum Tw226 to Formulate Functional Cinnamon Bark Essential Oil Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Culture Media
2.2. Microorganisms and Inocula Preparation
2.3. Biosurfactant Production
2.4. Emulsion Formulations
2.5. Characterization of the Emulsions
2.5.1. Drop Size Analysis
2.5.2. Physical Stability
2.5.3. Antioxidant Activity Determination
2.5.4. Antimicrobial Activity Determination
2.6. Data Analysis
3. Results and Discussion
3.1. Emulsions Characterization
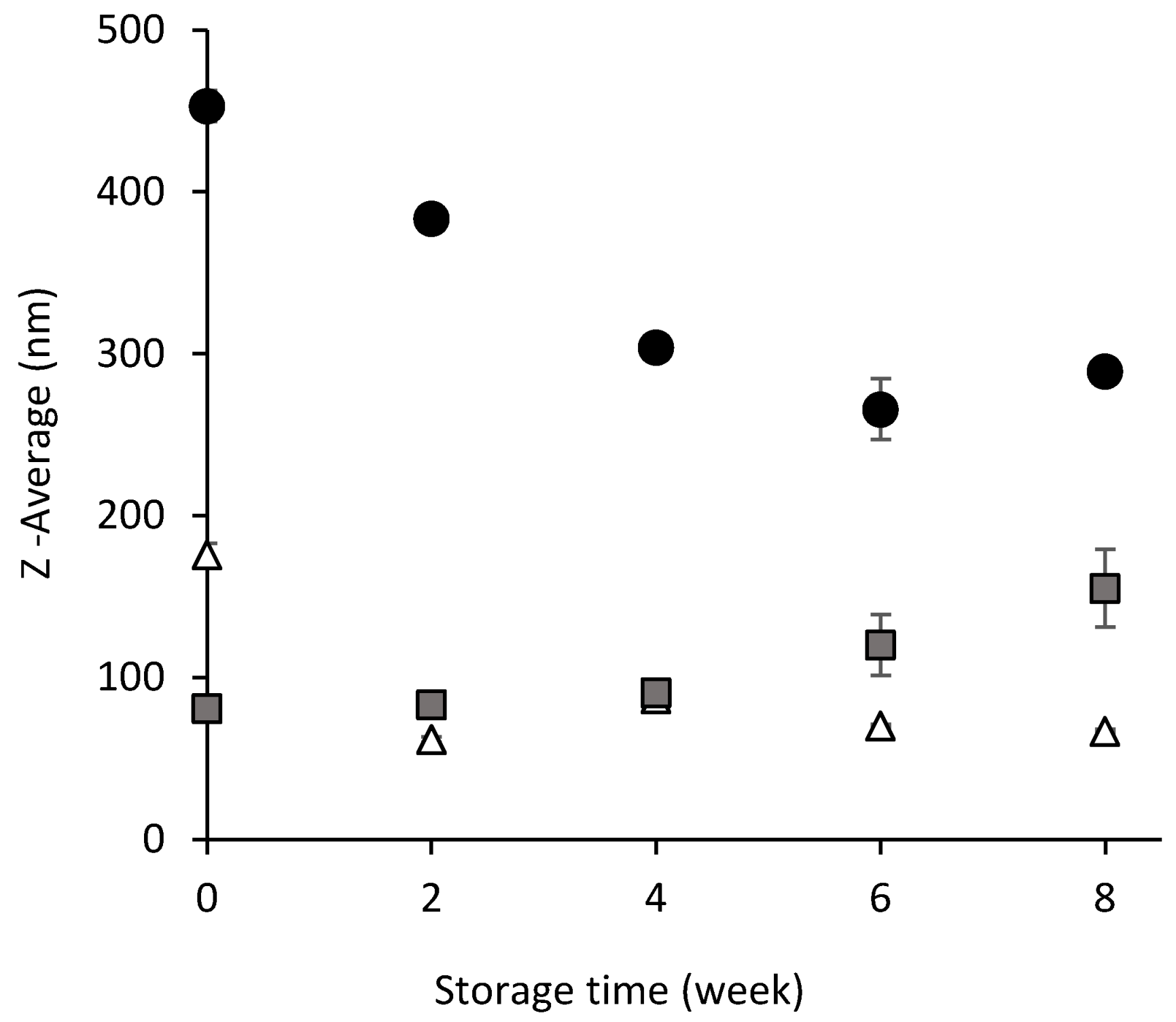
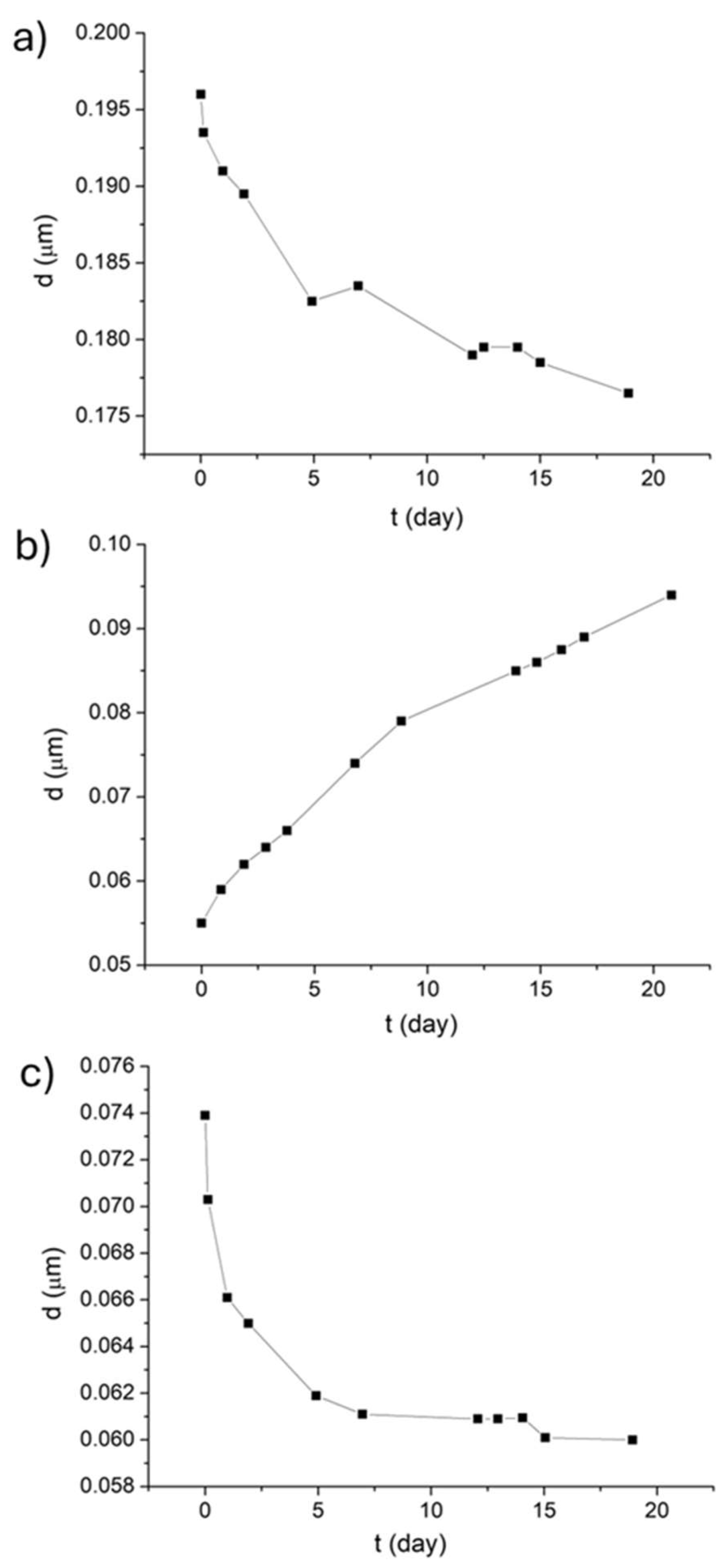
3.1.1. Droplet Size
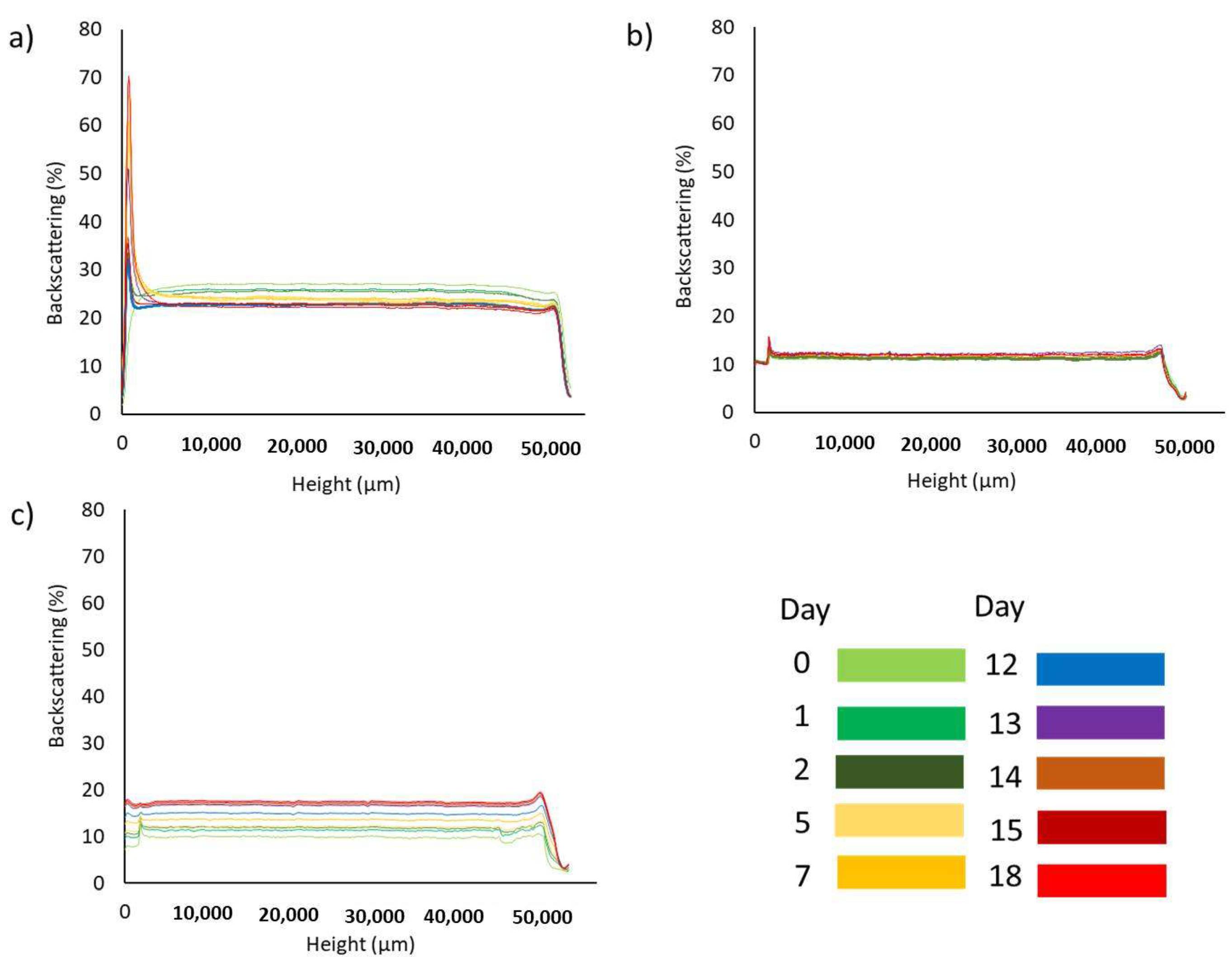
3.1.2. Turbidimetry Analysis
3.2. Functional Activity of Emulsion
3.2.1. Antioxidant Activity
3.2.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Khorram, S.B.; Sohrabi, M. Antioxidant activity of essential oils in foods. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Berger, R.G., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 247–265. [Google Scholar]
- Osimani, A.; Garofalo, C.; Harasym, J.; Aquilanti, L. Use of essential oils against foodborne spoilage yeasts: Advantages and drawbacks. Curr. Opin. Food Sci. 2022, 45, 100821. [Google Scholar]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; de Oliveira, M.S. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar]
- Guzmán, E.; Lucia, A. Essential oils and their individual components in cosmetic products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonça, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar]
- LaLonde, T.; Bowser, T.; Jadeja, R. Essential oils as antimicrobials. Madridge J. Food Technol. 2019, 4, 163–169. [Google Scholar]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crops Prod. 2020, 157, 112935. [Google Scholar]
- Barzegar, F.; Nabizadeh, S.; Kamankesh, M.; Ghasemi, J.B.; Mohammadi, A. Recent advances in natural product-based nanoemulsions as promising substitutes for hazardous synthetic food additives: A new revolution in food processing. Food Bioprocess Technol. 2023, 17, 1087–1108. [Google Scholar]
