Abstract
Coconut (Cocos nucifera L.) is a nutrient-rich plant extensively cultivated in tropical and subtropical regions. Coconut water (CW), the primary edible component of the fruit, has gained significant attention due to its nutritional value and increasing popularity as a functional beverage. In addition to its hydrating properties, CW is rich in essential nutrients such as sugars, minerals, and vitamins, which contribute to its diverse biological activities, including antioxidant, anti-inflammatory, anti-cancer, cardioprotective, and antimicrobial effects. However, CW’s high perishability and susceptibility to rapid deterioration present significant challenges for its preservation. The growing demand for natural and fresh CW has driven the development of innovative technologies aiming at extending its shelf life while maintaining its nutritional quality and sensory attributes. This review highlights recent research advancements in CW, focusing on its nutritional composition, biological activities, and innovations in preservation technologies. The aim is to facilitate the optimization of CW beverage formulations, promote the adoption of effective preservation methods, and drive the development of high-quality and consumer-appealing CW products.
1. Introduction
Coconut (Cocos nucifera L.) is a tropical fruit from the areca family, widely distributed in regions such as West and East Africa, Southeast Asia, the Pacific islands, as well as the Americas [1,2,3]. Globally, coconut cultivation covers 12.3 million hectares, yielding 61.4 million tons annually. The main producers include Indonesia, the Philippines, India, Brazil, and Sri Lanka, where coconut palm grows naturally [4]. Although the precise number of coconut tree species remains uncertain, three primary groups have emerged through both natural and artificial selection: the tall (or typical) variety, the dwarf (or nana) variety, and hybrid varieties [2]. Tall coconut species are often cultivated for oil production, whereas dwarf species are favored for the production of fresh coconut water (CW) and use in desserts. Hybrid coconut species combine the advantageous traits of both tall and dwarf coconut cultivars, meaning they exhibit a greater nut yield, higher oil extraction rates, and markedly improved pest/disease resistance versus conventional varieties, making them superior for commercial cultivation [2,5]. Coconut cultivation serves as a crucial strategy for enhancing food security in tropical regions, providing essential nutrients such as protein and fat, as well as a sustainable source of energy, while simultaneously generating valuable employment opportunities [6].
A mature coconut fruit consists of approximately 51.7% kernel, 38.5% shell, and 9.8% water. The kernel, which is the solid endosperm, is enclosed within a hollow shell that contains CW, alternatively referred to as the liquid endosperm. CW constitutes approximately 25% of the total weight of the fruit [7]. Tender coconut water (TCW) refers to the clear, slightly sweet, and aqueous component of an immature coconut [8]. It contains about 95.5% water, 0.1% fat, 4% carbohydrate, 0.02% calcium, 0.01% phosphorus, 0.5% iron, and some amino acids, vitamin B complex, vitamin C, and mineral salts [9]. TCW is rich in natural phytonutrients and has gained popularity as a natural energy drink [10]. Furthermore, TCW can be preserved for a duration of up to 15 days in ambient conditions after being harvested from trees. As the coconut fruit matures, the volume of CW decreases, and its chemical composition undergoes changes: the total soluble solids (TSS), ash content, and mineral content decrease, whereas the fat and protein content increase.
CW possesses various functional properties, including antioxidant [11], antimicrobial [12], antidiabetic [13], anti-inflammatory [14], anti-hyperlipidemic, anti-cancer [15], anti-ulcerogenic, and cardioprotective effects [16]. It has been incorporated into traditional medicine and dietary interventions in numerous cultures, particularly in Africa, India, and the Philippines, for the treatment of a multitude of conditions, including gastroenteritis, coronary heart disease, kidney diseases such as urolithiasis, and urinary tract infections. As a result of its nutritious and functional attributes, CW has gained popularity as a natural beverage, attracting increasing consumer interest [17]. CW has solidified its status as a premium functional beverage, with surging global demand propelling it to become one of the most rapidly expanding categories in the plant-based drinks sector [2,18].
Although CW is a popular natural functional beverage, it is highly susceptible to deterioration due to microbial contamination and enzymatic activity once exposed to the external environment [8]. This susceptibility drastically shortens its shelf life and can lead to substantial changes in its biochemical composition and organoleptic properties, resulting in the loss of nutritional values [19]. Therefore, it is imperative to utilize food processing technologies to ensure the preservation of CW and the successful commercialization of its related products. Over the past few decades, the pursuit of pioneering food processing technologies has intensified, with a focus on techniques such as high-hydrostatic-pressure processing, high-pressure carbon dioxide treatment, cold plasma application, pulsed electric fields, pulsed light technology, UV-C irradiation, ultrasonic processing, ozone treatment, and microfiltration. These innovative approaches are meticulously designed to enhance the preservation of both the nutritional integrity and sensory qualities of food products.
In summary, the coconut stands as a multifaceted fruit, boasting remarkable nutritional benefits and a plethora of natural nutrients. To the best of our knowledge, no recent reviews have systematically summarized the diverse aspects of coconut water (CW). This article provides a comprehensive overview of CW, emphasizing its nutritional constituents, bioactive properties, and the latest progress in processing technologies (Figure 1). The insights garnered are intended to steer the advancement, intricate processing, and enhanced value creation within the CW industry.
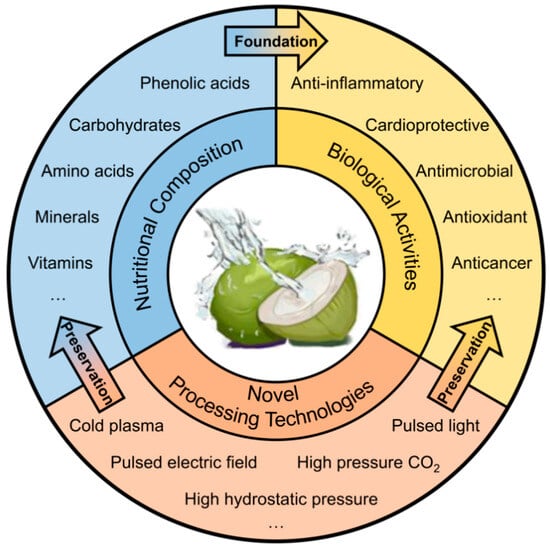
Figure 1.
Overview of the nutritional composition, biological activities, and novel processing technologies on coconut water.
2. Nutritional Composition of CW
CW is widely recognized as a functional beverage due to its rich and balanced nutritional composition. It provides a well-balanced blend of various compounds, such as carbohydrates, vital minerals (such as potassium (K), calcium (Ca), and magnesium (Mg)), B-complex vitamins and vitamin C, phenolic acids, and amino acids (Figure 2). These components not only contribute to its hydrating properties but also enhance its potential health benefits, making CW a naturally refreshing and nutritionally valuable drink.
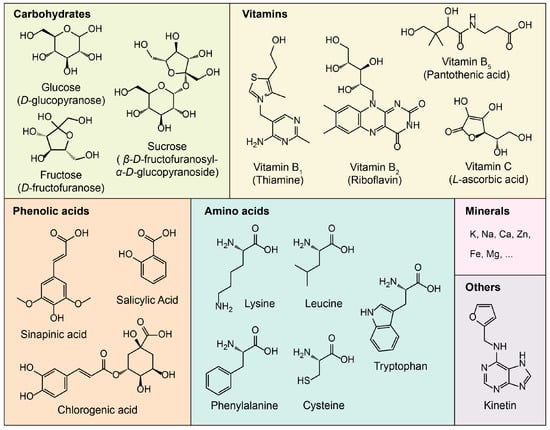
Figure 2.
Primary nutritional compounds of coconut water.
2.1. Carbohydrates
The carbohydrates in CW are predominantly composed of natural sugars, with glucose, fructose, and sucrose being the dominant types [20]. These sugars serve as an immediate and accessible source of energy, with their content varying depending on the coconut variety and its stage of maturity. According to USDA data in 2018, one hundred milliliters of pure CW contain 3.39 g of carbohydrates, including 1.27 g of sugars. TCW, obtained from immature coconuts aged between 5 and 7 months, exhibits TSS ranging from 3.8 to 6.9 °Bx, with a total sugar content varying from 2.08 to 6.52% [20]. The levels of sugar and TSS fluctuate during maturation, initially increasing and then decreasing [10]. During the initial stages, specifically 4 to 5 months post-pollination, CW exhibits a sour and astringent taste profile. At 7–8 months, CW acquires a sweet flavor profile, accompanied by an increase in sugar content to approximately 5 to 6% [21]. A typical 100 mL sample of TCW comprises approximately 95% water, 5.5–7% non-reducing sugars, and 4–4.5% reducing sugars. Due to its relatively high sugar content, TCW is prone to microbial contamination, necessitating processing methods to eliminate pathogens and prolong shelf life [22]. The total sugar content in mature CW varies depending on cultivar and geographic location, typically ranging between 1.8 and 4.4 g/100 mL. In comparison, mature CW contains 0.2% reducing sugar, while TCW has a higher sugar content of 4.4% [23].
