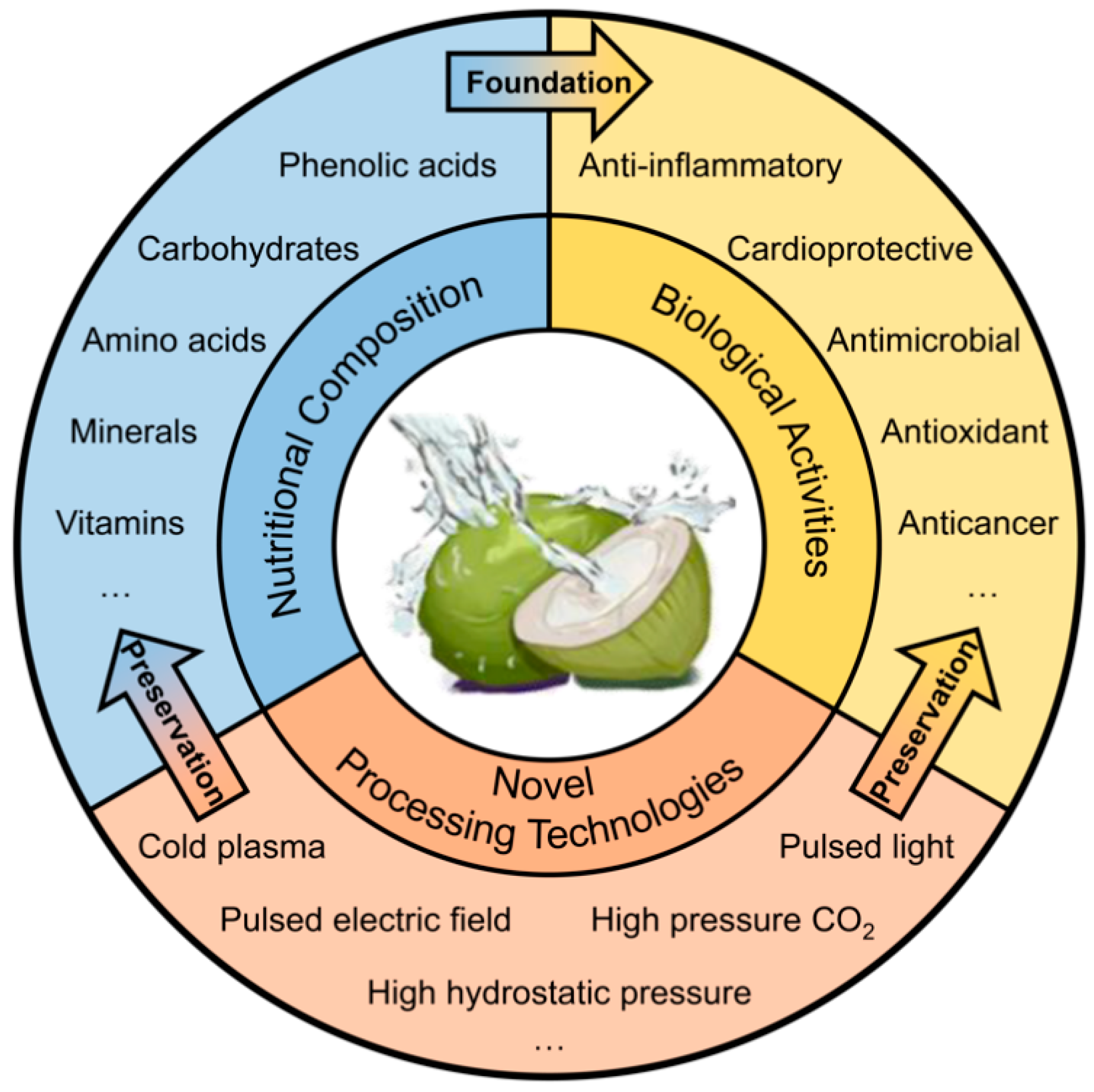
Research Progress in Coconut Water: A Review of Nutritional Composition, Biological Activities, and Novel Processing Technologies
Abstract
1. Introduction
2. Nutritional Composition of CW
2.1. Carbohydrates
2.2. Minerals
2.3. Vitamins
2.4. Phenolic Compounds
2.5. Amino Acids
2.6. Other Compounds
3. Biological Activities of Coconut Water
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Ant-Proliferative Activity
3.4. Cardioprotective Protect
3.5. Antimicrobial Activity
3.6. Other Health Benefits
4. Novel Processing Technologies for Coconut Water
4.1. High-Hydrostatic-Pressure (HHP) Processing
4.2. High-Pressure Carbon Dioxide (HPCD)
4.3. Cold Plasma (CP)
4.4. Pulsed Electric Field (PEF)
4.5. Pulsed Light (PL)
4.6. UV (UV-C) Irradiation
4.7. Ultrasound
4.8. Ozone
4.9. Microfiltration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| USDA | United States Department of Agriculture |
| TSS | Total Soluble Solids |
| CW | Coconut Water |
| TCW | Tender Coconut Water |
| HHP | High Hydrostatic Pressure |
| HPCD | High-Pressure Carbon Dioxide |
| CP | Cold Plasma |
| PPO | Polyphenol Oxidase |
| POD | Peroxidase |
| PEF | Pulsed Electric Field |
| PL | Pulsed Light |
| UV | Ultraviolet |
| MF | Microfiltration |
References
- Carvalho de Castro, J.M.; Nascimento Alves, C.A.; de LimaSantos, K.; de Oliveira Silva, E.; Maria da Silva Araújo, Í.; Barros deVasconcelos, L. Elaboration of a mixed beverage from hibiscus and coconut water: An evaluation of bioactive and sensory properties. Int. J. Gastron. Food Sci. 2021, 23, 100284. [Google Scholar] [CrossRef]
- Ignacio, I.-F.; Miguel, T.-S. Research opportunities on the coconut (Cocos nucifera L.) using new technologies. S. Afr. J. Bot. 2021, 141, 414–420. [Google Scholar] [CrossRef]
- Segura-Badilla, O.; Lazcano-Hernández, M.; Kammar-García, A.; Vera-López, O.; Aguilar-Alonso, P.; Ramírez-Calixto, J.; Navarro-Cruz, A.R. Use of coconut water (Cocus nucifera L.) for the development of a symbiotic functional drink. Heliyon 2020, 6, e03653. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, E.V.; Phan, B.N.; Onuoha, E.; Diggins, S.; Aguilar, V.; Swanson, S.; Lee, A. The use of High-Pressure Processing (HPP) to improve the safety and quality of raw coconut (Cocos nucifera L.) water. Int. J. Food Microbiol. 2020, 331, 108697. [Google Scholar] [CrossRef]
- Labouisse, J.-P.; Sileye, T.; Bonnot, F.o.; Baudouin, L. Achievements in breeding coconut hybrids for tolerance to coconut foliar decay disease in Vanuatu, South Pacific. Euphytica 2011, 177, 1–13. [Google Scholar] [CrossRef]
- Mat, K.; Abdul Kari, Z.; Rusli, N.D.; Che Harun, H.; Wei, L.S.; Rahman, M.M.; Mohd Khalid, H.N.; Mohd Ali Hanafiah, M.H.; Mohamad Sukri, S.A.; Raja Khalif, R.I.A.; et al. Coconut Palm: Food, Feed, and Nutraceutical Properties. Animals 2022, 12, 2107. [Google Scholar] [CrossRef]
- Alchoubassi, G.; Kinska, K.; Bierla, K.; Lobinski, R.; Szpunar, J. Speciation of essential nutrient trace elements in coconut water. Food Chem. 2021, 339, 127680. [Google Scholar] [CrossRef]
- Prithviraj, V.; Pandiselvam, R.; Babu, A.C.; Kothakota, A.; Manikantan, M.R.; Ramesh, S.V.; Beegum, P.P.S.; Mathew, A.C.; Hebbar, K.B. Emerging non-thermal processing techniques for preservation of tender coconut water. LWT 2021, 149, 111850. [Google Scholar] [CrossRef]
- Lamdande, A.G.; Mittal, R.; Raghavarao, K.S.M.S. Flux evaluation based on fouling mechanism in acoustic field-assisted ultrafiltration for cold sterilization of tender coconut water. Innov. Food Sci. Emerg. Technol. 2020, 61, 102312. [Google Scholar] [CrossRef]
- Kumar, M.; Saini, S.S.; Agrawal, P.K.; Roy, P.; Sircar, D. Nutritional and metabolomics characterization of the coconut water at different nut developmental stages. J. Food Compos. Anal. 2021, 96, 103738. [Google Scholar] [CrossRef]
- Bhagya, D.; Prema, L.; Rajamohan, T. Therapeutic effects of tender coconut water on oxidative stress in fructose fed insulin resistant hypertensive rats. Asian Pac. J. Trop. Med. 2012, 5, 270–276. [Google Scholar] [CrossRef]
- Rukmini, J.N.; Manasa, S.; Rohini, C.; Sireesha, L.; Ritu, S.; Umashankar, G.K. Antibacterial efficacy of tender coconut water (Cocos nucifera L.) on Streptococcus mutans: An in-vitro study. J. Int. Soc. Prev. Community Dent. 2017, 7, 130–134. [Google Scholar] [CrossRef]
- Preetha, P.P.; Devi, V.G.; Rajamohan, T. Mature coconut water exhibits antidiabetic and antithrombotic potential via L-arginine-nitric oxide pathway in alloxan induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Najam, R. Coconut water of different maturity stages ameliorates inflammatory processes in model of inflammation. J. Intercult. Ethnopharmacol. 2016, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Dennison, S.R.; Mura, M.; Lea, R.W.; Snape, T.J.; Harris, F. Cn-AMP2 from green coconut water is an anionic anticancer peptide. J. Pept. Sci. 2014, 20, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Anurag, P.; Rajamohan, T. Cardioprotective effect of tender coconut water in experimental myocardial infarction. Plant Foods Hum. Nutr. 2003, 58, 1–12. [Google Scholar] [CrossRef]