- Sharma, D.; Saharan, B.S.; Kapil, S. Biosurfactants of Lactic Acid Bacteria, 1st ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Satpute, S.; Das, P.; Pardesi, K.; Mone, N.; Sharma, D.; Banat, I. Biosurfactants from lactic acid bacteria. In Microbial Biosurfactants: Environmental and Industrial Applications; Singh, A., Prakash, O., Ghosh, S., Eds.; CRC Press: Florida, FL, USA, 2023; pp. 111–136. [Google Scholar]
- Lara, V.M.; Vallejo, M.; Parada, R.; Henao Ossa, J.S.; Gliemmo, M.F.; Campos, C.A. Characterization of the emulsifying activity of biosurfactants produced by lactic acid bacteria isolated from the Argentinian Patagonia. J. Dispersion Sci. Technol. 2020, 43, 902–909. [Google Scholar] [CrossRef]
- Lara, V.M.; Mendonça, C.M.N.; Silva, F.V.S.; Marguet, E.R.; Vallejo, M.; Converti, A.; Varani, A.M.; Gliemmo, M.F.; Campos, C.A.; Oliveira, R.P.S. Characterization of Lactiplantibacillus plantarum Tw226 strain and its use for the production of a new membrane-bound biosurfactant. J. Mol. Liq. 2022, 363, 119889. [Google Scholar]
- Lara, V.M.; Vallejo, M.; Gliemmo, M.F.; Campos, C.A. Caracterización de la actividad estabilizante de emulsiones provista por el biosurfactante producido por Lactiplantibacillus plantarum Tw226. In Tecnología de los Alimentos, 1st ed.; Alzamora, S.M., Buera, M.P., Castellano, R., Raffellini, S.M., Raimondo, E.E., Socolovsky, S.E., Vaudagna, S.R., Vidales, S.L., Zuleta, A., Eds.; Asociación Argentina de Tecnólogos Alimentarios—AATA: Ciudad Autónoma de Buenos Aires, Argentina, 2023. [Google Scholar]
- Dammak, I.; Sobral, P.J.D.A.; Aquino, A.; Neves, M.A.D.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Barbosa-Pereira, L.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Optimization of extraction conditions and fatty acid characterization of Lactobacillus pentosus cell-bound biosurfactant/bioemulsifier. J. Sci. Food Agric. 2015, 95, 313–320. [Google Scholar] [CrossRef]
- Ferreira, A.; Vecino, X.; Ferreira, D.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Novel cosmetic formulations containing a biosurfactant from Lactobacillus paracasei. Colloids Surf. B Biointerfaces 2017, 155, 522–539. [Google Scholar]