2.2. Minerals
CW is highly valued for its rich mineral content, which is essential for maintaining hydration and supporting various bodily functions. The primary minerals present in CW include K, sodium (Na), Ca, Mg, zinc (Zn), and iron (Fe), with K being the most prevalent among them [10]. A 100 mL serving of TCW typically consists of 95% water and notable mineral concentrations, such as K (290 mg), Fe (106 mg), Ca (44 mg), Na (42 mg), and copper (Cu, 26 mg) [22]. In another work, fresh TCW, a naturally sterile liquid harvested from immature coconuts aged 5 to 7 months, showed the following mineral composition: Na (1.75–31.4 mg/100 g), K (203.7–249.0 mg/100 g), Ca (3.6–27.35 mg/100 g), Mg (6.4–25.0 mg/100 g), and Fe (0.02–4.2 mg/100 g) [20]. After an 8-month period, the flavor intensity of CW diminishes, whereas its mineral content increases. Furthermore, the mineral and nutrient compositions of CW can vary depending on regional factors [21].
2.3. Vitamins
Vitamins are essential for various physiological functions in the human body. CW contains several water-soluble B-complex vitamins, including thiamine (B1), riboflavin (B2), pantothenic acid (B5), pyridoxine (B6), biotin (B7), and folate (B9). In addition to these B vitamins, CW also contains vitamin C, a key dietary antioxidant that supports immune function and aids in tissue repair [24]. These vitamins play crucial roles in energy production, metabolism, and maintaining overall health [24].
2.4. Phenolic Compounds
The composition of phenolic catechins in CW has scarcely been reported, with salicylic acid identified as the predominant phenolic compound. A comprehensive GC-MS analysis has revealed the presence of 19 water metabolites, encompassing phenols, within CW [10]. Among the 41 compounds identified, the major phenolic compounds include chlorogenic acid, caffeic acids, epicatechin, L-epicatechin, proanthocyanidin B1 and B2, catechin, tocopherols, and salicylic acid [25,26]. Additionally, the content of phenolic compounds in CW increases with maturity, enhancing its antioxidant properties and nutritional value [27].
2.5. Amino Acids
Amino acids are essential for various metabolic pathways crucial to life [28]. CW contains abundant free amino acids, highlighting its potential as a valuable nutritional resource, including essential ones such as lysine, leucine, cysteine, phenylalanine, histidine, and tryptophan [29]. Yannam et al. found that CW contains nine essential amino acids, with tryptophan being the most abundant [18]. Halim et al. used high-performance liquid chromatography (HPLC) to demonstrate that the total amino acid content increases as the coconut matures [28]. The primary amino acids identified were glutamine, alanine, and tyrosine [28]. Interestingly, CW contains higher levels of alanine, arginine, cysteine, and serine compared to milk, and these amino acids might be associated with the discoloration of CW [30]. TCW, in particular, is rich in arginine, alanine, cysteine, and serine [31]. Among these, arginine plays a significant role in producing nitric oxide, which enhances blood flow, promotes vasodilation, and boosts endurance [28]. Furthermore, L-arginine supports growth hormone secretion, aids muscle protein synthesis, and has been found to transform pancreatic cells into insulin-producing cells, potentially reversing diabetes-related effects [32].
2.6. Other Compounds
In addition to sugars (e.g., glucose, fructose, and sucrose), minerals, vitamins, phenolic acids, and amino acids, CW also contains certain amounts of phytohormones, particularly cytokinin, as well as other compounds like lipids, nitrogenous compounds, and enzymes [29]. Furthermore, CW has been found to contain a variety of volatile aromatic compounds, including esters, alcohols, aldehydes, phenols, and ketone, with ester compounds contributing the most to its aroma profile [25].
3. Biological Activities of Coconut Water
CW is a refreshing and rehydrating beverage rich in vitamins, minerals, electrolytes, amino acids, growth-promoting factors, and proteins [33]. It is free from fat and low in calories. CW possesses various therapeutic properties as listed in Table 1, with various applications ranging from foods to cosmetics. Numerous scientific studies have reported antioxidant activities in tender coconut water, but the comprehensive information on other biological activities of CW is scare. Therefore, the bioactivities of CW are reviewed in this article to provide an integrated understanding.

Table 1.
Bioactivities of coconut water and potential mechanisms.
3.1. Antioxidant Activity
The aromatic dwarf variety of CW has been reported to contain various phenolic compounds. Key bioactive constituents include catechin and several phenolic acids, such as syringic acid, salicylic acid, m-coumaric acid, p-coumaric acid, and p-hydroxybenzoic acid, which serve as significant sources of antioxidants in the prevention of diseases [43]. Caffeic acid, identified in CW from the Malayan Green Dwarf variety, has been reported to contribute to its antioxidant activities [44]. CW from young Malayan Yellow Dwarf exhibited antioxidant and anti-aging effects, with its rich bioactive composition holding promise as a natural functional ingredient for anti-aging skincare, underscoring its cosmetic industry potential [28]. The catechin extracted from CW showed antioxidant, antibacterial, and anti-cancer effects [45]. Interestingly, green CW exhibits a stronger free radical scavenging activity, higher antioxidant and anti-aging activities, and a richer profile of bioactive compounds compared to mature CW [28,46]. Furthermore, the DPPH and ABTS radical scavenging activities of CW were found to be comparable to or even higher than those of ascorbic acid, while its anti-collagenase activity surpassed that of epigallocatechin gallate (EGCG) [28].
In addition, CW also contains a group of phytohormones, namely cytokinins such as kinetin, kinetin riboside, trans-zeatin, trans-zeatin riboside, and trans-zeatin glucoside [47,48]. A previous study showed that kinetin and zeatin are used in cosmetic products due to their antioxidant and anti-aging properties [24]. Moreover, the essential oil extracted from CW, primarily comprising esters (58.3%) and ketones (33.5%), has also demonstrated notable free radical scavenging activity and antioxidant potential [49]. An in vivo study on Wistar albino rats confirmed that CW extract can enhance antioxidant capacity, highlighting its potential health benefits [34].
Interestingly, a recent study also showed that CW could inhibit the browning of ‘Gala’ apple wedges during storage at 4 ± 1 °C for 9 days, suggesting its promising application as a natural anti-browning agent in fresh-cut products [50]. Therefore, the richness of beneficial bioactive compounds in CW makes it a potent natural source of antioxidants and anti-aging compounds.
3.2. Anti-Inflammatory Activity
Rao et al. explored the anti-inflammatory activity of CW of different maturation stages (young and mature) with a rat paw edema model (4 mL/100 g dose orally) of inflammation using plethysmometer [14]. The results revealed that the maximum percentage inhibition observed was 42.52% for young CW and 25.94% for mature CW, respectively. Notably, the anti-inflammatory effect of young CW was significantly greater than that of orally administered ibuprofen at a dose of 400 mg/70 kg. These findings strongly indicate the potential use of young CW for a potent anti-inflammatory effect and mature CW for a moderate anti-inflammatory effect. Furthermore, the effect of TCW on primary rat hepatocyte viability, cytokine-induced gene expression, and proinflammatory signaling in an in vitro model of sepsis was also verified. TCW could represses hepatocyte IL-1β-mediated inflammatory damage by inhibiting Nos2 mRNA and iNOS protein expression through the AKT and JNK signaling pathways in vitro, with decreasing hepatocyte expression of pro-inflammatory cytokines and increased expression of acute-phase proteins Serpine1 and HMOX1, demonstrating that TCW could potentially be beneficial as a therapeutic agent in conditions where hepatic Nos2 expression is upregulated [35].
3.3. Ant-Proliferative Activity
Anionic host defense peptides (AHDPs) are gaining attention as key elements of the innate immune system and as potential antimicrobial agents with unique modes of action. Cn-AMP2 (TESYFVFSVGM), an AHDP from green CW of the plant Cocos nucifera, showed anti-proliferative activity against the human glioma cell lines 1321N1 and U87MG, with IC50 values of 1.25 and 1.85 mM, respectively [15].