- Rajashri, K.; Roopa, B.S.; Negi, P.S.; Rastogi, N.K. Effect of ozone and ultrasound treatments on polyphenol content, browning enzyme activities, and shelf life of tender coconut water. J. Food Process. Preserv. 2019, 44, e14363. [Google Scholar] [CrossRef]
- Yannam, S.K.; Patras, A.; Pendyala, B.; Vergne, M.; Ravi, R.; Gopisetty, V.V.S.; Sasges, M. Effect of UV-C irradiation on the inactivation kinetics of oxidative enzymes, essential amino acids and sensory properties of coconut water. J. Food Sci. Technol. 2020, 57, 3564–3572. [Google Scholar] [CrossRef]
- Prades, A.; Dornier, M.; Diop, N.; Pain, J.-P. Coconut water preservation and processing: A review. Fruits 2012, 67, 157–171. [Google Scholar] [CrossRef]
- Divya, P.M.; Roopa, B.S.; Manusha, C.; Balannara, P. A concise review on oil extraction methods, nutritional and therapeutic role of coconut products. J. Food Sci. Technol. 2022, 60, 441–452. [Google Scholar] [CrossRef]
- Jayawardena, J.A.E.C.; Vanniarachchy, M.P.G.; Wansapala, M.A.J. Progressive Freeze Concentration of Coconut Water and Use of Partial Ice Melting Method for Yield Improvement. Int. J. Food Sci. 2020, 2020, 4292013. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.B.P.; Buvaneswaran, M.; Anto, A.M.; Sinija, V.R. High-pressure processing for inactivation of enzymes, microbes, and its effect on nutritional composition of tender coconut water. J. Food Process Eng. 2024, 47, e14624. [Google Scholar] [CrossRef]
- Burns, D.T.; Johnston, E.L.; Walker, M.J. Authenticity and the Potability of Coconut Water—A Critical Review. J. AOAC Int. 2020, 103, 800–806. [Google Scholar] [CrossRef]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Yun, Y.; Li, C.; Fang, Y.; Zhang, W. Discrimination and characterization of different coconut water (CW) by their phenolic composition and volatile organic compounds (VOCs) using LC-MS/MS, HS-SPME-GC-MS, and HS-GC-IMS. J. Food Sci. 2023, 88, 3758–3772. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Y.; Ran, L.; Liu, R.; Sun, X.; Hu, L.; Xiao, Y.; Chen, F. Flavor deterioration of liquid endosperm in postharvest tender coconut revealed by LC-MS-based metabolomics, GC-IMS and E-tongue. Postharvest Biol. Technol. 2022, 187, 111866. [Google Scholar] [CrossRef]
- Appaiah, P.; Sunil, L.; Kumar, P.K.; Krishna, A.G. Physico-chemical characteristics and stability aspects of coconut water and kernel at different stages of maturity. J. Food Sci. Technol. 2015, 52, 5196–5203. [Google Scholar] [CrossRef]
- Halim, H.H.; Pak Dek, M.S.; Hamid, A.A.; Saari, N.; Mohd Lazim, M.I.; Abas, F.; Ngalim, A.; Ismail, A.; Jaafar, A.H. Novel sources of bioactive compounds in coconut (Cocos nucifera L.) water from different maturity levels and varieties as potent skin anti-aging strategies and anti-fatigue agents. Food Biosci. 2023, 51, 102326. [Google Scholar] [CrossRef]
- Manna, K.; Khan, A.; Das, D.K.; Bandhu Kesh, S.; Das, U.; Ghosh, S.; Sharma Dey, R.; Das Saha, K.; Chakraborty, A.; Chattopadhyay, S.; et al. Protective effect of coconut water concentrate and its active component shikimic acid against hydroperoxide mediated oxidative stress through suppression of NF-κB and activation of Nrf2 pathway. J. Ethnopharmacol. 2014, 155, 132–146. [Google Scholar] [CrossRef]
- Prades, A.; Dornier, M.; Diop, N.; Pain, J.-P. Coconut water uses, composition and properties: A review. Fruits 2012, 67, 87–107. [Google Scholar] [CrossRef]
- Shayanthavi, S.; Kapilan, R.; Wickramasinghe, I. Comprehensive analysis of physicochemical, nutritional, and antioxidant properties of various forms and varieties of tender coconut (Cocos nucifera L.) water in Northern Sri Lanka. Food Chem. Adv. 2024, 4, 100645. [Google Scholar] [CrossRef]
- Preetha, P.P.; Girija Devi, V.; Rajamohan, T. Effects of coconut water on carbohydrate metabolism and pancreatic pathology of alloxan induced diabetic rats. Eur. J. Integr. Med. 2013, 5, 234–240. [Google Scholar] [CrossRef]
- Lima, E.B.C.; Sousa, C.N.S.; Meneses, L.N.; Ximenes, N.C.; Santos Júnior, M.A.; Vasconcelos, G.S.; Lima, N.B.C.; Patrocínio, M.C.A.; Macedo, D.; Vasconcelos, S.M.M. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz. J. Med. Biol. Res. 2015, 48, 953–964. [Google Scholar] [CrossRef]
- Ndukwe, M.K.; Atiaetuk, I.E.; Aja, O.A.; Edward, U.I.; Mba, O.J. In vivo Antioxidant Effects of Coconut (Cocos nucifera) Water Extract in Wistar Albino Rats. Asian J. Res. Biochem. 2020, 7, 28–35. [Google Scholar] [CrossRef]
- Lakshmanan, J.; Zhang, B.; Wright, K.; Motameni, A.T.; Jaganathan, V.L.; Schultz, D.J.; Klinge, C.M.; Harbrecht, B.G. Tender coconut water suppresses hepatic inflammation by activating AKT and JNK signaling pathways in an in vitro model of sepsis. J. Funct. Foods 2020, 64, 103637. [Google Scholar] [CrossRef] [PubMed]
- Prathapan, A.; Rajamohan, T. Antioxidant and Antithrombotic Activity of Tender Coconut Water in Experimental Myocardial Infarction. J. Food Biochem. 2010, 35, 1501–1507. [Google Scholar] [CrossRef]
- Adebolu, T.T.; Akinjayeju, D.O. Effects of Coconut (Cocos nucifera Linn.) Water and Oil on the Ileal Bacterial Flora of Apparently Healthy Wistar Albino Rats. J. Adv. Microbiol. 2020, 20, 94–102. [Google Scholar] [CrossRef]