- Tarifa, M.; Brugnoni, L.; Lozano, J. Role of hydrophobicity in adhesion of wild yeast isolated from the ultrafiltration membranes of an apple juice processing plant. Biofouling 2013, 29, 841–853. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactant-producing lactobacilli: Screening, production profiles, and effect of medium composition. Appl. Environ. Soil Sci. 2011, 2011, 201254. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Mao, L.; Miao, S. Structuring food emulsions to improve nutrient delivery during digestion. Food Eng. Rev. 2015, 7, 439–451. [Google Scholar] [CrossRef]
- Syed, U.T.; Dias, A.M.; de Sousa, H.C.; Crespo, J.; Brazinha, C. Greening perfluorocarbon based nanoemulsions by direct membrane emulsification: Comparative studies with ultrasound emulsification. J. Clean. Prod. 2022, 357, 131966. [Google Scholar] [CrossRef]
- Kothekar, S.C.; Ware, A.M.; Waghmare, J.T.; Momin, S.A. Comparative analysis of the properties of Tween-20, Tween-60, Tween-80, Arlacel-60, and Arlacel-80. J. Dispersion Sci. Technol. 2007, 28, 477–484. [Google Scholar]
- Zheng, H.; Mao, L.; Yang, J.; Zhang, C.; Miao, S.; Gao, Y. Effect of oil content and emulsifier type on the properties and antioxidant activity of sea buckthorn oil-in-water emulsions. J. Food Qual. 2020, 1, 1540925. [Google Scholar]
- Hashtjin, A.M.; Abbasi, S. Nano-emulsification of orange peel essential oil using sonication and native gums. Food Hydrocoll. 2015, 44, 40–48. [Google Scholar]
- González, M.M.; Zalazar, A.L.; Pedreira, J.D.; Campos, C.A.; Gliemmo, M.F. Lemongrass and cinnamon oil nanoemulsions: Formulation and study of their physical stability and activity against Zygosaccharomyces bailii. Food Sci. Technol. Int. 2021, 27, 485–498. [Google Scholar] [PubMed]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [PubMed]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physical Properties and Antimicrobial Efficacy of Thyme Oil Nanoemulsions: Influence of Ripening Inhibitors. J. Agric. Food Chem. 2012, 60, 12056–12063. [Google Scholar]