3.4. Cardioprotective Protect
The richness of macro- and micro-nutrients in TCW is reported to have hypolipidemic, cardioprotective, and hepatoprotective effects [51]. The therapeutic properties of TCW make it useful as a remedy for a lot of ailments. Male Sprague–Dawley rats fed with a fructose-rich diet and treated with TCW (4 mL/100 g of body weight) for 3 subsequent weeks showed a significantly lowered systolic blood pressure, serum triglycerides, and free fatty acids. Plasma glucose and insulin levels and lipid peroxidation markers such as MDA, hydroperoxides, and conjugated dienes were also significantly reduced [11]. Oral administration of mature CW in diabetic rats showed a significant reduction in blood glucose and glycated hemoglobin levels, with an improvement in plasma insulin levels, which exerted significant antihyperglycemic potential and could be developed as a potent drug candidate or nutraceutical for the management of diabetes and associated complications [32]. Furthermore, both chronic oral administration of TCW (4 mL/100 g/day for 30 days) and acute intravenous treatment with lyophilized TCW (250 mg/100 g, single dose) significantly attenuated isoproterenol-induced oxidative stress and demonstrated notable antithrombotic effects [36]. When compared to streptokinase, one of the most effective thrombolytic drugs, TCW demonstrated a superior antioxidant activity and comparable antithrombotic efficacy. Similarly, oral MCW treatment (4 mL/100 g/day, 45 days) significantly lowered both blood glucose and HbA1c levels in diabetic rats while displaying dual antidiabetic and antithrombotic effects, likely via the L-arginine-NO pathway [13].
TCW treatment (oral: 4 mL/100 g/day for 30 days; 250 mg/100 g single dose) significantly reduced ISO-mediated oxidative stress and exhibited marked antithrombotic activity. Recent studies with TCW indicated that it is a rich source of cardioprotective factors viz. L-arginine [52], magnesium, potassium, calcium, and vitamin C, which are known to reduce the risk of coronary heart disease.
3.5. Antimicrobial Activity
The biosafety of CW and coconut oil was investigated by assessing their effects on the types and population of bacterial flora in the ileum of apparently healthy Wistar (AHW) rats. After four weeks of daily orogastric administration of various volumes of CW (0.5 mL, 1.0 mL, 1.5 mL, and 2.0 mL, respectively), it was observed that the CW exhibited inhibitory effects on the growth of all tested bacteria (Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Shigella flexneri, Serratia marscense, and Morganella morganii) isolated from the ileum of the rats with diameter zones of inhibition ranging from 7.50 ± 0.50 mm to 23.00 ± 2.00 mm [37]. In the in vivo assay, CW reduced the population of the ileal bacterial flora of the rats, with the highest effect on Escherichia coli from 2.39 × 10 to 1.23 × 10 cfu/mL after 28 days of administration [37].
An in vitro experimental study was conducted to evaluate the antimicrobial efficacy of TCW in its natural state on Streptococcus mutans [12]. However, neither fresh nor pasteurized TCW exhibited antibacterial activity, whereas chlorhexidine, used as a positive control, demonstrated effective S. mutans inactivation. Moreover, natural bioactive antimicrobial peptides have been found from green coconut water [53]. CW is antiseptic and acts as a mouth cleanser. The antibacterial peptides termed Cn-AMPs have great potential to become new natural antibiotics [54].
3.6. Other Health Benefits
CW was also used to treat various ailments, including hepatic disorders, renal disorders, gastric disorders, and reproductive disorders. The use of TCW was recommended in cases of gastroenteritis and for urinary stone dissolution [55,56]. A recent anti-urolithiatic study involving male Wistar rats proved that CW prevented the adherence of crystals on renal tissues and reduced the number of crystals formed in the urine [38]. Other research suggests that CW increases the urinary excretion of potassium chloride, and citrate in humans, thus lowering the likelihood of stones [39]. In addition, it was reported that increasing concentrations of CW and fermented CW could decrease the number and size of struvite crystals that grew in a gel medium, indicating the antioxidant property and a marginal inhibitory effect during in vitro struvite crystallization, but anti-uropathogenic effects of CW were not found; meanwhile, fermented CW showed potential antioxidant, anti-uropathogenic, and anti-struvite urolithiatic properties [40].
TCW was also proven to have protective efficacy on heat stress-induced testicular damage in a murine system of male Wistar rats. The results indicated that TCW treatment could restore excess generation of oxygen radicals following the suppression of antioxidant capacity and augmentation of lipid peroxidation in murine testicles. The intervention also mitigated HS-induced inflammation via Nrf2 pathway activation, subsequently ameliorating testicular damage [41]. Furthermore, concentrated CW and its active constituent, shikimic acid, mitigated the oxidative damage induced by H2O2 in freshly isolated murine hepatocytes. This cytoprotection was mediated through coordinated regulation of three critical pathways: NF-κB inhibition (anti-inflammatory), PI3K/Akt/Nrf2 activation (antioxidant), and SAPK/JNK/Bax modulation (anti-apoptotic) [29].
Previous studies have reported that the oral administration of young coconut juice to ovariectomized rats can accelerate wound healing, which is associated with a significantly higher density of immunostaining for ER-α an ER-β in keratinocytes, fibroblasts, white blood cells, fat cells, sebaceous gland, skeletal muscles, and hair shafts and follicles. These findings demonstrated the significant wound-healing potential of young coconut juice, suggesting its therapeutic value for cutaneous repair [42]. In addition, studies have determined the biochemical properties of CW during the sprouting process, indicating that CW can be used as an adjuvant therapeutic food for children with mineral deficiencies.
4. Novel Processing Technologies for Coconut Water
Conventional thermal processing methods, such as pasteurization and sterilization, have been widely used to produce CW products by major commercial manufacturers like Coco-Cola, Pepsi CO, and Vita Coco [4]. Nevertheless, these methods can lead to the inactivation of heat-sensitive nutrients, degradation of volatile flavor compounds, and formation of undesirable chemical derivatives, which can make the CW less appealing to consumers [57,58]. In recent decades, there has been a growing interest in the development of innovative food processing technologies, including high-hydrostatic-pressure processing, high-pressure carbon dioxide, cold plasma, pulsed electric field, pulsed light, UV-C irradiation, ultrasound, ozone, and microfiltration, aimed at producing food products with superior preservation of nutritional and organoleptic properties. In this section, we systemically review the studies on the applications of these technologies to CW, focusing specifically on quality preservation, microbial reduction, and enzyme inactivation (Table 2).

Table 2.
Effects of nonthermal processing technologies on the quality attributes, microbial inactivation, and enzyme inactivation of coconut water.
4.1. High-Hydrostatic-Pressure (HHP) Processing
HHP processing applies pressures ranging from 100 to 800 MPa at moderate or elevated temperatures, typically employing water as the pressure transfer medium, in order to apply pressure instantaneously and uniformly throughout the food system [83]. It has been proven effective in reducing microbial counts with minimal effects on quality attributes and is now commercially applied to various food products, such as juices and beverages, meat products, and fruit and vegetable preparations [84,85].
Unfortunately, HHP is not considered a validated process for eliminating the spores of Clostridium botulinum in low-acid products (pH > 4.6), including CW with a pH range between 4.8 and 5.7 [59]. After HHP treatment at 550 MPa and 10 °C for 3 min, the total counts of inoculated non-toxigenic C. botulinum spores in CW were not significantly reduced and remained constant during the 61 days of storage at 4 °C, regardless of the initial dissolved oxygen content [59]. Storage of HHP-treated CW at 10 and 20 °C also showed no growth of C. botulinum, but there was a significant increase in total aerobic counts within 10 and 4 days, respectively, suggesting the limited capacity of HHP to inactivate all spoilage microorganisms. By supplementing CW with selected germinants, amino acids, and nutrient-rich laboratory media (TPGY broth), the authors concluded that CW was deficient in some essential nutrients required by C. botulinum to grow.
Raghubeer et al. [4] processed CW at 593 MPa and 4 °C for 3 min and also detected no growth of C. botulinum or toxin production during storage at 4 and 10 °C for 45 days. However, this absence of growth and toxin production was also noted in untreated samples, indicating the presence of naturally occurring inhibitory compounds in CW. Despite this observation, HHP was demonstrated to be effective in eliminating inoculated strains of E. coli O157:H7, Salmonella, and Listeria monocytogenes to <1 CFU/mL and producing a microbiologically stable (<2 log) product during storage at 4 °C for 120 days without the development of off odors and while maintaining a taste similar to fresh CW. However, given the concerns about the presence of C. botulinum in HHP-treated CW, further research is needed to explore hurdle approaches to enhancing the inactivation efficiency of C. botulinum and fully accomplish the potential of HHP technology for CW products.
4.2. High-Pressure Carbon Dioxide (HPCD)
HPCD technology utilizes sub- or supercritical carbon dioxide (CO2, Tc = 31.1 °C, Pc = 7.38 MPa), which exhibits properties that combine the low viscosity of a gas, intermediate diffusivity, and the high density of a liquid. These unique characteristics have led to the development of HPCD for a wide range of food processing applications, including preservation, extraction, encapsulation, and drying [86]. Furthermore, CO2 offers several advantageous properties. This Generally Recognized as Safe (GRAS)-certified material is non-toxic, cost-efficient, widely accessible, and easily removable.