- Gandhi, M.; Aggarwal, M.; Puri, S.; Singla, S.K. Prophylactic effect of coconut water (Cocos nucifera L.) on ethylene glycol induced nephrocalcinosis in male wistar rat. Int. Braz. J. Urol. 2013, 39, 108–117. [Google Scholar] [CrossRef]
- Patel, R.M.; Jiang, P.; Asplin, J.; Granja, I.; Capretz, T.; Osann, K.; Okhunov, Z.; Landman, J.; Clayman, R.V. Coconut Water: An Unexpected Source of Urinary Citrate. BioMed Res. Int. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Ct, D.R.; Palaninathan, V.; James, R.A. Anti-uropathogenic, antioxidant and struvite crystallization inhibitory potential of fresh and fermented coconut water. Biocatal. Agric. Biotechnol. 2023, 47, 102555. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manna, K.; Das, A. Tender coconut water attenuates heat stress-induced testicular damage through modulation of the NF-κB and Nrf2 pathways. Food Funct. 2018, 9, 5463–5479. [Google Scholar] [CrossRef] [PubMed]
- Radenahmad, N.; Saleh, F.; Sayoh, I.; Sawangjaroen, K.; Subhadhirasakul, P.; Boonyoung, P.; Rundorn, W.; Mitranun, W. Young coconut juice can accelerate the healing process of cutaneous wounds. BMC Complement. Altern. Med. 2012, 12, 252. [Google Scholar]
- Mahayothee, B.; Koomyart, I.; Khuwijitjaru, P.; Siriwongwilaichat, P.; Nagle, M.; Müller, J. Phenolic Compounds, Antioxidant Activity, and Medium Chain Fatty Acids Profiles of Coconut Water and Meat at Different Maturity Stages. Int. J. Food Prop. 2016, 19, 2041–2051. [Google Scholar] [CrossRef]
- Luiz, J.; Bispo, V.; Chaves Filho, A.; Dantas, L.; Vasconcelos, D.; Abreu, F.; Melo, D.; Araujo Matos, I.; Freitas, F.; Gomes, O.; et al. Evaluation of Chemical Constituents and Antioxidant Activity of Coconut Water (Cocus nucifera L.) and Caffeic Acid in Cell Culture. An. Da Acad. Bras. De Ciencias 2013, 85, 1235–1247. [Google Scholar] [CrossRef]
- Manoharan, P.; Dhanabalan, S.C.; Alagan, M.; Muthuvijayan, S.; Ponraj, J.S.; Somasundaram, C.K. Facile synthesis and characterisation of green luminescent carbon nanodots prepared from tender coconut water using the acid-assisted ultrasonic route. Micro Nano Lett. 2020, 15, 920–924. [Google Scholar] [CrossRef]
- Arzeta-Ríos, A.J.; Guerra-Ramírez, D.; Reyes-Trejo, B.; Ybarra-Moncada, M.C.; Zuleta-Prada, H. Microwave heating effect on total phenolics and antioxidant activity of green and mature coconut water. Int. J. Food Eng. 2020, 16, 20190378. [Google Scholar] [CrossRef]
- Ge, L.; Yong, J.W.H.; Tan, S.N.; Yang, X.H.; Ong, E.S. Analysis of cytokinin nucleotides in coconut (Cocos nucifera L.) water using capillary zone electrophoresis-tandem mass spectrometry after solid-phase extraction. J. Chromatogr. A 2006, 1133, 322–331. [Google Scholar] [CrossRef]
- Mohd Lazim, M.I.; Badruzaman, B. Quantification of Cytokinins in Coconut Water from Different Maturation Stages of Malaysia’s Coconut (Cocos nucifera L.) Varieties. J. Food Process. Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Fonseca, V.A. Defining and Characterizing the Progression of Type 2 Diabetes. Diabetes Care 2009, 32, S151–S156. [Google Scholar] [CrossRef]
- Supapvanich, S.; Yimpong, A.; Srisuwanwichan, J. Browning inhibition on fresh-cut apple by the immersion of liquid endosperm from mature coconuts. J. Food Sci. Technol. 2020, 57, 4424–4431. [Google Scholar] [CrossRef]
- Sandhya, V.G.; Rajamohan, T. Comparative evaluation of the hypolipidemic effects of coconut water and lovastatin in rats fed fat–cholesterol enriched diet. Food Chem. Toxicol. 2008, 46, 3586–3592. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.P.; Becker, L.C.; Kass, D.A.; Champion, H.C.; Terrin, M.L.; Forman, S.; Ernst, K.V.; Kelemen, M.D.; Townsend, S.N.; Capriotti, A.; et al. L-Arginine Therapy in Acute Myocardial InfarctionThe Vascular Interaction with Age in Myocardial Infarction (VINTAGE MI) Randomized Clinical Trial. JAMA 2006, 295, 58–64. [Google Scholar] [CrossRef]
- Anaya, K.; Podszun, M.; Franco, O.L.; de Almeida Gadelha, C.A.; Frank, J. The Coconut Water Antimicrobial Peptide CnAMP1 Is Taken up into Intestinal Cells but Does Not Alter P-Glycoprotein Expression and Activity. Plant Foods Hum. Nutr. 2020, 75, 396–403. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dey, S.; Mandal, M.; Sarkar, S.; Maria-Neto, S.; Franco, O.L. Identification and structural insights of three novel antimicrobial peptides isolated from green coconut water. Peptides 2009, 30, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Macalalag, E.V., Jr.; Macalalag, A.L. Bukolysis: Young coconut water renoclysis for urinary stone dissolution. Int. Surg. 1987, 72, 247. [Google Scholar]
- Adams, W.; Bratt, D.E. Young coconut water for home rehydration in children with mild gastroenteritis. Trop. Geogr. Med. 1992, 44, 149–153. [Google Scholar] [PubMed]
- Naik, M.; Sunil, C.K.; Rawson, A.; Venkatachalapathy, N. Tender Coconut Water: A Review on Recent Advances in Processing and Preservation. Food Rev. Int. 2022, 38, 1215–1236. [Google Scholar] [CrossRef]