- Kaur, G.; Singh, P.; Sharma, S. Physical, morphological, and storage studies of cinnamon based nanoemulsions developed with Tween 80 and soy lecithin: A comparative study. J. Food Meas. Charact. 2021, 15, 2386–2398. [Google Scholar]
- Taylor, P. Ostwald Ripening in Emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar]
- Santos, J.; Alfaro-Rodríguez, M.C.; Vega, L.; Muñoz, J. Relationship between HLB number and predominant destabilization process in microfluidized nanoemulsions formulated with lemon essential oil. Appl. Sci. 2023, 13, 5208. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Physicochemical characterization and antimicrobial activity of food-grade emulsions and nanoemulsions incorporating essential oils. Food Hydrocoll. 2015, 43, 547–556. [Google Scholar]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. Characterisation of instability of concentrated dispersions by a new optical analyser: The TURBISCAN MA 1000. Colloids Surf. A. 1999, 152, 111–123. [Google Scholar]
- Muñoz, J.; Prieto-Vargas, P.; García, M.C.; Alfaro-Rodríguez, M.C. Effect of a Change in the CaCl2/Pectin Mass Ratio on the Particle Size, Rheology and Physical Stability of Lemon Essential Oil/W Emulgels. Foods 2023, 12, 1137. [Google Scholar] [CrossRef]
- Decker, E.A.; McClements, D.J.; Bourlieu-Lacanal, C.; Durand, E.; Figueroa-Espinoza, M.C.; Lecomte, J.; Villeneuve, P. Hurdles in predicting antioxidant efficacy in oil-in-water emulsions. Trends Food Sci. Technol. 2017, 67, 183–194. [Google Scholar]
- Lima, R.; Ribeiro, F.C.; Colombo, A.L.; de Almeida, J.N., Jr. The emerging threat antifungal-resistant Candida tropicalis in humans, animals, and environment. Front. Fungal Biol. 2022, 3, 957021. [Google Scholar]
- Miao, Y.; Ding, T.; Liu, Y.; Zhou, X.; Du, J. The Yeast and Hypha Phases of Candida krusei Induce the Apoptosis of Bovine Mammary Epithelial Cells via Distinct Signaling Pathways. Animals 2023, 13, 3222. [Google Scholar] [CrossRef]