To achieve the pasteurization of CW using HPCD, Cappelletti et al. [61] optimized the processing conditions, including the pressure, temperature, and treatment time. The optimized HPCD treatment, conducted at 12 MPa and 40 °C for 30 min, proved effective in achieving a 5-log (CFU/mL) reduction in mesophilic microorganisms, lactic acid bacteria, yeasts, and molds, as well as a 7-log (CFU/mL) reduction in total coliforms. Moreover, despite HPCD causing a decrease in pH and volatile fractions of CW, it retained nutritional and volatile compounds, as well as sensory attributes, significantly better than thermal treatment at 90 °C for 1 min. A detailed analysis of the volatile compound composition revealed that HPCD treatment reduced short- and medium-chain alcohols. In contrast, thermal treatment led to an increase in oxidated compounds, such as aldehydes and 2-acetyl-1-pyrroline, characterized by low odor thresholds with aromas of “cooked rice” and “popcorn” [62]. Additionally, sensory discrimination analysis showed no significant difference between HPCD-treated and untreated CW. However, when CW was treated solely with HPCD under the aforementioned optimized conditions, it exhibited microbiological instability after only 7 days of storage at 4 °C [63]. In contrast, combining HPCD with ultrasound at 12 MPa, 40 °C, and 10 W for 15 min extended the shelf life to 4 weeks [63].
4.3. Cold Plasma (CP)
Plasma, considered the fourth state of matter alongside solid, liquid, and gas, is generated when a single or combination of gases is excited by a high electric field strength using various electric discharge methods [87]. Among these methods, dielectric barrier discharge (DBD) is the most commonly employed. Plasma primarily consists of partially or fully ionized gases, encompassing the coexistence of positively and negatively charged ions, free radicals, excited molecules, UV photons, and other reactive species [88]. CP is generated at ambient temperature and atmospheric pressure and has been extensively utilized for food preservation. It enables the inactivation of a range of foodborne pathogenic and spoilage microorganisms and enzymes without significantly compromising the nutritional and organoleptic properties of foods [87].
Several studies have demonstrated that CP treatments have minimal or negligible effects on several physiochemical properties of CW, such as pH, total titratable acid (TTA), total soluble acid, ascorbic acid, and color parameters [64,65,66,67]. Nevertheless, the magnitude of these effects is highly dependent on the treatment conditions, including voltage, treatment time, and gas composition. For instance, Chutia and Mahanta [64] observed a significant reduction in the total phenolic content of CW when the CP treatment time was increased to 2 min, with a more pronounced decrease at higher voltages. Mahnot, Mahanta, Farkas, Keener, and Misra [67] demonstrated that processing CW with cold plasma generated in air did not affect the pH but reduced the TTA, whereas CP generated in M65 (65% O2, 30% CO2, 5%N2) lowered the pH without affecting the TTA.
When considering microbial safety, CP has been shown to achieve a reduction of approximately 1–2 log (CFU/mL) in natural or inoculated microbial load [66,67,68]. However, this level of inactivation is insufficient to ensure a satisfactory shelf life for CW under refrigerated conditions [64,67]. To enhance the microbial inactivation efficiency of CP on CW, the addition of citric acid has been investigated. A minimum reduction of 5 log (CFU/mL) in inoculated E. coli, L. monocytogenes, and Salmonella, as well as a shelf life of 48 days at 5 °C, was achieved with the addition of 400 ppm of citric acid to CW prior to CP treatment [66,67]. Additionally, Chutia and Mahanta [64] demonstrated that a blended beverage comprising CP-treated CW and orange juice, with the addition of ascorbic acid, maintained a shelf life of 35 days when stored at 6 °C in a glass bottle.
CP processing has also been demonstrated to achieve an inactivation rate of above 70% for POD in CW, with the effectiveness being influenced by processing parameters, such as frequency, voltage, and treatment time [64,65]. Moreover, it was found that compared to PPO, POD has stronger resistance to CP; when aiming for half of the maximum activity value, the processing time required for PPO is always shorter than that for POD, at 18 kV, 23 kV, and 28 kV [68].
4.4. Pulsed Electric Field (PEF)
PEF technology operates by applying intermittent high-voltage direct-current pulses, typically ranging from 100 to 300 V/cm up to 20–80 kV/cm, for very short durations (microseconds to milliseconds) [89,90]. These pulses pass through a food product positioned between two electrodes. When the electrical pulses reach a sufficient intensity, they can induce electroporation and potentially lead to dielectric breakdown [88]. This can cause leakage of intracellular compounds towards the external environment, ultimately resulting in cellular rupture [88]. Numerous studies have demonstrated the potential of PEF in various food processing applications, such as cold pasteurization, extraction of bioactive compounds, as well as drying, dehydration, and freezing processes [90].
Despite extensive research on the application of PEF technology for cold pasteurization of various juices, there is limited literature on its application to CW. Tongdonyod et al. [69] demonstrated the feasibility of combining PEF with mild heat treatment to achieve microbial inactivation while preserving the fresh-like characteristics of CW. The optimal PEF conditions, at 22.5 kV/cm, 119 kJ/L, and 40 °C, were found to inactivate the counts of E. coli K12 and Listeria innocua counts by 6.6 and 5.9 log (CFU/mL), respectively, and extend the shelf life to over 35 days at around 8 °C, comparable to thermal pasteurization at 85 °C for 10 min. Furthermore, PEF treatment resulted in fewer changes in physiochemical properties, sensorial qualities, and volatile flavor profiles of CW compared to thermal pasteurization. However, PEF treatment increased the PPO activity of CW by 17%, while the POD activity decreased by 78%.
4.5. Pulsed Light (PL)
PL relies on the application of a series of high-intensity light pulses ranging from 0.01 to 50 J/cm2 and short durations, typically between 1 μs and 0.1 s [91]. These pulses encompass an intense, broad spectrum spanning from 200 to 1100 nm, which includes ultraviolet (UV) light, visible light (VL), and infrared (IR). The lethal effect of PL on microorganisms is generally attributed to photo-chemical, photo-thermal, and photo-physical mechanisms. Among these, the photo-chemical effect plays a significant role, wherein light photons of UV-C wavelengths, notably 253.7 nm, are absorbed by microbial DNA, thus leading to cell death [91].
By optimizing pulsed light (PL) processing conditions for CW, including the PL fluence rate, exposure time, input voltage to the lamp, and distance between the lamp and the sample, a significant reduction of 5.33 log (CFU/mL) in inoculated E. coli was achieved. Additionally, there were reductions of 5.54 log (CFU/mL) and 4.67 log (CFU/mL) in the aerobic plate count and yeast and mold populations, respectively [70,72]. Furthermore, Basak et al. [71] demonstrated that PL treatment at 2.5 kV for 2.5 min (1073 J⋅cm2) ensured at least a 5-log (CFU/mL) reduction in E. coli, Bacillus cereus, and L. monocytogenes. Nevertheless, complete inactivation of POD and PPO enzymes required more intense PL treatment, at 2.9 kV for 5 min (1073 J/cm2) and 2.9 kV for 6 min (3586 J/cm2), respectively. The milder PL conditions for microbial safety of CW had no adverse effects on its physiochemical properties and sensory attributes. In contrast, more intense PL conditions aimed at achieving both microbial safety and enzymatic stability caused some damage, although they still resulted in higher total phenolics, ascorbic acid, and sensory scores compared to thermal processing at 90 °C for 3 min.
4.6. UV (UV-C) Irradiation
Ultraviolet (UV) light, which spans the electromagnetic spectrum from 100 and 400 nm, has been approved by the United States Food and Drug Administration (FDA) as an alternative technology for pasteurizing fruits and vegetable juices [75]. The germicidal effectiveness of UV light stems from its ability to initiate a complex series of chain reactions within cellular structures, leading to DNA damage [88]. As mentioned earlier, the highest microbial inactivation efficiency is achieved with light in the UV-C region (200–280 nm), as the absorbance of DNA at 253.7 nm closely matches the emitted wavelength.
Several studies have examined the efficiency of UV-C irradiation at 254 nm for nonthermal pasteurization of CW. Bhullar et al. [74] reported that UV-C treatment at 30 mJ/cm2 inactivated E. coli, Salmonella Typhimurium, and L. monocytogenes by above 5 log (CFU/mL) and bacteriophage T1UV by 4.73 log (CFU/mL). However, bacteriophage MS2 exhibited greater resistance to UV-C, requiring approximately 120 mJ/cm2 to achieve nearly 5-log inactivation. Notably, UV-C irradiation ranging from 100 to 400 mJ/cm2 did not produce cytotoxic effects on normal human intestinal cells or normal mouse liver cells. In terms of enzyme activity, UV-C treatment at 400 mJ/cm2 reduced the PPO and POD activity by 94 and 93%, respectively, without significant loss of essential amino acids, although there were slight changes in sensory attributes [18]. In contrast, Augusto et al. [77] discovered that PPO exhibited higher resistance compared to POD to UV treatment of CW within the wavelength range between 250 and 740 nm, with a peak between 400 and 420 nm. Specifically, PPO and POD activity decreased by 98 and 99%, respectively, after 30 min of UV processing. Additionally, Gabriel [76] discovered that common physicochemical stresses, such as acidity, desiccation, or their combinations, increased the exposure times and UV-C energy dose values required to achieve a 90% reduction in the population of inoculated cocktails of E. coli O157:H7, S. enterica, and L. monocytogenes in coconut liquid endosperm beverages, indicating increased resistance to UV-C treatment.