- Cunha, A.G.; Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; Rodrigues, T.H.S.; Brito, E.S.d.; Miranda, M.R.A.d. Chemical composition of thermally processed coconut water evaluated by GC–MS, UPLC-HRMS, and NMR. Food Chem. 2020, 324, 126874. [Google Scholar] [CrossRef]
- González-Angulo, M.; Clauwers, C.; Harastani, R.; Tonello, C.; Jaime, I.; Rovira, J.; Michiels, C.W. Evaluation of factors influencing the growth of non-toxigenic Clostridium botulinum type E and Clostridium sp. in high-pressure processed and conditioned tender coconut water from Thailand. Food Res. Int. 2020, 134, 109278. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, L.; Wang, S.; Xu, Z.; Liao, X.; Cheng, Y. Comparison of the quality attributes of coconut waters by high-pressure processing and high-temperature short time during the refrigerated storage. Food Sci. Nutr. 2019, 7, 1512–1519. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ferrentino, G.; Endrizzi, I.; Aprea, E.; Betta, E.; Corollaro, M.L.; Charles, M.; Gasperi, F.; Spilimbergo, S. High Pressure Carbon Dioxide pasteurization of coconut water: A sport drink with high nutritional and sensory quality. J. Food Eng. 2015, 145, 73–81. [Google Scholar] [CrossRef]
- De Marchi, F.; Aprea, E.; Endrizzi, I.; Charles, M.; Betta, E.; Corollaro, M.L.; Cappelletti, M.; Ferrentino, G.; Spilimbergo, S.; Gasperi, F. Effects of Pasteurization on Volatile Compounds and Sensory Properties of Coconut (Cocos nucifera L.) Water: Thermal vs. High-Pressure Carbon Dioxide Pasteurization. Food Bioprocess Technol. 2015, 8, 1393–1404. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ferrentino, G.; Spilimbergo, S. Supercritical carbon dioxide combined with high power ultrasound: An effective method for the pasteurization of coconut water. J. Supercrit. Fluids 2014, 92, 257–263. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Influence of cold plasma voltage and time on quality attributes of tender coconut water (Cocos nucifera L.) and degradation kinetics of its blended beverage. J. Food Process. Preserv. 2021, 45, e15372. [Google Scholar] [CrossRef]
- Porto, E.; Alves Filho, E.G.; Silva, L.M.A.; Fonteles, T.V.; do Nascimento, R.B.R.; Fernandes, F.A.N.; de Brito, E.S.; Rodrigues, S. Ozone and plasma processing effect on green coconut water. Food Res. Int. 2020, 131, 109000. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Mahanta, C.L.; Keener, K.M.; Misra, N.N. Strategy to achieve a 5-log Salmonella inactivation in tender coconut water using high voltage atmospheric cold plasma (HVACP). Food Chem. 2019, 284, 303–311. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Mahanta, C.L.; Farkas, B.E.; Keener, K.M.; Misra, N.N. Atmospheric cold plasma inactivation of Escherichia coli and Listeria monocytogenes in tender coconut water: Inoculation and accelerated shelf-life studies. Food Control. 2019, 106, 106678. [Google Scholar] [CrossRef]
- Chutia, H.; Kalita, D.; Mahanta, C.L.; Ojah, N.; Choudhury, A.J. Kinetics of inactivation of peroxidase and polyphenol oxidase in tender coconut water by dielectric barrier discharge plasma. LWT 2019, 101, 625–629. [Google Scholar] [CrossRef]
- Tongdonyod, S.; Thikham, S.; Kittiwachana, S.; Wichaphon, J.; Klangpetch, W. Optimization of pulsed electric fields combined with mild heat treatment on microbial inactivation of tender coconut water and evaluation of quality attributes during storage. Innov. Food Sci. Emerg. Technol. 2023, 90, 103507. [Google Scholar] [CrossRef]
- Preetha, P.; Pandiselvam, R.; Varadharaju, N.; Kennedy, Z.J.; Balakrishnan, M.; Kothakota, A. Effect of pulsed light treatment on inactivation kinetics of Escherichia coli (MTCC 433) in fruit juices. Food Control 2021, 121, 107547. [Google Scholar] [CrossRef]
- Basak, S.; Jha, T.; Chakraborty, S. Pasteurization of tender coconut water by pulsed light treatment: Microbial safety, enzymatic inactivation, and impact on physicochemical properties. Innov. Food Sci. Emerg. Technol. 2023, 84, 103302. [Google Scholar] [CrossRef]
- Preetha, P.; Varadharaju, N.; Jeevarathinam, G.; Deepa, J.; Kumar, A.P.M.; Balakrishnan, M.; Rajkumar, P.; Pandiselvam, R. Optimization of continuous flow pulsed light system process parameters for microbial inactivation in tender coconut water, pineapple and orange juice. J. Food Process Eng. 2023, 46, e14254. [Google Scholar] [CrossRef]
- Maguluri, R.K.; Dasalkar, A.H.; Singam, S.S.R.; Yannam, S.K. Impact of 280 nm UV-C LEDs on the microbial safety and nutritional quality in tender coconut water. Food Humanit. 2023, 1, 864–872. [Google Scholar] [CrossRef]
- Bhullar, M.S.; Patras, A.; Kilanzo-Nthenge, A.; Pokharel, B.; Yannam, S.K.; Rakariyatham, K.; Pan, C.; Xiao, H.; Sasges, M. Microbial inactivation and cytotoxicity evaluation of UV irradiated coconut water in a novel continuous flow spiral reactor. Food Res. Int. 2018, 103, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Umagiliyage, A.L.; Dhital, R.; Joshi, P.; Watson, D.G.; Fisher, D.J.; Choudhary, R. Nonthermal pasteurization of tender coconut water using a continuous flow coiled UV reactor. LWT—Food Sci. Technol. 2017, 83, 127–131. [Google Scholar] [CrossRef]