- Diaz de Castro, R.; Lima, E.O. Anti-Candida activity and chemical composition of Cinnamomum zeylanicum Blume essential oil. Braz. Arch. Biol. Technol. 2013, 56, 749–755. [Google Scholar]
- Jantan, I.B.; Karim Moharam, B.A.; Santhanam, J.; Jamal, J.A. Correlation between chemical composition and antifungal activity of the essential oils of eight Cinnamomum species. Pharm. Biol. 2008, 46, 406–412. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Gil, G.A.; Kakuda, L.; Tonani, L.; von Zeska Kress, M.R.; Oliveira, W.P. Surfactant-Driven Effects on the Antifungal Activity of Lippia origanoides Kunth Essential Oil Encapsulated in Lipid-Based Nanosystems. ACS Omega 2025, 10, 7876–7887. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara, V.M.; Gliemmo, M.F.; Vallejo, M.; García González, M.d.C.; Alfaro Rodríguez, M.d.C.; Campos, C.A. Use of the Glycolipopeptid Biosurfactant Produced by Lactiplantibacillus plantarum Tw226 to Formulate Functional Cinnamon Bark Essential Oil Emulsions. Foods 2025, 14, 1540. https://doi.org/10.3390/foods14091540
Lara VM, Gliemmo MF, Vallejo M, García González MdC, Alfaro Rodríguez MdC, Campos CA. Use of the Glycolipopeptid Biosurfactant Produced by Lactiplantibacillus plantarum Tw226 to Formulate Functional Cinnamon Bark Essential Oil Emulsions. Foods. 2025; 14(9):1540. https://doi.org/10.3390/foods14091540
Chicago/Turabian StyleLara, Virginia M., María F. Gliemmo, Marisol Vallejo, María del Carmen García González, María del Carmen Alfaro Rodríguez, and Carmen A. Campos. 2025. "Use of the Glycolipopeptid Biosurfactant Produced by Lactiplantibacillus plantarum Tw226 to Formulate Functional Cinnamon Bark Essential Oil Emulsions" Foods 14, no. 9: 1540. https://doi.org/10.3390/foods14091540
APA StyleLara, V. M., Gliemmo, M. F., Vallejo, M., García González, M. d. C., Alfaro Rodríguez, M. d. C., & Campos, C. A. (2025). Use of the Glycolipopeptid Biosurfactant Produced by Lactiplantibacillus plantarum Tw226 to Formulate Functional Cinnamon Bark Essential Oil Emulsions. Foods, 14(9), 1540. https://doi.org/10.3390/foods14091540