Maguluri et al. [73] investigated the potential of UV-C irradiation at 280 nm for the inactivation and photoreactivation of common food pathogens in CW. They found out that the UV-C doses required to achieve a 5-log reduction in the counts of E. coli and L. monocytogenes were 71 and 20.3 mJ/cm2, respectively. This study also demonstrated that UV treatment at 71 mJ/cm2 resulted in minor changes in the color, ascorbic acid concentration, and some sensory attributes of CW, while having no impact on other physiochemical properties, including pH, TTA, Brix, total phenolic content, mineral content, and sugars.
4.7. Ultrasound
Ultrasound is defined as a sound wave that exceeds the audible frequency range (20 kHz). It operates by inducing acoustic cavitation, which occurs when larger bubbles are generated, undergo expansion, and subsequently undergo rapid collapse, resulting in the release of a significant amount of energy [88]. Ultrasound possesses numerous advantages, including its eco-friendliness, cost-effectiveness, and non-destructiveness, and has been applied in various food processing techniques, such as filtration, freezing and crystallization, drying, sterilization/pasteurization, and extraction [92]. However, using ultrasound alone for food treatment has limited efficiency in achieving enzyme and microbial inactivation [78,79]. For instance, while ultrasound treatment at 60% amplitude with a 6 s pulse at a 20 kHz frequency for 6 min retained the initial quality parameters of CW, spoilage occurred within one week under both ambient and refrigerated conditions [17]. The addition of nisin at a final concentration of 5000 IU/200 mL to ultrasound-treated CW extended its shelf life to two weeks under refrigerated conditions but significantly decreased its nutritional and nutraceutical contents [17].
Combining ultrasound with other processing technologies, particularly thermal treatments, has demonstrated promise and potential in enhancing microbiological safety while maintaining the quality of food products [93]. POD has higher resistance to both ultrasound and thermal treatments, with its activity decreasing by 27 and 50% after ultrasound treatments at 286 W/L and 20 kHz for 30 min and thermal treatments at 80 °C for >30 min, respectively [78]. Pretreating CW with ultrasound has been demonstrated to sensitize POD before thermal treatments, resulting in more uniform heat resistance [78]. Ribeiro, Valdramidis, Nunes, and de Souza [79] also showed the significant additive effect of ultrasound on thermal treatment in inactivating both POD and PPO. Total inactivation of PPO and POD can be achieved after ultrasound treatment with energies of 629.90 (Tmax > 60 °C) and 655.80 (Tmax > 60 °C) W/L, respectively. However, due to the potential generation of off-flavors caused by oxidation reactions, decomposition of bioactive compounds, and negative impacts on physiochemical properties (e.g., pH, color, texture) induced by ultrasound, further investigation is required to evaluate the quality attributes of CW, as well as its microbial safety.
4.8. Ozone
Ozone is a triatomic allotrope of oxygen known for its high oxidizing power, which can effectively inactivate a wide range of microorganisms such as bacteria, viruses, algae, and fungi [65]. Due to its ability to spontaneously decompose into oxygen without leaving hazardous residues on foods, ozone is considered a GRAS chemical. It has been approved by the FDA as an antimicrobial agent for direct application to food since 2001 [88]. The antimicrobial mechanism of ozone involves its interaction with polyunsaturated fatty acids (PUFAs) in the cell envelope, converting them into acid peroxides. Additionally, ozone oxidizes amino acids, peptides, proteins, and enzymes by attacking sulfhydryl groups within these molecules [88].
Ozone treatment, utilizing concentrations within the range of 0.075 to 0.37 mg/mL and achieving over 50% absorption by CW, effectively decreased the activity of POD to undetectable levels, without altering its physicochemical properties or chemical compositions [65]. Nevertheless, Rajashri, Roopa, Negi, and Rastogi [17] demonstrated that ozone processing at 0.02 mg/mL led to spoilage of CW within one week of refrigerated storage. On the other hand, the combination of nisin and ozone processing extended the shelf life of CW to three weeks and inactivated both POD and PPO activity to undetectable levels while preserving its physicochemical, nutritional, and nutraceutical properties.
4.9. Microfiltration
Microfiltration (MF) is a process that ideally only rejects suspended solids while allowing proteins to pass freely through the membrane [94]. The operational principle of MF relies on physical size exclusion, where the membrane’s pore structure acts as a selective barrier based on particle dimensions [8]. This process has the potential to achieve pasteurization and clarification simultaneously. The combination of MF and additives has shown promise in preserving CW while maintaining its physiochemical properties. Purkayastha et al. [80] used a two-stage filtration system, including membrane filters with pore sizes of 0.8 and 0.45 μm, to process CW, followed by the addition of L-ascorbic acid. During storage at 4 °C for 21 days, methyl-α-D-rhamnopyranoside (the primary glycoside detected in micro-filtered CW) was retained, while the formation of free fatty acids was controlled, and rancidity was delayed. The same research group also investigated the preservation of CW using MF with additional additives, including citric acid (200 mg/L), ascorbic acid (180 mg/L), and L-cysteine (90 mg/L), using glass and plastic bottle packaging [81]. The results showed that both package methods could store CW at 4 °C for 46 days with acceptable sensory results, while CW remained sterile for 180 days in glass bottles. Additionally, MF followed by the addition of citric acid (200 mg/L), ascorbic acid (180 mg/L), and orange honey (5%, w/v) and packaging in glass bottles with headspace flushed with nitrogen achieved sterility of CW at 4 °C for 190 days, while maintaining its sensory attributes for 90 days [82].
5. Conclusions
CW has emerged as a popular functional beverage due to its rich nutritional profile, such as sugars, minerals, and vitamins, as well as its diverse biological activities, including antioxidant anti-inflammatory, anti-cancer, cardioprotective, and antimicrobial effects. To develop CW products with superior nutritional and organoleptic properties, innovative food processing technologies, such as high-hydrostatic-pressure processing, high-pressure carbon dioxide, cold plasma, pulsed electric field, pulsed light, UV-C irradiation, ultrasound, ozone, and microfiltration, have been explored. These methods offer promising alternatives to traditional thermal processing by preserving CW’s quality attributes while achieving microbial and enzyme inactivation.
Coconut is a vital agricultural commodity in tropical regions, contributing significantly to global trade and economic growth. However, in some areas, the utilization of coconut resources remains underdeveloped, with mature CW often discarded, leading to environmental pollution, resource wastage, and challenges for sustainable industry growth. To address these challenges, efforts should focus on enhancing the technological content of coconut products, expanding market opportunities, and improving coconut meat processing capabilities. Additionally, identifying natural enzyme inhibitors to prevent enzymatic degradation in CW offers a promising research direction for better maintaining its nutritional value, taste, and flavor. Further exploration of coconut protein’s nutritional and health benefits could support the development of functional food products, advancing the food processing industry and contributing to the global tropical economy. By-products from the coconut industry also hold significant potential as nutraceuticals for managing metabolic disorders, offering both health and economic benefits on a global scale.