- Gabriel, A.A. Previous physicochemical stress exposures influence subsequent resistance of Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes to ultraviolet-C in coconut liquid endosperm beverage. Int. J. Food Microbiol. 2015, 201, 7–16. [Google Scholar] [CrossRef]
- Augusto, P.E.D.; Ibarz, R.; Garvín, A.; Ibarz, A. Peroxidase (POD) and polyphenol oxidase (PPO) photo-inactivation in a coconut water model solution using ultraviolet (UV). Food Res. Int. 2015, 74, 151–159. [Google Scholar] [CrossRef]
- Rojas, M.L.; Trevilin, J.H.; Funcia, E.d.S.; Gut, J.A.W.; Augusto, P.E.D. Using ultrasound technology for the inactivation and thermal sensitization of peroxidase in green coconut water. Ultrasonics Sonochem. 2017, 36, 173–181. [Google Scholar] [CrossRef]
- Ribeiro, M.d.M.; Valdramidis, V.P.; Nunes, C.A.; de Souza, V.R. Synergistic effect of thermosonication to reduce enzymatic activity in coconut water. Innov. Food Sci. Emerg. Technol. 2017, 41, 404–410. [Google Scholar] [CrossRef]
- Purkayastha, M.D.; Kalita, D.; Das, V.K.; Mahanta, C.L.; Thakur, A.J.; Chaudhuri, M.K. Effects of L-ascorbic acid addition on micro-filtered coconut water: Preliminary quality prediction study using 1H-NMR, FTIR and GC-MS. Innov. Food Sci. Emerg. Technol. 2012, 13, 184–199. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Kalita, D.; Mahanta, C.L.; Chaudhuri, M.K. Effect of additives on the quality of tender coconut water processed by nonthermal two stage microfiltration technique. LWT—Food Sci. Technol. 2014, 59, 1191–1195. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Gupta, K.; Mahanta, C.L. Shelf life enhancement and associated quality and sensory changes on refrigerated storage of tender coconut water subjected to non-thermal microfiltration and treated with additives. J. Food Sci. Technol. 2019, 56, 3408–3421. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; Rao, L.; Zhao, L.; Wu, X.; Wang, Y.; Liao, X. Effect of high hydrostatic pressure processing on the structure, functionality, and nutritional properties of food proteins: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4640–4682. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Rao, L.; Zhao, L.; Wu, X.; Wang, Y.; Liao, X. High pressure processing combined with selected hurdles: Enhancement in the inactivation of vegetative microorganisms. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1800–1828. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical Carbon Dioxide Applications in Food Processing. Food Eng. Rev. 2021, 13, 570–591. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]
- Bigi, F.; Maurizzi, E.; Quartieri, A.; De Leo, R.; Gullo, M.; Pulvirenti, A. Non-thermal techniques and the “hurdle” approach: How is food technology evolving? Trends Food Sci. Technol. 2023, 132, 11–39. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Gavahian, M.; Franco, D.; Zhang, W.; Mousavi Khaneghah, A.; Guerrero-Sánchez, Y.; Lorenzo, J.M. Impact of pulsed light processing technology on phenolic compounds of fruits and vegetables. Trends Food Sci. Technol. 2021, 115, 1–11. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K. Opportunities and Challenges in Application of Ultrasound in Food Processing. Crit. Rev. Food Sci. Nutr. 2011, 51, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Urošević, T.; Povrenović, D.; Vukosavljević, P.; Urošević, I.; Stevanović, S. Recent developments in microfiltration and ultrafiltration of fruit juices. Food Bioprod. Process. 2017, 106, 147–161. [Google Scholar] [CrossRef]

| Bioactivity | In Vivo/In Vitro Model | Treatments | Results | Potential Mechanisms | Reference |
|---|---|---|---|---|---|
| Antioxidant | Wistar albino rats | Orally given 10 mL, 20 mL, 30 mL, and 40 mL of the coconut water extract for 28 days | Increased MDA, SOD, GSH, and vitamin C | / | [34] |
| Murine hepatocytes | CWC (200 and 400 μg/mL) | Mitigated the oxidative damage induced by H2O2 | Inhibition of NF-κB, activation of PI3K/Akt/Nrf2, reduction in apoptosis by the SAPK/JNK/Bax pathway | [29] | |
| Anti-inflammatory | Rat paw edema model | 4 mL/100 g dose oral treatment | Inhibition of 42.52% for young CW and 25.94% for mature CW | Inhibition of Nos2 mRNA and iNOS protein expression through AKT and JNK signaling pathways | [14] |
| Hepatocytes isolated from male Sprague–Dawley rats | TCW (v/v) and sterile water mixed to 2 X William E | Improved hepatocyte viability and protected hepatocytes against cytokine-mediated cell death | Suppressed IL-1β-mediated increase in Nos2, Tnf, and Il6 mRNA and increased heme oxygenase 1 (HMOX1) protein Inhibited iNOS expression through activation of AKT and JNK pathways | [35] | |
| Anti-proliferative | Human glioma cell lines 1321N1 and U87MG | Incubation with concentrations from 0.25 to 2.0 mM for 72 h | IC50 values of 1.25 and 1.85 mM | The long hydrophobic C-terminal sequence penetrates the membrane core region, driving the translocation of Cn-AMP2 across the cancer cell membrane to attack intracellular targets and induce anti-proliferative mechanisms | [15] |
| Cardioprotective effects | Male Sprague–Dawley rats | 4 mL/100 g of body weight | Lowered the systolic blood pressure, reduced serum triglycerides and free fatty acids | Inhibition of lipid peroxidation, upregulation of antioxidant status, and improved insulin sensitivity | [11] |