Author Contributions
Conceptualization, S.S. and W.W.; software, P.Y.; investigation, F.W. and X.H.; resources, F.W. and H.Y.; writing—original draft preparation, S.S. and W.W.; writing—review and editing, X.L.; visualization, F.W. and P.Y.; supervision, X.L.; project administration, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the 2115 Talent Development Program of China Agricultural University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| USDA | United States Department of Agriculture |
| TSS | Total Soluble Solids |
| CW | Coconut Water |
| TCW | Tender Coconut Water |
| HHP | High Hydrostatic Pressure |
| HPCD | High-Pressure Carbon Dioxide |
| CP | Cold Plasma |
| PPO | Polyphenol Oxidase |
| POD | Peroxidase |
| PEF | Pulsed Electric Field |
| PL | Pulsed Light |
| UV | Ultraviolet |
| MF | Microfiltration |
References
- Carvalho de Castro, J.M.; Nascimento Alves, C.A.; de LimaSantos, K.; de Oliveira Silva, E.; Maria da Silva Araújo, Í.; Barros deVasconcelos, L. Elaboration of a mixed beverage from hibiscus and coconut water: An evaluation of bioactive and sensory properties. Int. J. Gastron. Food Sci. 2021, 23, 100284. [Google Scholar] [CrossRef]
- Ignacio, I.-F.; Miguel, T.-S. Research opportunities on the coconut (Cocos nucifera L.) using new technologies. S. Afr. J. Bot. 2021, 141, 414–420. [Google Scholar] [CrossRef]
- Segura-Badilla, O.; Lazcano-Hernández, M.; Kammar-García, A.; Vera-López, O.; Aguilar-Alonso, P.; Ramírez-Calixto, J.; Navarro-Cruz, A.R. Use of coconut water (Cocus nucifera L.) for the development of a symbiotic functional drink. Heliyon 2020, 6, e03653. [Google Scholar] [CrossRef] [PubMed]
- Raghubeer, E.V.; Phan, B.N.; Onuoha, E.; Diggins, S.; Aguilar, V.; Swanson, S.; Lee, A. The use of High-Pressure Processing (HPP) to improve the safety and quality of raw coconut (Cocos nucifera L.) water. Int. J. Food Microbiol. 2020, 331, 108697. [Google Scholar] [CrossRef]
- Labouisse, J.-P.; Sileye, T.; Bonnot, F.o.; Baudouin, L. Achievements in breeding coconut hybrids for tolerance to coconut foliar decay disease in Vanuatu, South Pacific. Euphytica 2011, 177, 1–13. [Google Scholar] [CrossRef]
- Mat, K.; Abdul Kari, Z.; Rusli, N.D.; Che Harun, H.; Wei, L.S.; Rahman, M.M.; Mohd Khalid, H.N.; Mohd Ali Hanafiah, M.H.; Mohamad Sukri, S.A.; Raja Khalif, R.I.A.; et al. Coconut Palm: Food, Feed, and Nutraceutical Properties. Animals 2022, 12, 2107. [Google Scholar] [CrossRef]
- Alchoubassi, G.; Kinska, K.; Bierla, K.; Lobinski, R.; Szpunar, J. Speciation of essential nutrient trace elements in coconut water. Food Chem. 2021, 339, 127680. [Google Scholar] [CrossRef]
- Prithviraj, V.; Pandiselvam, R.; Babu, A.C.; Kothakota, A.; Manikantan, M.R.; Ramesh, S.V.; Beegum, P.P.S.; Mathew, A.C.; Hebbar, K.B. Emerging non-thermal processing techniques for preservation of tender coconut water. LWT 2021, 149, 111850. [Google Scholar] [CrossRef]
- Lamdande, A.G.; Mittal, R.; Raghavarao, K.S.M.S. Flux evaluation based on fouling mechanism in acoustic field-assisted ultrafiltration for cold sterilization of tender coconut water. Innov. Food Sci. Emerg. Technol. 2020, 61, 102312. [Google Scholar] [CrossRef]
- Kumar, M.; Saini, S.S.; Agrawal, P.K.; Roy, P.; Sircar, D. Nutritional and metabolomics characterization of the coconut water at different nut developmental stages. J. Food Compos. Anal. 2021, 96, 103738. [Google Scholar] [CrossRef]
- Bhagya, D.; Prema, L.; Rajamohan, T. Therapeutic effects of tender coconut water on oxidative stress in fructose fed insulin resistant hypertensive rats. Asian Pac. J. Trop. Med. 2012, 5, 270–276. [Google Scholar] [CrossRef]
- Rukmini, J.N.; Manasa, S.; Rohini, C.; Sireesha, L.; Ritu, S.; Umashankar, G.K. Antibacterial efficacy of tender coconut water (Cocos nucifera L.) on Streptococcus mutans: An in-vitro study. J. Int. Soc. Prev. Community Dent. 2017, 7, 130–134. [Google Scholar] [CrossRef]
- Preetha, P.P.; Devi, V.G.; Rajamohan, T. Mature coconut water exhibits antidiabetic and antithrombotic potential via L-arginine-nitric oxide pathway in alloxan induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Najam, R. Coconut water of different maturity stages ameliorates inflammatory processes in model of inflammation. J. Intercult. Ethnopharmacol. 2016, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Dennison, S.R.; Mura, M.; Lea, R.W.; Snape, T.J.; Harris, F. Cn-AMP2 from green coconut water is an anionic anticancer peptide. J. Pept. Sci. 2014, 20, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Anurag, P.; Rajamohan, T. Cardioprotective effect of tender coconut water in experimental myocardial infarction. Plant Foods Hum. Nutr. 2003, 58, 1–12. [Google Scholar] [CrossRef]
- Rajashri, K.; Roopa, B.S.; Negi, P.S.; Rastogi, N.K. Effect of ozone and ultrasound treatments on polyphenol content, browning enzyme activities, and shelf life of tender coconut water. J. Food Process. Preserv. 2019, 44, e14363. [Google Scholar] [CrossRef]
- Yannam, S.K.; Patras, A.; Pendyala, B.; Vergne, M.; Ravi, R.; Gopisetty, V.V.S.; Sasges, M. Effect of UV-C irradiation on the inactivation kinetics of oxidative enzymes, essential amino acids and sensory properties of coconut water. J. Food Sci. Technol. 2020, 57, 3564–3572. [Google Scholar] [CrossRef]
- Prades, A.; Dornier, M.; Diop, N.; Pain, J.-P. Coconut water preservation and processing: A review. Fruits 2012, 67, 157–171. [Google Scholar] [CrossRef]
- Divya, P.M.; Roopa, B.S.; Manusha, C.; Balannara, P. A concise review on oil extraction methods, nutritional and therapeutic role of coconut products. J. Food Sci. Technol. 2022, 60, 441–452. [Google Scholar] [CrossRef]
- Jayawardena, J.A.E.C.; Vanniarachchy, M.P.G.; Wansapala, M.A.J. Progressive Freeze Concentration of Coconut Water and Use of Partial Ice Melting Method for Yield Improvement. Int. J. Food Sci. 2020, 2020, 4292013. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.B.P.; Buvaneswaran, M.; Anto, A.M.; Sinija, V.R. High-pressure processing for inactivation of enzymes, microbes, and its effect on nutritional composition of tender coconut water. J. Food Process Eng. 2024, 47, e14624. [Google Scholar] [CrossRef]
- Burns, D.T.; Johnston, E.L.; Walker, M.J. Authenticity and the Potability of Coconut Water—A Critical Review. J. AOAC Int. 2020, 103, 800–806. [Google Scholar] [CrossRef]
- Yong, J.W.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Yun, Y.; Li, C.; Fang, Y.; Zhang, W. Discrimination and characterization of different coconut water (CW) by their phenolic composition and volatile organic compounds (VOCs) using LC-MS/MS, HS-SPME-GC-MS, and HS-GC-IMS. J. Food Sci. 2023, 88, 3758–3772. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Y.; Ran, L.; Liu, R.; Sun, X.; Hu, L.; Xiao, Y.; Chen, F. Flavor deterioration of liquid endosperm in postharvest tender coconut revealed by LC-MS-based metabolomics, GC-IMS and E-tongue. Postharvest Biol. Technol. 2022, 187, 111866. [Google Scholar] [CrossRef]
- Appaiah, P.; Sunil, L.; Kumar, P.K.; Krishna, A.G. Physico-chemical characteristics and stability aspects of coconut water and kernel at different stages of maturity. J. Food Sci. Technol. 2015, 52, 5196–5203. [Google Scholar] [CrossRef]
- Halim, H.H.; Pak Dek, M.S.; Hamid, A.A.; Saari, N.; Mohd Lazim, M.I.; Abas, F.; Ngalim, A.; Ismail, A.; Jaafar, A.H. Novel sources of bioactive compounds in coconut (Cocos nucifera L.) water from different maturity levels and varieties as potent skin anti-aging strategies and anti-fatigue agents. Food Biosci. 2023, 51, 102326. [Google Scholar] [CrossRef]
- Manna, K.; Khan, A.; Das, D.K.; Bandhu Kesh, S.; Das, U.; Ghosh, S.; Sharma Dey, R.; Das Saha, K.; Chakraborty, A.; Chattopadhyay, S.; et al. Protective effect of coconut water concentrate and its active component shikimic acid against hydroperoxide mediated oxidative stress through suppression of NF-κB and activation of Nrf2 pathway. J. Ethnopharmacol. 2014, 155, 132–146. [Google Scholar] [CrossRef]