| Male Sprague–Dawley rats | Oral administration of mature CW (4 mL/100 g body weight) for 45 days | Significant reduction in blood glucose and glycated hemoglobin levels, increased plasma insulin levels | Reduction in pancreatic damage induced by alloxan Stimulated cell regeneration | [32] | |
| Male albino rats (Sprague–Dawley) | Oral administration with TCW (4 mL/100 g/day for 30 days) and single-dose (250 mg/100 g) intravenous post-treatment with lyophilized TCW | Reduced oxidative stress and exerted antithrombotic effects | Scavenging free radicals; inhibition of platelet aggregation; and adhesion by inhibiting fibrinogen and enhancing the release of anti-aggregatory nitric oxide | [36] | |
| Male Sprague–Dawley rats | Oral MCW treatment (4 mL/100 g body weight) for 45 days | Reduce the concentration of blood glucose and HbA1c; increased contents of nitric oxide synthase, liver and plasma arginine, and urinary nitrite | Mediated by L-arginine-nitric oxide pathway | [13] | |
| Antimicrobial | Apparently healthy Wistar (AHW) rats | Daily orogastric administration of various volumes of CW (0.5–2.0 mL) for four weeks | Inhibitory effects on the growth of Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, and so on | / | [37] |
| Anti-urolithiatic | Male Wistar rats | / | Prevented the adherence of crystals on renal tissues and reduced the number of crystals formed in the urine | / | [38] |
| Adult volunteers with no prior history of nephrolithiasis | Consumed 1.92 L of either Taste of Nirvana pure coconut water or tap water daily for four days | Significantly increased urinary citrate (29%), urinary potassium (130%), and urinary chloride (37%), without affecting urine pH, lowering the likelihood of stones | / | [39] | |
| Decreased the number and size of struvite crystals in the gel medium | [40] | ||||
| Protection on murine system | Male Wistar rats | Protective effects on heat stress-induced testicular damage | Reversed the HS-induced proinflammatory state through activation of the Nrf2-assisted antioxidant response | [41] | |
| Cutaneous wound healing | Ovariectomized rats | Oral administration of young coconut juice | Beneficial effects on cutaneous wound healing | Significantly increased density of immunostaining for ER-α and ER-β in keratinocytes, fibroblasts, white blood cells, fat cells, and so on | [42] |
| Processing Technology | Treatment Conditions | Quality Attributes | Microbial Inactivation | Enzyme Inactivation | Reference |
|---|---|---|---|---|---|
| High hydrostatic pressure (HHP) | Pressure: 550 MPa; Time: 3 min; Temperature: 10 °C (Initial) | - | The counts of inoculated Type E C. botulinum and Group II Clostridium sp. were not significantly reduced and remained constant regardless of the initial dissolved oxygen content or storage temperature (4 and 20 °C). | - | [59] |
| Pressure: 593 MPa; Time: 3 min; Temperature: 4 °C | - | The counts of inoculated E. coli O157:H7, Salmonella, and L. monocytogenes were reduced to <1 CFU/mL during storage at 4 °C for 54 and 75 days for Florida CW and Brazil CW, respectively. Additionally, the uninoculated CW sample was microbiologically stable (<2 log CFU/mL) during storage at 4 °C for 120 days. | - | [4] | |
| Pressure: 500 MPa; Time: 5 min; Temperature: Room temperature | The amino acids, total protein, sugars, phenols, ascorbic acid, and antioxidant capacity were retained after storage at 4 °C for 25 days. The color, aroma, flavor, and overall acceptability were retained. | The counts of total aerobic bacteria (TAB) and molds and yeasts (M&Y) were reduced to undetectable levels and remained below 2 and 1.3 log (CFU/mL), respectively, during storage at 4 °C for 25 days. | - | [60] | |
| High-pressure carbon dioxide (HPCD) | Pressure: 8 and 12 MPa; Time: 5–60 min; Temperature: 22, 30, 35, 40, and 45 °C | The dry matter, soluble solids, sugars, and vitamin content were retained while the pH and volatile fraction were reduced. The sensory attributes were not affected. | 12 MP, 40 °C, and 30 min were the optimal HPCD conditions to induce 5-log (CFU/mL) reductions in mesophilic microorganisms, lactic acid bacteria, yeasts, and molds and a 7-log reduction in total coliforms. | - | [61] |
| Pressure: 12 MPa; Time: 30 min; Temperature: 40 °C | The short- and medium-chain alcohols were depleted and the sensory attributes were not affected. | - | - | [62] | |
| Pressure: 12 MPa; Time: 1–60 min; Temperature: 25, 30, 35, and 40 °C | - | 2-log (CFU/mL) reductions in inoculated S. enterica were achieved when HPCD was conducted at 40 °C. For natural microbial flora, HPCD at 12 MP, 40 °C, and 30 min assured sufficient microbial inactivation, but the product was microbiologically unstable during storage at 4 °C. Combining HPCD with ultrasound (12 MPa, 40 °C, 15 min, and 10 W) assured a shelf life at 4 °C for 4 weeks. | - | [63] | |