- Prades, A.; Dornier, M.; Diop, N.; Pain, J.-P. Coconut water uses, composition and properties: A review. Fruits 2012, 67, 87–107. [Google Scholar] [CrossRef]
- Shayanthavi, S.; Kapilan, R.; Wickramasinghe, I. Comprehensive analysis of physicochemical, nutritional, and antioxidant properties of various forms and varieties of tender coconut (Cocos nucifera L.) water in Northern Sri Lanka. Food Chem. Adv. 2024, 4, 100645. [Google Scholar] [CrossRef]
- Preetha, P.P.; Girija Devi, V.; Rajamohan, T. Effects of coconut water on carbohydrate metabolism and pancreatic pathology of alloxan induced diabetic rats. Eur. J. Integr. Med. 2013, 5, 234–240. [Google Scholar] [CrossRef]
- Lima, E.B.C.; Sousa, C.N.S.; Meneses, L.N.; Ximenes, N.C.; Santos Júnior, M.A.; Vasconcelos, G.S.; Lima, N.B.C.; Patrocínio, M.C.A.; Macedo, D.; Vasconcelos, S.M.M. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz. J. Med. Biol. Res. 2015, 48, 953–964. [Google Scholar] [CrossRef]
- Ndukwe, M.K.; Atiaetuk, I.E.; Aja, O.A.; Edward, U.I.; Mba, O.J. In vivo Antioxidant Effects of Coconut (Cocos nucifera) Water Extract in Wistar Albino Rats. Asian J. Res. Biochem. 2020, 7, 28–35. [Google Scholar] [CrossRef]
- Lakshmanan, J.; Zhang, B.; Wright, K.; Motameni, A.T.; Jaganathan, V.L.; Schultz, D.J.; Klinge, C.M.; Harbrecht, B.G. Tender coconut water suppresses hepatic inflammation by activating AKT and JNK signaling pathways in an in vitro model of sepsis. J. Funct. Foods 2020, 64, 103637. [Google Scholar] [CrossRef] [PubMed]
- Prathapan, A.; Rajamohan, T. Antioxidant and Antithrombotic Activity of Tender Coconut Water in Experimental Myocardial Infarction. J. Food Biochem. 2010, 35, 1501–1507. [Google Scholar] [CrossRef]
- Adebolu, T.T.; Akinjayeju, D.O. Effects of Coconut (Cocos nucifera Linn.) Water and Oil on the Ileal Bacterial Flora of Apparently Healthy Wistar Albino Rats. J. Adv. Microbiol. 2020, 20, 94–102. [Google Scholar] [CrossRef]
- Gandhi, M.; Aggarwal, M.; Puri, S.; Singla, S.K. Prophylactic effect of coconut water (Cocos nucifera L.) on ethylene glycol induced nephrocalcinosis in male wistar rat. Int. Braz. J. Urol. 2013, 39, 108–117. [Google Scholar] [CrossRef]
- Patel, R.M.; Jiang, P.; Asplin, J.; Granja, I.; Capretz, T.; Osann, K.; Okhunov, Z.; Landman, J.; Clayman, R.V. Coconut Water: An Unexpected Source of Urinary Citrate. BioMed Res. Int. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Ct, D.R.; Palaninathan, V.; James, R.A. Anti-uropathogenic, antioxidant and struvite crystallization inhibitory potential of fresh and fermented coconut water. Biocatal. Agric. Biotechnol. 2023, 47, 102555. [Google Scholar] [CrossRef]
- Kumar, S.S.; Manna, K.; Das, A. Tender coconut water attenuates heat stress-induced testicular damage through modulation of the NF-κB and Nrf2 pathways. Food Funct. 2018, 9, 5463–5479. [Google Scholar] [CrossRef] [PubMed]
- Radenahmad, N.; Saleh, F.; Sayoh, I.; Sawangjaroen, K.; Subhadhirasakul, P.; Boonyoung, P.; Rundorn, W.; Mitranun, W. Young coconut juice can accelerate the healing process of cutaneous wounds. BMC Complement. Altern. Med. 2012, 12, 252. [Google Scholar]
- Mahayothee, B.; Koomyart, I.; Khuwijitjaru, P.; Siriwongwilaichat, P.; Nagle, M.; Müller, J. Phenolic Compounds, Antioxidant Activity, and Medium Chain Fatty Acids Profiles of Coconut Water and Meat at Different Maturity Stages. Int. J. Food Prop. 2016, 19, 2041–2051. [Google Scholar] [CrossRef]
- Luiz, J.; Bispo, V.; Chaves Filho, A.; Dantas, L.; Vasconcelos, D.; Abreu, F.; Melo, D.; Araujo Matos, I.; Freitas, F.; Gomes, O.; et al. Evaluation of Chemical Constituents and Antioxidant Activity of Coconut Water (Cocus nucifera L.) and Caffeic Acid in Cell Culture. An. Da Acad. Bras. De Ciencias 2013, 85, 1235–1247. [Google Scholar] [CrossRef]
- Manoharan, P.; Dhanabalan, S.C.; Alagan, M.; Muthuvijayan, S.; Ponraj, J.S.; Somasundaram, C.K. Facile synthesis and characterisation of green luminescent carbon nanodots prepared from tender coconut water using the acid-assisted ultrasonic route. Micro Nano Lett. 2020, 15, 920–924. [Google Scholar] [CrossRef]
- Arzeta-Ríos, A.J.; Guerra-Ramírez, D.; Reyes-Trejo, B.; Ybarra-Moncada, M.C.; Zuleta-Prada, H. Microwave heating effect on total phenolics and antioxidant activity of green and mature coconut water. Int. J. Food Eng. 2020, 16, 20190378. [Google Scholar] [CrossRef]
- Ge, L.; Yong, J.W.H.; Tan, S.N.; Yang, X.H.; Ong, E.S. Analysis of cytokinin nucleotides in coconut (Cocos nucifera L.) water using capillary zone electrophoresis-tandem mass spectrometry after solid-phase extraction. J. Chromatogr. A 2006, 1133, 322–331. [Google Scholar] [CrossRef]
- Mohd Lazim, M.I.; Badruzaman, B. Quantification of Cytokinins in Coconut Water from Different Maturation Stages of Malaysia’s Coconut (Cocos nucifera L.) Varieties. J. Food Process. Technol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Fonseca, V.A. Defining and Characterizing the Progression of Type 2 Diabetes. Diabetes Care 2009, 32, S151–S156. [Google Scholar] [CrossRef]
- Supapvanich, S.; Yimpong, A.; Srisuwanwichan, J. Browning inhibition on fresh-cut apple by the immersion of liquid endosperm from mature coconuts. J. Food Sci. Technol. 2020, 57, 4424–4431. [Google Scholar] [CrossRef]
- Sandhya, V.G.; Rajamohan, T. Comparative evaluation of the hypolipidemic effects of coconut water and lovastatin in rats fed fat–cholesterol enriched diet. Food Chem. Toxicol. 2008, 46, 3586–3592. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.P.; Becker, L.C.; Kass, D.A.; Champion, H.C.; Terrin, M.L.; Forman, S.; Ernst, K.V.; Kelemen, M.D.; Townsend, S.N.; Capriotti, A.; et al. L-Arginine Therapy in Acute Myocardial InfarctionThe Vascular Interaction with Age in Myocardial Infarction (VINTAGE MI) Randomized Clinical Trial. JAMA 2006, 295, 58–64. [Google Scholar] [CrossRef]
- Anaya, K.; Podszun, M.; Franco, O.L.; de Almeida Gadelha, C.A.; Frank, J. The Coconut Water Antimicrobial Peptide CnAMP1 Is Taken up into Intestinal Cells but Does Not Alter P-Glycoprotein Expression and Activity. Plant Foods Hum. Nutr. 2020, 75, 396–403. [Google Scholar] [CrossRef]
- Mandal, S.M.; Dey, S.; Mandal, M.; Sarkar, S.; Maria-Neto, S.; Franco, O.L. Identification and structural insights of three novel antimicrobial peptides isolated from green coconut water. Peptides 2009, 30, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Macalalag, E.V., Jr.; Macalalag, A.L. Bukolysis: Young coconut water renoclysis for urinary stone dissolution. Int. Surg. 1987, 72, 247. [Google Scholar]
- Adams, W.; Bratt, D.E. Young coconut water for home rehydration in children with mild gastroenteritis. Trop. Geogr. Med. 1992, 44, 149–153. [Google Scholar] [PubMed]
- Naik, M.; Sunil, C.K.; Rawson, A.; Venkatachalapathy, N. Tender Coconut Water: A Review on Recent Advances in Processing and Preservation. Food Rev. Int. 2022, 38, 1215–1236. [Google Scholar] [CrossRef]
- Cunha, A.G.; Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; Rodrigues, T.H.S.; Brito, E.S.d.; Miranda, M.R.A.d. Chemical composition of thermally processed coconut water evaluated by GC–MS, UPLC-HRMS, and NMR. Food Chem. 2020, 324, 126874. [Google Scholar] [CrossRef]
- González-Angulo, M.; Clauwers, C.; Harastani, R.; Tonello, C.; Jaime, I.; Rovira, J.; Michiels, C.W. Evaluation of factors influencing the growth of non-toxigenic Clostridium botulinum type E and Clostridium sp. in high-pressure processed and conditioned tender coconut water from Thailand. Food Res. Int. 2020, 134, 109278. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, L.; Wang, S.; Xu, Z.; Liao, X.; Cheng, Y. Comparison of the quality attributes of coconut waters by high-pressure processing and high-temperature short time during the refrigerated storage. Food Sci. Nutr. 2019, 7, 1512–1519. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ferrentino, G.; Endrizzi, I.; Aprea, E.; Betta, E.; Corollaro, M.L.; Charles, M.; Gasperi, F.; Spilimbergo, S. High Pressure Carbon Dioxide pasteurization of coconut water: A sport drink with high nutritional and sensory quality. J. Food Eng. 2015, 145, 73–81. [Google Scholar] [CrossRef]