| Cold plasma | Voltage: 18 to 28 kV; Gap of dielectric barriers: 1.5 cm; Working gas: air; Time: 1–3 min | The total fatty acid was increased while the ascorbic acid, DPPH free radical scavenging activity, and transmittance were decreased. The total phenol content was decreased until the treatment time reached 3 min. | Around a 2-log (CFU/mL) reduction in microbial load was achieved. | Treatment at 18 kV and 2.85 min resulted in a residual POD activity of 22%. | [64] |
| Frequency: 200, 400, 550, and 730 Hz; Voltage: 15 and 20 kV; Gap of dielectric barriers: 1.5 cm; Working gas: air; Time: 15 min | The physicochemical properties (pH, total soluble acids, titratable acidity, and color) were not significantly affected. | - | Increase in frequency promoted the inactivation of POD activity, with the smallest residual activity of 28% at 730 Hz. | [65] | |
| Voltage: 90 kV; Gap of dielectric barriers: 5 cm; Working gas: dry air; Time: 0.5–2 min | The pH, total titratable acid (TTA), and L* value were significantly decreased, and the conductivity, total soluble solids (TSS), ascorbic acid, transmission, and a* value were not affected. | At a plasma treatment time of 2 min, a 1.3-log (CFU/mL) reduction in inoculated S. enterica was achieved. | - | [66] | |
| Voltage: 90 kV; Gap of dielectric barriers: 5 cm; Working gas: dry air (78% N2, 21% O2 and traces of other gases) and modified air M65 (65% O2, 30% CO2, 5% N2); Time: 2 min | The pH was not affected but decreased using air and M65 gas, respectively. The total titratable acid (TTA) was decreased but not affected using air and M65 gas, respectively. The total soluble solids (TSS) was not significantly affected. The L* and b* values were not greatly impacted while the a* values were decreased. | For E. coli, 1.09- and 1.79-log (CFU/mL) reductions were achieved using air and M65 gas; while for L. monocytogenes, a 2.03-log (CFU/mL) reduction was achieved using air, and no microbial recoveries were obtained with M65 air by 24 h post refrigerated storage. | - | [67] | |
| Voltage: 18, 23, and 28 kV; Gap of dielectric barriers: 1.5 cm; Working gas: air; Time: 1–5 min | - | - | The times required for half-maximal activity values for POD were 0.84, 1.67, and 2.53 min at 18 kV, 23 kV, and 28 kV, respectively, and for PPO were 0.67, 1.18, and 1.35 min, respectively. POP was more resistant to cold plasma than PPO. | [68] | |
| Pulsed electric field (PEF) | Electric field strength: 20–30 kV/cm; Specific energy: 80–120 kJ/L; Pulse width: 4 µs; Flow rate: 100 L/h; Pulse frequency: 130–350 Hz; Temperature: 30–40 °C | PEF treatment decreased the scores for sensory attributes and changed the volatile flavor profiles. | The optimized PEF treatment combining 40 °C with a field strength of 22.5 kV/cm and specific energy of 119 kJ/L decreased the count of inoculated E. coli K12 and Listeria innocua by 6.60 and 5.90 (CFU/mL), respectively. | PEF treatment increased the PPO activity by 17%, while it decreased the POD activity by 78%. | [69] |
| Pulsed light (PL) | Fluence rate: 8.1–756 J/cm2; Time: 0–0.25 min | - | PL treatment at 95.2 J/cm2 decreased the count of inoculated E. coli by 5.33-log (CFU/mL). | - | [70] |
| Fluence rate: 127–3586 J/cm2; Time: 0–6 min | - | PL treatment at 465 J/cm2 decreased the counts of inoculated E. coli, Bacillus cereus, and L. monocytogenes by 5.12, 2.97, and 3.40 log (CFU/mL), respectively. | PL treatment at 2988 J/cm2 inactivated PPO and POD to <1% residual activity. PPO was more resistant to PL than POD. | [71] | |
| Fluence rate: 12.6–756 J/cm2; Time: 0.25–0.75 min | - | The optimized PL treatment with an input voltage of 1492 V, distance of 7.6 cm, and treatment time of 43 s (12 passes) decreased the aerobic plate count (APC) and yeast and mold (Y&M) by 5 and 4 log (CFU/mL), respectively. | - | [72] | |
| UV (UV-C) irradiation | UV-C light wavelength: 254 nm; Reduction equivalent fluence (REF): 0–400 mJ/cm2 | No significant loss in nine essential amino acids and sensory attributes were observed after UV-C treatment at 400 and 200 mJ/cm2, respectively. | - | UV-C treatment at 400 mJ/cm2 decreased the PPO and POD activity by 94 and 93%, respectively. PPO was more resistant to UV-C than POD. | [18] |
| UV-C light wavelength: 280 nm; Reduction equivalent fluence (REF): 0–70 mJ/cm2 | UV treatment did not affect the pH, titratable acidity, Brix, total phenolic content, mineral content, and sugars, but brought slight changes in the color measurement and ascorbic acid concentration. The sensory attributes were slightly decreased. | UV-C treatment at 71 and 20.3 mJ/cm2 decreased the counts of inoculated E. coli and L. monocytogenes by ≥5 log (CFU/mL), respectively. | - | [73] | |
| UV-C light wavelength: 254 nm; Reduction equivalent fluence (REF): 0–400 mJ/cm2 | - | UV-C treatment at 30 mJ/cm2 can decrease the counts of inoculated E. coli, S. Typhimurium, and L. monocytogenes by ≥5 log (CFU/mL). UV-C treatment at 30 and 120 mJ/cm2 decreased the contents of T1UV-C and MS2 surrogate viruses by ≥5 log (CFU/mL), respectively. | - | [74] | |
| UV-C light wavelength: 254 nm; UV dose: 802 and 425.46 mJ/mL in 1.6 and 3.2 mm reactor, respectively; Reynolds number: 198.8, 397.7, 596.4 | The physicochemical properties (pH, soluble solids, and density) were not significantly affected. | UVC treatment at Re 596.4 and 1.6 mm reactor decreased the counts of E. coli and L. monocytogenes by 5.27 and 4.18 log (CFU/mL), respectively. | - | [75] | |
| UV-C light wavelength: 254 nm; UV-C dose: 2.6 mW/cm2; Time: 0–0.4 min | - | Exposures to different sublethal stresses (i.e., acidification, desiccation) increased the exposure times (D) and UV-C energy dose values (D_UVC) necessary to reduce 90% of the population of E. coli, S. enterica, and L. monocytogenes. | - | [76] | |
| Light wavelength: 250–740 nm (peak between 400 and 420 nm); Nominal power: 400 W; Time: 1–60 min; Temperature: 25 °C | - | - | Treatment for 15 min decreased the POD and PPO activity by ~5% and ~8%, respectively. Treatment for 30 min decreased the POD and PPO activity by ~1% and ~2%, respectively. PPO was more resistant than POD. | [77] | |
| Ultrasound | Ultrasonic power: 60% amplitude with 6 s pulse; Frequency: 20 kHz; Time: 1–10 min; Temperature: 20 °C | Ultrasound treatment for 6 min retained the initial quality attributes. | The samples stored at room and refrigerated temperatures were spoiled within a week. The addition of nisin reduced the bacterial population by 2 log (CFU/mL) and the counts of yeast, molds, and E. coli to undetectable levels, and achieved a storage time of 2 weeks in a refrigerated condition. | Combining ultrasound treatment with nisin inactivated the PPO, POD, and PAL activity by 50, 30, and 35%, respectively. | [17] |
| Acoustic energy: 286 W/L; Frequency: 20 kHz; Time: 0–30 min; Temperature: 25 °C | - | - | Ultrasonic treatment for 30 min decreased the POD activity by around 27%. | [78] | |
| Acoustic energy: 448–717 W/L; Frequency: 20 kHz; Time: 5–15 min; Temperature: 20–80 °C | - | - | Ultrasonic treatment with acoustic energy > 500–550 W/L is required to promote a significant decrease in PPO and POD activity. Acoustic treatment with energies of 629.90 and 655.80 W/L can achieve total inactivation of PPO and POD, respectively. | [79] | |
| Ozone | Ozone loads: 0.075–0.37 mg/mL; Temperature: 10–30 °C | The physicochemical properties (pH, total soluble acids, titratable acidity, and color) were not significantly affected. | - | No detectable activity of POD was found after all ozone treatments. | [65] |
| Ozone loads: 20 mg/L at a flow rate of 1 L/min; Time: 1–10 min; Temperature: 25 °C | Ozone treatment for 5 min retained the initial quality attributes. | The samples stored at room and refrigerated temperatures were spoiled within a week. The addition of nisin achieved a storage time of 3 weeks in a refrigerated condition. | Combining ozone treatment with nisin inactivated PPO and POD activity to undetectable levels. | [17] | |
| Microfiltration | Filter: 0.8 and 0.45 μm pore size; Additive: L-ascorbic acid | The addition of L-ascorbic acid to micro-filtered coconut water retained methyl-α-D-rhamnopyranoside (prime glycoside detected in micro-filtered coconut water), controlled the formation of free fatty acids, and delayed rancidity during storage at 4 °C. | - | - | [80] |
| Filter: 0.8 and 0.45 μm pore size; Additive: ascorbic acid, citric acid, and L-cysteine | The sensory qualities remained stable during storage at 4 °C for 46 days. The pH and total soluble solids did not change significantly, while total titratable acidity and simple sugars increased. | The microbial load of coconut water in plastic bottles and glass bottles remained sterile during storage at 4 °C for 7 and 180 days, respectively. | - | [81] | |
| Filter: 0.8 and 0.45 μm pore size; Additive: citric acid, ascorbic acid, and orange honey | The physicochemical properties (pH, titratable acidity, total soluble solids, total simple sugars, free fatty acid, total reducing sugars) were not significantly affected. | No microbial count was detected on the total plate count agar and coliform plates during storage at 4 °C for 190 days. | The PPO activity was reduced while the PO and SNI activity were not changed. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Wang, W.; Wang, F.; Yang, P.; Yang, H.; He, X.; Liao, X. Research Progress in Coconut Water: A Review of Nutritional Composition, Biological Activities, and Novel Processing Technologies. Foods 2025, 14, 1503. https://doi.org/10.3390/foods14091503
Shi S, Wang W, Wang F, Yang P, Yang H, He X, Liao X. Research Progress in Coconut Water: A Review of Nutritional Composition, Biological Activities, and Novel Processing Technologies. Foods. 2025; 14(9):1503. https://doi.org/10.3390/foods14091503
Chicago/Turabian StyleShi, Shaoran, Wenxin Wang, Fengzhang Wang, Peiqing Yang, Huanzhi Yang, Xiyu He, and Xiaojun Liao. 2025. "Research Progress in Coconut Water: A Review of Nutritional Composition, Biological Activities, and Novel Processing Technologies" Foods 14, no. 9: 1503. https://doi.org/10.3390/foods14091503
APA StyleShi, S., Wang, W., Wang, F., Yang, P., Yang, H., He, X., & Liao, X. (2025). Research Progress in Coconut Water: A Review of Nutritional Composition, Biological Activities, and Novel Processing Technologies. Foods, 14(9), 1503. https://doi.org/10.3390/foods14091503