- De Marchi, F.; Aprea, E.; Endrizzi, I.; Charles, M.; Betta, E.; Corollaro, M.L.; Cappelletti, M.; Ferrentino, G.; Spilimbergo, S.; Gasperi, F. Effects of Pasteurization on Volatile Compounds and Sensory Properties of Coconut (Cocos nucifera L.) Water: Thermal vs. High-Pressure Carbon Dioxide Pasteurization. Food Bioprocess Technol. 2015, 8, 1393–1404. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ferrentino, G.; Spilimbergo, S. Supercritical carbon dioxide combined with high power ultrasound: An effective method for the pasteurization of coconut water. J. Supercrit. Fluids 2014, 92, 257–263. [Google Scholar] [CrossRef]
- Chutia, H.; Mahanta, C.L. Influence of cold plasma voltage and time on quality attributes of tender coconut water (Cocos nucifera L.) and degradation kinetics of its blended beverage. J. Food Process. Preserv. 2021, 45, e15372. [Google Scholar] [CrossRef]
- Porto, E.; Alves Filho, E.G.; Silva, L.M.A.; Fonteles, T.V.; do Nascimento, R.B.R.; Fernandes, F.A.N.; de Brito, E.S.; Rodrigues, S. Ozone and plasma processing effect on green coconut water. Food Res. Int. 2020, 131, 109000. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Mahanta, C.L.; Keener, K.M.; Misra, N.N. Strategy to achieve a 5-log Salmonella inactivation in tender coconut water using high voltage atmospheric cold plasma (HVACP). Food Chem. 2019, 284, 303–311. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Mahanta, C.L.; Farkas, B.E.; Keener, K.M.; Misra, N.N. Atmospheric cold plasma inactivation of Escherichia coli and Listeria monocytogenes in tender coconut water: Inoculation and accelerated shelf-life studies. Food Control. 2019, 106, 106678. [Google Scholar] [CrossRef]
- Chutia, H.; Kalita, D.; Mahanta, C.L.; Ojah, N.; Choudhury, A.J. Kinetics of inactivation of peroxidase and polyphenol oxidase in tender coconut water by dielectric barrier discharge plasma. LWT 2019, 101, 625–629. [Google Scholar] [CrossRef]
- Tongdonyod, S.; Thikham, S.; Kittiwachana, S.; Wichaphon, J.; Klangpetch, W. Optimization of pulsed electric fields combined with mild heat treatment on microbial inactivation of tender coconut water and evaluation of quality attributes during storage. Innov. Food Sci. Emerg. Technol. 2023, 90, 103507. [Google Scholar] [CrossRef]
- Preetha, P.; Pandiselvam, R.; Varadharaju, N.; Kennedy, Z.J.; Balakrishnan, M.; Kothakota, A. Effect of pulsed light treatment on inactivation kinetics of Escherichia coli (MTCC 433) in fruit juices. Food Control 2021, 121, 107547. [Google Scholar] [CrossRef]
- Basak, S.; Jha, T.; Chakraborty, S. Pasteurization of tender coconut water by pulsed light treatment: Microbial safety, enzymatic inactivation, and impact on physicochemical properties. Innov. Food Sci. Emerg. Technol. 2023, 84, 103302. [Google Scholar] [CrossRef]
- Preetha, P.; Varadharaju, N.; Jeevarathinam, G.; Deepa, J.; Kumar, A.P.M.; Balakrishnan, M.; Rajkumar, P.; Pandiselvam, R. Optimization of continuous flow pulsed light system process parameters for microbial inactivation in tender coconut water, pineapple and orange juice. J. Food Process Eng. 2023, 46, e14254. [Google Scholar] [CrossRef]
- Maguluri, R.K.; Dasalkar, A.H.; Singam, S.S.R.; Yannam, S.K. Impact of 280 nm UV-C LEDs on the microbial safety and nutritional quality in tender coconut water. Food Humanit. 2023, 1, 864–872. [Google Scholar] [CrossRef]
- Bhullar, M.S.; Patras, A.; Kilanzo-Nthenge, A.; Pokharel, B.; Yannam, S.K.; Rakariyatham, K.; Pan, C.; Xiao, H.; Sasges, M. Microbial inactivation and cytotoxicity evaluation of UV irradiated coconut water in a novel continuous flow spiral reactor. Food Res. Int. 2018, 103, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gautam, D.; Umagiliyage, A.L.; Dhital, R.; Joshi, P.; Watson, D.G.; Fisher, D.J.; Choudhary, R. Nonthermal pasteurization of tender coconut water using a continuous flow coiled UV reactor. LWT—Food Sci. Technol. 2017, 83, 127–131. [Google Scholar] [CrossRef]
- Gabriel, A.A. Previous physicochemical stress exposures influence subsequent resistance of Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes to ultraviolet-C in coconut liquid endosperm beverage. Int. J. Food Microbiol. 2015, 201, 7–16. [Google Scholar] [CrossRef]
- Augusto, P.E.D.; Ibarz, R.; Garvín, A.; Ibarz, A. Peroxidase (POD) and polyphenol oxidase (PPO) photo-inactivation in a coconut water model solution using ultraviolet (UV). Food Res. Int. 2015, 74, 151–159. [Google Scholar] [CrossRef]
- Rojas, M.L.; Trevilin, J.H.; Funcia, E.d.S.; Gut, J.A.W.; Augusto, P.E.D. Using ultrasound technology for the inactivation and thermal sensitization of peroxidase in green coconut water. Ultrasonics Sonochem. 2017, 36, 173–181. [Google Scholar] [CrossRef]
- Ribeiro, M.d.M.; Valdramidis, V.P.; Nunes, C.A.; de Souza, V.R. Synergistic effect of thermosonication to reduce enzymatic activity in coconut water. Innov. Food Sci. Emerg. Technol. 2017, 41, 404–410. [Google Scholar] [CrossRef]
- Purkayastha, M.D.; Kalita, D.; Das, V.K.; Mahanta, C.L.; Thakur, A.J.; Chaudhuri, M.K. Effects of L-ascorbic acid addition on micro-filtered coconut water: Preliminary quality prediction study using 1H-NMR, FTIR and GC-MS. Innov. Food Sci. Emerg. Technol. 2012, 13, 184–199. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Kalita, D.; Mahanta, C.L.; Chaudhuri, M.K. Effect of additives on the quality of tender coconut water processed by nonthermal two stage microfiltration technique. LWT—Food Sci. Technol. 2014, 59, 1191–1195. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Gupta, K.; Mahanta, C.L. Shelf life enhancement and associated quality and sensory changes on refrigerated storage of tender coconut water subjected to non-thermal microfiltration and treated with additives. J. Food Sci. Technol. 2019, 56, 3408–3421. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; Rao, L.; Zhao, L.; Wu, X.; Wang, Y.; Liao, X. Effect of high hydrostatic pressure processing on the structure, functionality, and nutritional properties of food proteins: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4640–4682. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Rao, L.; Zhao, L.; Wu, X.; Wang, Y.; Liao, X. High pressure processing combined with selected hurdles: Enhancement in the inactivation of vegetative microorganisms. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1800–1828. [Google Scholar] [CrossRef]
- Aganovic, K.; Hertel, C.; Vogel, R.F.; Johne, R.; Schlüter, O.; Schwarzenbolz, U.; Jäger, H.; Holzhauser, T.; Bergmair, J.; Roth, A.; et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3225–3266. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical Carbon Dioxide Applications in Food Processing. Food Eng. Rev. 2021, 13, 570–591. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]
- Bigi, F.; Maurizzi, E.; Quartieri, A.; De Leo, R.; Gullo, M.; Pulvirenti, A. Non-thermal techniques and the “hurdle” approach: How is food technology evolving? Trends Food Sci. Technol. 2023, 132, 11–39. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Gavahian, M.; Franco, D.; Zhang, W.; Mousavi Khaneghah, A.; Guerrero-Sánchez, Y.; Lorenzo, J.M. Impact of pulsed light processing technology on phenolic compounds of fruits and vegetables. Trends Food Sci. Technol. 2021, 115, 1–11. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K. Opportunities and Challenges in Application of Ultrasound in Food Processing. Crit. Rev. Food Sci. Nutr. 2011, 51, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Urošević, T.; Povrenović, D.; Vukosavljević, P.; Urošević, I.; Stevanović, S. Recent developments in microfiltration and ultrafiltration of fruit juices. Food Bioprod. Process. 2017, 106, 147–161. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).